Abstract
Leukocytes migrate from the blood into areas of inflammation by interacting with various adhesion molecules on endothelial cells. Vascular adhesion protein-1 (VAP-1) is a glycoprotein expressed on inflamed endothelium where it plays a dual role: it is both an enzyme that oxidizes primary amines and an adhesin that is involved in leukocyte trafficking to sites of inflammation. Although VAP-1 was identified more than 15 years ago, the counterreceptor(s) for VAP-1 on leukocytes has remained unknown. Here we have identified Siglec-10 as a leukocyte ligand for VAP-1 using phage display screenings. The binding between Siglec-10 and VAP-1 was verified by different adhesion assays, and this interaction was also consistent with molecular modeling. Moreover, the interaction between Siglec-10 and VAP-1 led to increased hydrogen peroxide production, indicating that Siglec-10 serves as a substrate for VAP-1. Thus, the Siglec-10–VAP-1 interaction seems to mediate lymphocyte adhesion to endothelium and has the potential to modify the inflammatory microenvironment via the enzymatic end products.
Introduction
Maintenance of an adequate immune defense is largely dependent on migration of circulating leukocytes from the vasculature into the surrounding tissue. Extravasation of leukocytes from the blood into the tissues is controlled by a multistep cascade, which involves numerous adhesion and signaling molecules. First, leukocytes tether and roll on the vascular endothelium, after which they adhere more strongly, arrest, and finally diapedese through the vessel wall.1,2
One of the endothelial molecules involved in this cascade is a 180-kDa homodimeric glycoprotein, vascular adhesion protein-1 (VAP-1).3 VAP-1 is mainly expressed on vascular endothelium, on smooth muscle cells, and on adipocytes.4 It is rapidly translocated to the endothelial cell surface on inflammation, where it supports recruitment of leukocytes.5,6 VAP-1 is involved in the rolling, firm adhesion, and transmigration phases of the extravasation cascade. Besides being an adhesin, VAP-1 is also an enzyme (semicarbazide sensitive amine oxidase, SSAO) and catalyzes oxidative deamination of a primary amine to an aldehyde with concomitant release of hydrogen peroxide and ammonium.4 We and others have shown that VAP-1 supports leukocyte recruitment to sites of inflammation via both enzyme-activity–dependent and enzyme-activity–independent ways. Monoclonal anti–VAP-1 antibodies, which do not block the enzymatic activity of VAP-1, still block the binding of leukocytes to the endothelium in vitro and in vivo, and inhibitors of the enzymatic function of VAP-1 are able to effectively prevent the interaction between leukocytes and endothelium in vitro and in vivo.7,8 Moreover, it has been recently shown that the end products generated by the catalytic activity of VAP-1, especially hydrogen peroxide, play a role in the regulation of other homing-associated molecules and thus enhance the inflammatory response.9,10
Although VAP-1 has been known already for a long time and the role of VAP-1 in extravasation is rather well characterized, its counterreceptor on leukocytes has remained elusive.
Methods
Purified proteins, antibodies, reagents, synthetic peptides
Recombinant VAP-1 protein was obtained from Chinese hamster ovary (CHO) cells stably transfected with the full-length human VAP-1 cDNA.11 The harvested cells were lysed using lysis buffer (150mM NaCl, 10mM Tris base, pH 7.2, 1.5mM MgCl2, 1% [vol/vol] NP40, 1mM phenylmethylsulfonyl fluoride, and 1% aprotinin). CnBr Sepharose 4B beads (GE Healthcare) armed with an anti–VAP-1 monoclonal antibody (mAb: Jg-2.10, a gift from Prof E. Butcher, Stanford University, CA) was used for the affinity purification of VAP-1 protein from the lysate. The bound VAP-1 protein was eluted from the column with 50mM triethanolamine after extensive washes of the column with the lysis buffer. After elution and lyophilization, the purity of the VAP-1 protein was analyzed on a silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis gradient gel (5%-12.5%). Amine oxidase activity was measured using a fluorometric Amplex Red assay.8
mAb TK-8-18 against human VAP-1 and the monoclonal (5G6) and polyclonal antibodies against Siglec-10 as well as Siglec-10–Ig and CD44-Ig chimeras have been described.12-14 mAbs, 3G6 against chicken T cells3 and 9B5 against human CD44,15 were used as negative control antibodies. Polyclonal rabbit anti–VAP-1 antibody was made against recombinant human VAP-1. Fluorescein isothiocyanate (FITC)–conjugated anti-CD19 was from eBioscience and anti–P-selectin from R&D Systems. The second-stage antibodies were purchased as follows: streptavidin-horseradish peroxidase (HRP)–conjugated anti–human-IgG (Fc-spesific) from BD Biosciences, FITC-conjugated anti–rabbit-IgG from Sigma-Aldrich, Alexa 488–conjugated anti-FITC, Alexa 546–conjugated anti–rat IgG, anti–sheep IgG, and Prolong Antifade Gold from Invitrogen. Carboxyfluorescein succinimidyl ester (CFSE) was from Invitrogen and BM chemiluminescence enzyme-linked immunosorbent assay (ELISA) substrate and neuraminidase from Roche Diagnostics. Semicarbazide and clorgyline were purchased from Sigma-Aldrich, and synthetic peptides from NeoMPS.
Phage display screening
Phage display screening with a peptide library displaying CX8C decapeptides (where X is any amino acid) was performed as described.16 Briefly, recombinant human VAP-1 (100 μg/mL in Tris-buffered saline) was coated onto a Nunc Maxisorp 96-well plate. Specifically bound phages were eluted from the wells and used to infect K91kan Escherichia coli. Three more rounds of panning were performed in the same manner. For colony sequencing, primers 5′ and 3′ to the peptide insertion site of the phage were used: the forward primer was 5′-TAATACGACTCACTATAGGGCAAGCTGATTAACCGATACAAT-3′ and the reverse primer 5′-CCCTCATAGTTAGCGTAACGATCT-3′.
Cell lines and cell culture
CHO cells stably transfected with VAP-1, vector only, and Siglec-10 cDNAs have been described.13,17 CHO cells expressing the extracellular regions of Siglec-11 and Siglec-G fused to the green fluorescent protein and membrane anchored through glycosylphosphatidylinositol were developed using a novel expression system (D.M.O. and P.R.C., manuscript in preparation). The enzymatically inactive mutant CHO-VAP-1Y471F and the RGD mutant CHO-VAP-1D720A have been generated as described by Koskinen et al7 and Salmi et al,17 respectively.
Mice
VAP-1 knockout (KO) and VAP-1 transgenic KO mice expressing human VAP-1 on endothelium (KOTG) mice (VAP-1–deficient mice expressing human VAP-1 as a transgene on endothelium under Tie-1 promoter9 ) were used with the approval of the ethical committee at the University of Turku.
Immunohistochemistry
Frozen sections of mesenteric lymph nodes of VAP-1 KO and VAP-1 KOTG mice were stained with anti–human VAP-1 (Jg-2.10) followed by FITC-conjugated anti–rat IgG as a second-stage reagent. Heart sections from KO and KOTG mice were first preincubated either with CD44-Ig chimera (control) or Siglec-10–Ig chimera (10 μg/mL) followed by anti–human VAP-1 (Jg-2.10) and Alexa 546–conjugated anti–rat IgG second-stage reagent.
Adhesion assays
Assays with peptides, recombinant proteins, chimeras.
Recombinant VAP-1 and bovine serum albumin (BSA; as a negative control) were immobilized in wells of Nunc Maxisorp 96-well plates overnight at 4°C, blocked with phosphate-buffered saline (PBS)/3% BSA, and incubated with biotinylated peptides (10 μg/mL or 100 μg/mL) for 2 hours at room temperature. Bound peptides were detected with HRP-conjugated streptavidin and measured with a luminometer (Tecan Ultra; Tecan).
Alternatively, anti–human IgG (Fc specific) antibody was coated onto Nunc Maxisorp wells overnight at 4°C. After blocking with PBS/2% BSA, Siglec-10–Ig chimera (1 μg/mL) was immobilized to wells via the Fc-tail and recombinant VAP-1 (1 μg/mL and 5 μg/mL) was incubated in the wells for 2 hours at room temperature. Bound VAP-1 was detected with biotinylated anti–human VAP-1 antibody (TK-8-18) followed by HRP-conjugated streptavidin. Analyses were performed with a luminometer.
Assays with transfectants.
CHO-Siglec-10 or CHO-mock cells were incubated with 20 μg/mL recombinant VAP-1 followed by a polyclonal anti–VAP-1 antibody, FITC-conjugated anti–rabbit IgG secondary antibody, and Alexa 488–conjugated anti-FITC antibody. Binding was determined using FACSCalibur and Cellquest software (BD Biosciences).
CHO-VAP-1, CHO-VAP-1Y471F, CHO-VAP-1D720A, or CHO-mock cells were cultured to confluence on 96-well plates. After blocking the wells with PBS/1% BSA, 2 × 105 CFSE-labeled CHO-Siglec-10, Siglec-11, or Siglec-G cells were added and incubated for 30 minutes at 37°C. After 9 washes, the adherence was quantified with a fluorometer (Tecan Infinite M200; Tecan). In the indicated assays, cells were treated with 0.1 mU sialidase/neuraminidase for 1 hour at 37°C.
Ex vivo frozen section assays.
These binding assays were done as described18 using lymph nodes from VAP-1 KO and VAP-1 KOTG mice. To enrich Siglec-10+ cells, B lymphocytes were isolated from human tonsils with a human B-cell enrichment kit according to the instructions of the manufacturer (Easy Sep; StemCell Technologies). The lymphocytes (> 98% pure CD19+ cells) were pretreated with anti–Siglec-10 mAb (5G6) and a negative control mAb (3G6) 10 μL/mL for 30 minutes before adding them onto the sections. The cells were let to bind to the vessels under rotating conditions for 30 minutes. Thereafter, the bound cells were fixed with glutaraldehyde and the number of vessel-bound lymphocytes was counted. The results are expressed as percentage of control binding. The number of lymphocytes bound per venules in VAP-1 KOTG mice treated with the control mAb was chosen to define 100% binding. At least 100 venules in each group were counted per assay.
Fluorescence-activated cell sorter stainings
Cell-surface stainings of CHO-VAP-1 and CHO-mock cells were performed using anti–VAP-1 (Jg-2.10) and negative control mAbs. Stainings were done in the presence of 10 μg/mL hIg, CD44-Ig, or Siglec-10–Ig. Alexa 546–conjugated goat anti–rat IgG was used as the secondary reagent. Purity of isolated B cells was evaluated by FITC-conjugated anti-CD19. Their Siglec-10 expression was analyzed by polyclonal sheep anti–Siglec-10 antibody. Polyclonal anti–P-selectin served as a negative control antibody. The second-stage antibody was Alexa 546–conjugated anti–sheep IgG. The cells were analyzed using FACSCalibur and Cellquest software.
Fluorometric detection of VAP-1-mediated H2O2 formation
The enzymatic activity of purified VAP-1 in the presence of cells expressing Siglec-10 or control cells were measured using Amplex Red reagent (Invitrogen). Purified VAP-1 was preincubated for 30 minutes at 37°C in 200 μL Krebs Ringer phosphate buffer with or without 1mM semicarbazide (inhibitor of VAP-1). Catalytic reaction was initiated by addition of purified VAP-1 onto Siglec-10 and control cells with or without semicarbazide and H2O2 detecting mixture containing HRP and Amplex Red reagent. Fluorescence intensity of the samples was measured with Tecan Infinite M200.
Modeling and docking simulation
The structural model of the second Siglec-10 C2 domain was made using the crystal structure of the first Siglec-5 C2 domain (pdb code: 2zg2).19 Ten models were generated with Modeller,20 and the model with the lowest objective function was chosen. The sequence alignments were performed using MALIGN and MALFORM21 within the Bodil visualization and modeling package.22 The peptide WVLQNRVLSSS from Siglec-10 was docked into a VAP-1 structure (pdb code: 1us1),23 in which the active site topaquinone was in an active conformation, and Leu469, the guardian amino acid,20 was made flexible during docking to allow access to the active site. The peptide was generated using SYBYL 7.3 (Tripos Inc) and docked to VAP-1 using GOLD 3.2.24,25 Ten dockings with Arg293 in the peptide covalently bound to the N5 atom of topaquinone were made, and the peptide-VAP-1 model with the highest fitness value derived by GOLD was chosen for further analysis. Energetically favorable binding sites in the binding groove of VAP-1 were calculated with both an aliphatic carboxylate and an aliphatic amide group as a probe using GRID (Molecular Discovery), and the maps were visualized at −6 kcal/mol using PYMOL Molecular Graphics System (DeLano Scientific). The sequence alignment in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) was generated using the program ALSCRIPT,26 the model was created with PYMOL, and the video was made using eMovie27 within PYMOL.
Statistical analyses
The Student t test (unpaired, 2-tailed) was used to compare numerical variables between 2 groups. The kinetic curves of the enzymatic activity were analyzed using variance analysis of repeated measurements with SAS Enterprise Guide 3.0 program.
Results
Siglec-10 and VAP-1 molecules interact
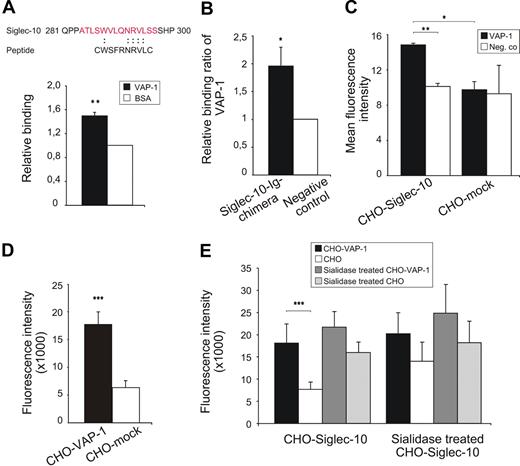
We used a cyclic CX8C phage peptide library to screen for potential ligands of VAP-1. After 4 rounds of panning, we obtained a 400-fold enrichment of phages bound to VAP-1 compared with the control (BSA-coated wells). Sequencing of randomly selected clones of the enriched phages gave a sequence corresponding to a peptide shown in Figure 1A. Binding of the peptide was confirmed by testing the adhesion of an identical synthetic peptide to recombinant VAP-1 (Figure 1A). Database searches with the sequence derived from the phage library revealed similarities to the amino acid sequence of Sialic acid binding Ig-like lectin-10, Siglec-10 (residues 288-295, Figure 1A).
Siglec-10 interacts with VAP-1. (A) The amino acid sequence obtained from randomly picked clones after 4 rounds of selection and its match to Siglec-10 and the binding of the corresponding synthetic peptide to recombinant VAP-1 (100 ng/well) in ELISA. The results are mean ± SEM from 3 separate experiments and triplicate wells in each experiment. (B) Binding of recombinant VAP-1 to Siglec-10–Ig chimera. Siglec-10–Ig chimera was immobilized onto ELISA microtiter wells via an anti-hIgG antibody. hIg was used as a negative control. The results are presented as relative binding ratios and are mean ± SEM from 3 separate experiments, each having triplicate wells. (C) Binding of recombinant VAP-1 to CHO cells expressing Siglec-10 and to mock-transfected control cells as detected by anti–VAP-1 antibody. Negative controls are incubations with antibodies without the addition of recombinant VAP-1. The results are mean ± SEM of mean fluorescence intensities measured by flow cytometer from 2 separate experiments. (D) Binding of CFSE-labeled Siglec-10 transfectants to CHO cells expressing VAP-1 and mock controls. The results are mean ± SEM of fluorescent intensities measured by fluorometer from 7 separate experiments, each having duplicate wells. (E) The contribution of sialic acids to the interaction was tested by treating either the cells expressing VAP-1 and or the cells expressing Siglec-10 with neuraminidase to remove the sialic acids. The results are means of fluorescent intensities ± SEM from 4 separate experiments, each having duplicate wells. *P < .05. **P < .01. ***P < .001.
Siglec-10 interacts with VAP-1. (A) The amino acid sequence obtained from randomly picked clones after 4 rounds of selection and its match to Siglec-10 and the binding of the corresponding synthetic peptide to recombinant VAP-1 (100 ng/well) in ELISA. The results are mean ± SEM from 3 separate experiments and triplicate wells in each experiment. (B) Binding of recombinant VAP-1 to Siglec-10–Ig chimera. Siglec-10–Ig chimera was immobilized onto ELISA microtiter wells via an anti-hIgG antibody. hIg was used as a negative control. The results are presented as relative binding ratios and are mean ± SEM from 3 separate experiments, each having triplicate wells. (C) Binding of recombinant VAP-1 to CHO cells expressing Siglec-10 and to mock-transfected control cells as detected by anti–VAP-1 antibody. Negative controls are incubations with antibodies without the addition of recombinant VAP-1. The results are mean ± SEM of mean fluorescence intensities measured by flow cytometer from 2 separate experiments. (D) Binding of CFSE-labeled Siglec-10 transfectants to CHO cells expressing VAP-1 and mock controls. The results are mean ± SEM of fluorescent intensities measured by fluorometer from 7 separate experiments, each having duplicate wells. (E) The contribution of sialic acids to the interaction was tested by treating either the cells expressing VAP-1 and or the cells expressing Siglec-10 with neuraminidase to remove the sialic acids. The results are means of fluorescent intensities ± SEM from 4 separate experiments, each having duplicate wells. *P < .05. **P < .01. ***P < .001.
Several types of adhesion assays were performed to demonstrate binding between Siglec-10 and VAP-1 molecules. The first experiments showed specific binding of recombinant VAP-1 to plate-immobilized Siglec-10–Ig chimera (Figure 1B). In additional studies, recombinant VAP-1 specifically adhered to Siglec-10 transfectants (Figure 1C). For further confirmation, the interaction between cells expressing VAP-1 and cells expressing Siglec-10 was studied. The results were consistent with those of the previous experiments and showed VAP-1-Siglec-10–dependent adhesion of the cells expressing these molecules (Figure 1D).
Because VAP-1 contains α2,3-linked sialic acids28 and it is known that Siglecs bind to sialic acids, we tested carbohydrate dependence of the interaction between VAP-1 and Siglec-10. Adhesion assays were carried out using cells expressing VAP-1 and cells expressing Siglec-10 subsequent to sialidase treatments of the cells. The sialidase used was from Clostridium perfringens, which cleaves α2,3-, α2,6-, and α2,8-linked sialic acids. Sialidase treatment as such increased the background binding. It is probably because removal of sialic acid changes the overall charge of the molecules and their binding properties. Their removal may also open binding sites that are not normally accessible. Nevertheless, because the sialidase treatment of both CHO-VAP-1 and CHO-Siglec-10 decreased VAP-1-Siglec-10–dependent binding between these cells, this suggests that sialic acids may contribute to the binding (Figure 1E).
Lymphocytes use Siglec-10 for binding to endothelial VAP-1
We then analyzed whether normal Siglec-10–expressing lymphocytes can bind endothelial VAP-1. As CD19+ B cells are practically all positive for Siglec-1013, we isolated tonsillar CD19+ B cells and tested their binding to lymph node vasculature of both VAP-1 KO mice and KOTG mice9 in ex vivo frozen section adhesion assays. This setting allowed us to measure binding between Siglec-10 and VAP-1 without the theoretical possibility that Siglec-10 binds to another (unknown) endothelial cell molecule and VAP-1 binds to another (unknown) leukocyte ligand, which we could not have been able to discriminate from the specific binding using frozen sections from wild-type murine or human lymph nodes. Expression of human VAP-1 in KOTG lymph nodes is demonstrated in Figure 2A and Siglec-10 expression of isolated B cells used in the assays in Figure 2B. The binding assays showed that the vasculature expressing human VAP-1 mediates significantly better adherence of B cells than vessels in KO lymph nodes. Moreover, the binding was largely mediated by Siglec-10 as anti–Siglec-10 antibody significantly reduced binding of B lymphocytes to KOTG vessels but did not have any inhibitory effect in KO lymph nodes (Figure 2C). These experiments demonstrate that the interaction between Siglec-10 and VAP-1 can take place in the binding of normal lymphocytes to the vessel wall.
Siglec-10+ lymphocytes bind to vessels using VAP-1. (A) Expression of human VAP-1 on mesenteric lymph node vasculature of KOTG mice (left) detected by FITC-Jg-2.10 antibody. A high endothelial venule is pointed out by a white arrow. Lack of expression shown in KO mice (right). Staining with a negative control antibody is shown in the inset. Scale bar represents 50 μm. (B) Purity of the B cells and their Siglec-10 expression used for ex vivo binding assays. Fluorescence-activated cell sorter histograms of CD19 and Siglec-10 expression are shown. Negative control (neg co) antibody was polyclonal anti–P-selectin antibody. (C) Ex vivo frozen section binding assays were used to analyze lymphocyte binding to vessels in mesenteric lymph nodes obtained from VAP-1 KO and VAP-1 KOTG mice. The function of Siglec-10 was blocked by incubating the cells before the assay with anti–Siglec-10 antibody. The results are shown as percentage of control binding (number of KOTG vessel-bound lymphocytes incubated with a nonblocking control mAb is defined as 100%; mean ± SEM). ***P < .001.
Siglec-10+ lymphocytes bind to vessels using VAP-1. (A) Expression of human VAP-1 on mesenteric lymph node vasculature of KOTG mice (left) detected by FITC-Jg-2.10 antibody. A high endothelial venule is pointed out by a white arrow. Lack of expression shown in KO mice (right). Staining with a negative control antibody is shown in the inset. Scale bar represents 50 μm. (B) Purity of the B cells and their Siglec-10 expression used for ex vivo binding assays. Fluorescence-activated cell sorter histograms of CD19 and Siglec-10 expression are shown. Negative control (neg co) antibody was polyclonal anti–P-selectin antibody. (C) Ex vivo frozen section binding assays were used to analyze lymphocyte binding to vessels in mesenteric lymph nodes obtained from VAP-1 KO and VAP-1 KOTG mice. The function of Siglec-10 was blocked by incubating the cells before the assay with anti–Siglec-10 antibody. The results are shown as percentage of control binding (number of KOTG vessel-bound lymphocytes incubated with a nonblocking control mAb is defined as 100%; mean ± SEM). ***P < .001.
Siglec-10 binds to the enzymatic groove of VAP-1 and the interaction results in production of hydrogen peroxide
To characterize the putative Siglec-10 binding site in VAP-1, we used an anti–VAP-1 antibody and the Siglec-10–Ig chimera in competitive staining experiments. When the cells expressing VAP-1 were incubated with a control chimera (CD44-Ig) or hIg followed by the anti–VAP-1 antibody, 84% plus or minus 9% (mean ± SEM, n = 3) of the cells stained positively, but when the cells were first incubated with the Siglec-10–Ig chimera, only 47% plus or minus 3% (mean ± SEM, n = 3) of the cells were positive (P = .025, Figure 3A). Siglec-10–Ig chimera pretreatment also partially blocked the anti–VAP-1 antibody binding to human VAP-1 expressed as a transgene in vasculature of VAP-1 KO mice (Figure 3B). Thus, the binding site of Siglec-10 is in the relative vicinity of the binding site of anti–VAP-1 antibody Jg-2.10, but it is not the same.
Siglec-10 binds to the enzymatic groove of VAP-1 and acts as a substrate. (A) Examples of competitive stainings with Siglec-10–Ig chimera and anti–VAP-1 antibody Jg-2.10. CHO-VAP-1 transfectants were stained with the anti–VAP-1 antibody either in the presence of Siglec-10–Ig chimera or CD44-Ig (negative control) and analyzed by fluorescence-activated cell sorter. Percentages of cells positively stained with anti–VAP-1 mAb or a negative class-matched control antibody are shown. (B) Heart sections of KOTG mice were first incubated either with a control (CD44-Ig) or Siglec-10–Ig chimera and stained thereafter with anti–VAP-1 mAb (Jg-2.10). Some brightly positive vessels in the control section and fewer and less bright ones in the section pretreated with Siglec-10–Ig chimera are pointed out by arrows. Scale bar represents 50 μm. (C) Enzymatic activity of VAP-1 was measured as H2O2 (pmol) produced in 1 hour in the presence of Siglec-10 transfectants or mock-transfected control cells. The results are presented as relative SSAO activity ± SEM from 3 separate experiments, each having duplicate wells. A representative experiment showing the amount of H2O2 produced at different time points during a 1-hour measurement is presented in the upper left corner. (D) Binding of Siglec-10 transfectants to CHO cells expressing enzymatically active VAP-1, enzymatically inactive VAP-1, or mock controls. The results are mean fluorescence intensities ± SEM from 7 separate experiments, each having duplicate wells. (E) Binding of Siglec-10 peptide (CATLSWVLQNRVLSSCK-biotin) and mutated Siglec-10 peptide (CATLSWVLQNAVLSSCK-biotin) to recombinant VAP-1 (400 ng/well). The results are mean of relative binding ± SEM from 3 separate experiments with triplicate wells *P < .05. **P < .01. ***P < .001.
Siglec-10 binds to the enzymatic groove of VAP-1 and acts as a substrate. (A) Examples of competitive stainings with Siglec-10–Ig chimera and anti–VAP-1 antibody Jg-2.10. CHO-VAP-1 transfectants were stained with the anti–VAP-1 antibody either in the presence of Siglec-10–Ig chimera or CD44-Ig (negative control) and analyzed by fluorescence-activated cell sorter. Percentages of cells positively stained with anti–VAP-1 mAb or a negative class-matched control antibody are shown. (B) Heart sections of KOTG mice were first incubated either with a control (CD44-Ig) or Siglec-10–Ig chimera and stained thereafter with anti–VAP-1 mAb (Jg-2.10). Some brightly positive vessels in the control section and fewer and less bright ones in the section pretreated with Siglec-10–Ig chimera are pointed out by arrows. Scale bar represents 50 μm. (C) Enzymatic activity of VAP-1 was measured as H2O2 (pmol) produced in 1 hour in the presence of Siglec-10 transfectants or mock-transfected control cells. The results are presented as relative SSAO activity ± SEM from 3 separate experiments, each having duplicate wells. A representative experiment showing the amount of H2O2 produced at different time points during a 1-hour measurement is presented in the upper left corner. (D) Binding of Siglec-10 transfectants to CHO cells expressing enzymatically active VAP-1, enzymatically inactive VAP-1, or mock controls. The results are mean fluorescence intensities ± SEM from 7 separate experiments, each having duplicate wells. (E) Binding of Siglec-10 peptide (CATLSWVLQNRVLSSCK-biotin) and mutated Siglec-10 peptide (CATLSWVLQNAVLSSCK-biotin) to recombinant VAP-1 (400 ng/well). The results are mean of relative binding ± SEM from 3 separate experiments with triplicate wells *P < .05. **P < .01. ***P < .001.
Next we investigated whether Siglec-10 can interfere with the enzymatic activity of VAP-1. These assays showed that Siglec-10–expressing cells functioned as a substrate for VAP-1 (Figure 3C); therefore, based on the catalytic reaction mechanism of SSAOs, a free amino group in Siglec-10 (arginine 293) interacts covalently with the topaquinone residue in the active site of VAP-1. The importance of the enzymatic activity in the binding between VAP-1 and Siglec-10 was revealed in further analyses, in which the binding of cells expressing Siglec-10 to cells expressing the inactive mutant of VAP-1 was analyzed. Siglec-10 transfectants failed to interact with the cells expressing the enzymatically inactive VAP-1 mutant, in which tyrosine 471, the precursor of topaquinone, had been mutated to phenylalanine (Figure 3D). Moreover, a circular peptide with a full match to the corresponding Siglec-10 amino acid sequence (residues 284-297, CATLSWVLQNRVLSSCK-biotin) bound to recombinant VAP-1. Instead, the mutated Siglec-10 peptide, in which the arginine had been replaced by alanine (CATLSWVLQNAVLSSCK-biotin) failed to bind recombinant VAP-1 in ELISA assays (Figure 3E). Taken together, these data indicate that Siglec-10 binds to the enzymatic groove and functions as a substrate of VAP-1 via arginine-topaquinone interaction.
To model the interaction between VAP-1 and Siglec-10, a structural model of the second C2-type domain of Siglec-10 was made based on the C2-set immunoglobulin domain of Siglec-5 (Protein Databank accession code 2zg2).19 The reliability of the model is good because the immunoglobulin fold is in general known to be highly conserved and all the C2-type immunoglobulin domains found in CD33-related Siglecs share a high sequence similarity (supplemental Figure 1). Normally, the C2-type immunoglobulin fold is composed of 7 β-strands forming an anti–parallel β barrel, but in Siglec-5 there are only 6 β-strands, ABE, and CFG. The seventh β-strand C′ is replaced by a highly flexible loop between the strands C and E that coils into 2 one-turn helices (the CE loop). Interestingly, the sequence corresponding to the peptide that binds to VAP-1 is located in this CE loop in our structural model.
Docking studies were carried out with peptides of different lengths derived from the CE loop, in which the arginine in the middle of the peptide was covalently bound to topaquinone. Of these peptides, the one carrying the sequence, WVLQNRVLSS, fits best into the active site cavity of VAP-1 (Figure 4A). Similarly, it has been reported earlier that lentil seedling copper-amine oxidase can use an arginine as a substrate.29 The sequence of the peptide used in the docking studies shares high sequence identity with Siglec-11 (supplemental Figure 1). There is only one amino acid difference: asparagine in Siglec-10 versus aspartate in Siglec-11. To verify whether the binding groove prefers an asparagine over an aspartate, favorable interactions were calculated with an aliphatic amide and an aliphatic carboxylate as a probe. Interestingly, the binding groove clearly has favorable interaction sites for amide groups and does not favor carboxylate binding, thus predicting better binding for the asparagine in Siglec-10 compared with the aspartate in Siglec-11 (Figure 4B). Leu469 in VAP-1, located in the middle of the tunnel leading to the active site, has been suggested to act as a guardian amino acid, and our docking results support this theory.23 Leu469 has to slightly move before the peptide has the possibility to reach and bind to topaquinone. Furthermore, manual docking of the Siglec-10 C2-type domain model to VAP-1 shows that this domain fits well into the surface groove of VAP-1 at the active site opening and makes contacts with the β-hairpin Arm1 and the RGD loop from the other subunit (Figure 4; supplemental Video 1), thus interacting with both monomers in the VAP-1 dimer.
Interaction of VAP-1 with Siglec-10 C2-type domain 2. (A) The 3-dimensional structure of VAP-1 (monomer A, yellow; and monomer B, green) with a peptide derived from Siglec-10 CE loop (purple ball-and-stick) docked into the active site of monomer B. Arg293 in the docked peptide is labeled and covalently bound to topaquinone (TPQ), which is in an active conformation. Arm1 and the RGD site in domain A are close to the opening of the active site in VAP-1. At the right, there is a 3-dimensional model of the second Siglec-10 C2 domain shown in an orientation fitting into VAP-1. The amino acids corresponding to the docked ligand are shown as purple ball-and-stick in the Siglec-10 model, and the topaquinone-binding arginine is colored yellow and labeled. (B) Stereo view of Siglec-10 (purple) binding to topaquinone (green) in VAP-1. Grid maps for the amide (blue wires) and carboxylate (red wires) probes are shown at a level of −6 kcal/mol. The asparagine (Asn) in Siglec-10 that corresponds to aspartate in Siglec-11 is labeled. (C) Binding of CHO cells expressing Siglec-10 to CHO cells expressing wild-type VAP-1, the RGD mutant of VAP-1, or mock controls was determined with cell-cell adhesion assay as explained in “Assays with transfectants.” The results are presented as a relative binding ratio ± SEM from 5 separate experiments, each having duplicate wells. *P < .05. (D) Binding of CHO cells expressing Siglec-11 to VAP-1 transfectants. (E) Binding of CHO cells expressing Siglec-G to VAP-1 transfectants. The results of panels D and E are relative binding ratio ± SEM from 2 separate experiments, each having quadruple wells.
Interaction of VAP-1 with Siglec-10 C2-type domain 2. (A) The 3-dimensional structure of VAP-1 (monomer A, yellow; and monomer B, green) with a peptide derived from Siglec-10 CE loop (purple ball-and-stick) docked into the active site of monomer B. Arg293 in the docked peptide is labeled and covalently bound to topaquinone (TPQ), which is in an active conformation. Arm1 and the RGD site in domain A are close to the opening of the active site in VAP-1. At the right, there is a 3-dimensional model of the second Siglec-10 C2 domain shown in an orientation fitting into VAP-1. The amino acids corresponding to the docked ligand are shown as purple ball-and-stick in the Siglec-10 model, and the topaquinone-binding arginine is colored yellow and labeled. (B) Stereo view of Siglec-10 (purple) binding to topaquinone (green) in VAP-1. Grid maps for the amide (blue wires) and carboxylate (red wires) probes are shown at a level of −6 kcal/mol. The asparagine (Asn) in Siglec-10 that corresponds to aspartate in Siglec-11 is labeled. (C) Binding of CHO cells expressing Siglec-10 to CHO cells expressing wild-type VAP-1, the RGD mutant of VAP-1, or mock controls was determined with cell-cell adhesion assay as explained in “Assays with transfectants.” The results are presented as a relative binding ratio ± SEM from 5 separate experiments, each having duplicate wells. *P < .05. (D) Binding of CHO cells expressing Siglec-11 to VAP-1 transfectants. (E) Binding of CHO cells expressing Siglec-G to VAP-1 transfectants. The results of panels D and E are relative binding ratio ± SEM from 2 separate experiments, each having quadruple wells.
As VAP-1 has an RGD motif on the surface of the molecule close to the substrate channel, its possible contribution to Siglec-10 binding was analyzed. The binding of Siglec-10 to a VAP-1 mutant, in which the aspartate residue of the RGD motif is substituted with alanine, was comparable with the binding to wild-type VAP-1, indicating that the RGD motif is not required in the VAP-1–Siglec-10 interaction (Figure 4C). We further tested whether VAP-1 can interact with Siglec-11 or Siglec-G that is the mouse ortholog of Siglec-10.28 These assays showed that neither Siglec-11 nor Siglec-G binds to VAP-1 (Figure 4D-E). Thus, the lack of arginine in Siglec-G and one amino acid difference in Siglec-11 compared with Siglec-10 in the binding peptide are enough to prevent the binding.
Discussion
Adhesion and enzymatic assays have suggested that VAP-1 has a leukocyte surface ligand(s) that may also serve as a substrate for VAP-1.30 However, despite intensive attempts using various approaches, no ligands have been identified. Thus, the identification of Siglec-10 as such a ligand in this work is a new opening in the research focusing on the role of ectoenzymes in leukocyte-endothelial cell interactions during leukocyte extravasation.
Siglec-10 is a member of the family of sialic acid-binding Ig-like lectins (Siglecs), which binds glycoconjugates carrying sialic acid in either the α2,3 or α2,6 linkage and is expressed on certain subsets of human leukocytes, including B cells, eosinophils, and monocytes.31,32 Taking into account the expression pattern and binding properties of Siglec-10, it has the characteristics to be a ligand for endothelial VAP-1. Although the sequence match between the enriched peptide and Siglec-10 is not perfect, the sequence contained several amino acids with similar properties. The amino acid sequence of Siglec-10 that binds to VAP-1 is located at the second C2-set immunoglobulin domain, which, however, does not interact with the known sialic acid ligands. More detailed analysis revealed that this sequence is specific for Siglec-10 and Siglec-11 with only one amino acid difference: Siglec-10 has an asparagine, whereas the corresponding amino acid is an aspartate in Siglec-11. The other Siglecs have crucial differences in the corresponding sequence; moreover, their CE loop is shorter and less flexible than in Siglec-10 and Siglec-11. Interestingly, this small difference seems to be enough to prevent proper interaction between VAP-1 and Siglec-11. The in vivo expression patterns of Siglec-10 and Siglec-11 also differ markedly as Siglec-11 is expressed on tissue macrophages, such as Kupffer cells in liver and on brain microglia and not on peripheral blood leukocytes as is Siglec-10.33
Our data show that Siglec-10 acts as a substrate for VAP-1. In the presence of Siglec-10–expressing cells, VAP-1 starts to produce hydrogen peroxide. This supports our previous observations that, when VAP-1 interacts with lymphocytes, a low amount of H2O2 is formed.7 Moreover, here we show that, when the conserved tyrosine in the active site of VAP-1 is changed into phenylalanine, the formation of topaquinone from its tyrosine precursor is prevented and Siglec-10 fails to interact efficiently with this enzymatically inactive form of VAP-1. These results, together with the docking studies, support the hypothesis that a free NH2 group in an amino acid on a lymphocyte surface molecule can serve as a substrate for VAP-1. In this case, the free NH2 group of an arginine residue of Siglec-10 expressed on the surface of a lymphocyte binds covalently to the topaquinone in the active site and serves as a substrate for VAP-1 forming a transient Schiff base during the reaction. The transient nature of the Schiff base formed between VAP-1 and Siglec-10 explains the specific, although relatively weak, binding observed in our assays.
The mouse ortholog of human Siglec-10, Siglec-G, has also been identified. Siglec-G and Siglec-10 share high sequence identity, similar chromosomal location of their genes, and a conserved protein structure.34 A recent study with Siglec-G–deficient mice revealed a B-cell-restricted expression pattern and B1 cell-specific functions of Siglec-G.25 However, Siglec-G has a glutamine residue instead of an arginine in the CE loop, which makes a similar interaction with VAP-1 impossible (Figure 4E; supplemental Figure 1). Because of this crucial difference in the critical sequence area, Siglec-G–deficient mice were not used in our studies.
In conclusion, we have identified a novel interaction between VAP-1 and Siglec-10. Siglec-10 binds to VAP-1 via a free NH2 group of an arginine side chain and serves as a substrate for VAP-1. During the catalytic reaction, a transient Schiff base is formed between the enzyme and the substrate, and it can mediate binding of the lymphocyte to the endothelium. During the subsequent oxidative half-reaction, hydrogen peroxide is released as also observed in our experiments. Hydrogen peroxide is a potent mediator modulating several adhesion and signaling molecules as well as transcription factors30 and, thus, the overall inflammatory microenvironment. Importantly, Siglec-10 is the first leukocyte ligand characterized for VAP-1; moreover, this interaction is the first protein-protein interaction reported for Siglec-10. The discovery of this novel interaction is crucial for the development of new anti-inflammatory drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anne Sovikoski-Georgieva for secretarial help and Mark Johnson for the excellent computing facilities at Åbo Akademi University.
This work was supported by the Finnish Academy, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, Arvo and Inkeri Suominen Foundation, Tor, Joe and Pentti Borg's Foundation, and Medicinska Understödsföreningen Liv och Hälsa.
Authorship
Contribution: E. Kivi performed the experiments and wrote the manuscript; K.E., K. Aalto, and K. Auvinen performed the experiments; Y.N. and T.A.S. did the molecular modeling; E. Koivunen planned the phage display screening; D.M.O. and P.R.C. provided valuable reagents and participated in planning of the work; M.S. planned the experiments and contributed to writing; and S.J. designed the work, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: A patent application has been filed. The authors declare no competing financial interests.
Correspondence: Sirpa Jalkanen, MediCity Research Laboratory, University of Turku, Tykistökatu 6 A, FIN-20520 Turku, Finland; e-mail: sirpa.jalkanen@utu.fi.
References
Author notes
K.E. and K. Aalto contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal