Abstract
Dasatinib is a BCR-ABL inhibitor with 325-fold higher potency than imatinib against unmutated BCR-ABL in vitro. Imatinib failure is commonly caused by BCR-ABL mutations. Here, dasatinib efficacy was analyzed in patients recruited to phase 2/3 trials with chronic-phase chronic myeloid leukemia with or without BCR-ABL mutations after prior imatinib. Among 1043 patients, 39% had a preexisting BCR-ABL mutation, including 48% of 805 patients with imatinib resistance or suboptimal response. Sixty-threedifferent BCR-ABL mutations affecting 49 amino acids were detected at baseline, with G250, M351, M244, and F359 most frequently affected. After 2 years of follow-up, dasatinib treatment of imatinib-resistant patients with or without a mutation resulted in notable response rates (complete cytogenetic response: 43% vs 47%) and durable progression-free survival (70% vs 80%). High response rates were achieved with different mutations except T315I, including highly imatinib-resistant mutations in the P-loop region. Impaired responses were observed with some mutations with a dasatinib median inhibitory concentration (IC50) greater than 3nM; among patients with mutations with lower or unknown IC50, efficacy was comparable with those with no mutation. Overall, dasatinib has durable efficacy in patients with or without BCR-ABL mutations. All trials were registered at http://www.clinicaltrials.gov as NCT00123474, NCT00101660, and NCT00103844.
Introduction
In chronic myeloid leukemia (CML), proliferation is driven by the constitutive tyrosine kinase activity of BCR-ABL, a chimeric oncoprotein encoded after the fusion of the BCR gene from chromosome 22 and ABL kinase gene from chromosome 9.1 Most patients (∼ 80%) are diagnosed with CML during the initial chronic phase (CP).2 Current therapeutic approaches for CML-CP aim to decrease leukemic cell numbers and prevent disease progression by inhibiting BCR-ABL kinase activity.
Dasatinib (SPRYCEL; Bristol-Myers Squibb) is a potent BCR-ABL inhibitor and is 325-fold more potent than imatinib (Glivec/Gleevec; Novartis) in vitro against unmutated BCR-ABL.3 Across a series of international phase 2/3 trials with more than 2 years of follow-up, dasatinib has demonstrated durable responses in patients with CML after resistance, suboptimal response, or intolerance to imatinib.4-8 After a phase 3 dose optimization trial showing equivalent efficacy and significantly fewer key side effects compared with alternative dosing schedules, dasatinib 100 mg once daily is now the approved dose in patients with CML-CP.8
BCR-ABL mutations are a common cause of inadequate response or relapse during imatinib treatment. In a recent study, a BCR-ABL mutation was detected in 4 of 34 patients (13%) with CML-CP who experienced a suboptimal response to first-line imatinib, and 18 of 72 (25%) who met criteria for imatinib failure.9 Among patients with CML-CP who developed imatinib resistance after prior interferon-alpha treatment, a BCR-ABL mutation was reported in 31% to 42% of patients.10,11 In studies of patients with imatinib-resistant advanced-phase CML or Philadelphia chromosome–positive acute lymphoblastic leukemia, a BCR-ABL mutation was detected in 30% to 83% of patients.10-12 Although more than 90 different BCR-ABL mutations have been reported affecting more than 55 amino acids, 15 amino acid substitutions account for more than 85% of reported mutations, and 7 amino acids (ABL G250, Y253, E255, T315, M351, F359, H396) are responsible for two-thirds.13,14
In clinical trials, dasatinib treatment responses have been similar in patients with or without BCR-ABL mutations, although a detailed analysis has not been performed.4-8 The objective of the current report was to analyze in detail the efficacy of dasatinib in patients with CML-CP recruited to phase 2/3 trials according to BCR-ABL mutation status.
Methods
Patients and treatment
Patient data were analyzed from 3 dasatinib trials in CML-CP: CA180-013 (START-C), a phase 2 trial of dasatinib 70 mg twice daily in 387 patients with resistance (n = 288) or intolerance (n = 99) to imatinib4,15 ; CA180-017 (START-R), a phase 2 randomized trial of dasatinib 70 mg twice daily versus imatinib 800 mg/day in patients with resistance to imatinib 400 to 600 mg/day (n = 150), including 101 patients who were randomized to dasatinib7 ; and CA180-034, a phase 3 dose-optimization trial in patients with resistance, suboptimal response, or intolerance to imatinib (N = 670), in which patients were randomized using a 2 × 2 factorial design to receive 1 of 4 dosing schedules: 100 mg once daily (n = 167), 50 mg twice daily (n = 168), 140 mg once daily (n = 167), or 70 mg twice daily (n = 168).8 In all 3 studies, dasatinib dose escalation (to a maximum of 180 mg once daily or 90 mg twice daily) was permitted for inadequate response, and dose interruption or reduction (to a minimum of 80 mg once daily or 40 mg twice daily) was permitted for adverse events. Trials were conducted in accordance with the Declaration of Helsinki and were approved by the responsible Institutional Review Board or Ethics Committee of all participating centers. Written informed consent was obtained from all patients.
Eligibility criteria differed between trials for patients with a BCR-ABL mutation. In Study 013, patients resistant to imatinib at a dose of 600 mg/day or less were eligible if they had any of the following BCR-ABL mutations associated with high-level imatinib resistance: L248V, G250E, Q252H/R, Y253F/H, E255K/V, T315I, F317L, or H396P/R; no specific mutation status was required for patients resistant to imatinib doses higher than 600 mg/day. In Study 017, patients known to have a BCR-ABL mutation associated with high-level imatinib resistance (as listed for Study 013) were excluded from enrollment; however, mutation screening prior to enrollment was not obligatory. In Study 034, patients who had any BCR-ABL mutation were eligible, including those who had achieved a major cytogenetic response (MCyR) with imatinib at a dose of 400 mg/day or higher.
Assessments
Patient samples were assayed at 1 of 4 laboratories: the University of Heidelberg; Institute of Medical and Veterinary Science; Fred Hutchinson Cancer Research Center (Studies 013 and 017); or ACGT Inc (Study 034, coordinated by Bristol-Myers Squibb). Peripheral blood cell mRNA was collected at baseline and analyzed for point mutations in the BCR-ABL kinase domain by reverse transcription–polymerase chain reaction and direct sequencing, with or without screening by denaturing high-performance liquid chromatography, according to the standard practices of the laboratories. All mutation data in the current analysis were based on conventional direct sequencing. For analyses of individual BCR-ABL mutations, patients who had more than 1 mutation were included in separate analyses for each mutation that was detected. Known single nucleotide polymorphisms within the BCR-ABL kinase domain16 were not considered to represent mutations and patients with only polymorphisms were included in analyses of patients with no mutation.
Standard efficacy assessments, including complete hematologic response (CHR), MCyR, and complete cytogenetic response (CCyR), were used to classify treatment responses.7,15 All cytogenetic responses were determined using conventional assessment of at least 20 metaphases. A major molecular response (MMR) was defined as a reduction of BCR-ABL transcripts to 0.1% or less according to the international scale.17
Analyses
Time to response was analyzed using a Kaplan-Meier technique in which all nonresponders were censored at the same maximum assessment time. The resulting curve represents the response rate over the observed period and reaches a maximal value corresponding to the overall observed rate. Standard Kaplan-Meier analysis was used to estimate duration of response, progression-free survival (PFS), transformation-free survival (TFS), and overall survival (OS), and 95% confidence intervals (CIs) were calculated. Progression was defined as any of the following events: confirmed accelerated- or blast-phase (AP/BP) disease, loss of a previous CHR or MCyR, increase in Philadelphia chromosome–positive metaphases of 30% or more (Study 034 only), increase in white blood cell count (doubling from lowest value to more than 20 ×109/L [20 000/mm3] or an increase of more than 50 ×109/L [50 000/mm3] on 2 occasions, performed at least 2 weeks apart), or death at any time regardless of cause8,15 ; occasional cases where disease progression was reported by the investigator but the progression event was not provided were also included for progression analyses. Loss of CHR was defined as no longer meeting all of the criteria for CHR for a consecutive 2-week period. The range of events considered in the PFS analysis was therefore similar to analyses of event-free survival presented elsewhere.18 Lack of response was not defined as a progression event, and progression in nonresponding patients was defined using the same events as in responding patients. For TFS analyses, transformation was defined as confirmed AP or BP disease or death at any time regardless of cause, and patients with other progression events were not considered in the analysis. Duration of response was measured from the time of response until progression event. For PFS/TFS/duration of response analysis, patients who neither progressed/lost response nor died were censored at last assessment prior to loss to follow-up. Patients were not followed for PFS/TFS/duration of response analysis after study drug discontinuation for protocol-stipulated reasons that included the following: withdrawal of informed consent; any adverse event, laboratory abnormality, or intercurrent illness that, in the investigator's opinion, indicated that continued treatment was not in the patient's best interest; pregnancy; and stem cell transplantation (Study 034). For OS analysis, living patients or patients lost to follow-up were censored on last known alive date. In Study 034, patients were followed for survival status regardless of whether they discontinued study drug. In START-R, OS was not assessed because of the crossover design of the study.
For analysis by median inhibitory concentration to dasatinib, cellular IC50 values for individual mutations were derived from the reports by O'Hare et al3 and Redaelli et al.19 Mutations were grouped according to whether the highest dasatinib IC50 value reported was greater than 3nM, or 3nM or less. Because the IC50 value for T315I is substantially higher than all other mutations, patients with T315I were not included in the IC50-based analysis. Using these criteria, mutations with a dasatinib IC50 greater than 3nM (intermediate IC50) were L248V, G250E, Q252H, E255K/V, V299L, F317L, L384M, and F486S; mutations with a dasatinib IC50 of 3nM or less (low IC50) were M244V, Y253F/H, D276G, E279K, F311L, M351T, F359V, V379I, L387M, and H396P/R; all other mutations were considered to have an unknown IC50. For analysis of responses according to IC50 in patients who had more than 1 baseline mutation and the different mutations belonged to separate IC50 groupings, patients were classified according to highest known IC50, that is, patients with a concurrent mutation with IC50 greater than 3nM were excluded from the group with IC50 of 3nM or less, and patients with a mutation with known IC50 (as listed in the current paragraph) were excluded from the unknown IC50 group.
Results
Patient characteristics
A total of 1158 patients with CML-CP after imatinib failure were randomized to (Studies 017 and 034) or treated with (Study 013) dasatinib. Of these, 1043 patients had a mutational assessment performed at baseline and were eligible for analysis, including 805 with imatinib resistance and 238 with imatinib intolerance. Patient characteristics were representative of the broader patient population with imatinib-resistant or -intolerant CML-CP (Table 1). Patients had a median duration of disease prior to dasatinib treatment of 58 months (68 vs 24 months in resistant/suboptimal vs intolerant population). Pretreatment included imatinib at a dose greater than 600 mg/day in 38% and prior interferon-α in 59% of patients. In all 3 studies, minimum follow-up was 24 months from last patient first visit to database lock. Median follow-up in each study (with range) was 25 months (0.1-32.4) for Study 013, 26 months (0.9-32.7) for Study 017, and 26 months (0.1-34.5) for Study 034.
Baseline characteristics and dosing for patients treated in dasatinib trials in chronic-phase CML who had a mutational assessment performed at baseline
| . | Total, n = 1043 . | Imatinib resistant, n = 805 . | Imatinib intolerant, n = 238 . |
|---|---|---|---|
| Male, no. (%) | 516 (49) | 429 (53) | 87 (37) |
| Median age, y (range) | 56 (18-85) | 55 (18-85) | 57 (19-84) |
| Median duration of CML, mo (range) | 58 (1-251) | 68 (3-251) | 24 (1-183) |
| Highest imatinib dose, no. (%) | |||
| Less than 400 mg/day | 1 (< 1) | 1 (< 1) | 0 (0) |
| 400 to 600 mg/day | 643 (62) | 422 (52) | 221 (93) |
| More than 600 mg/day | 398 (38) | 382 (48) | 16 (7) |
| Prior imatinib treatment, % | |||
| Less than 1 y | 213 (20) | 72 (9) | 141 (59) |
| 1 to 3 y | 356 (34) | 291 (36) | 65 (27) |
| More than 3 y | 473 (45) | 442 (55) | 31 (13) |
| Other prior therapy, no. (%) | |||
| Interferon-α | 614 (59) | 508 (63) | 106 (45) |
| Stem cell transplantation | 72 (7) | 62 (8) | 10 (4) |
| Response achieved prior to imatinib failure, no. (%) | |||
| CHR | 882 (85) | 709 (88) | 173 (73) |
| MCyR | 387 (37) | 289 (36) | 98 (41) |
| CCyR | 192 (18) | 135 (17) | 57 (24) |
| Baseline BCR-ABL mutation, no. (%) | |||
| Yes | 402 (39) | 384 (48) | 18 (8) |
| No | 641 (61) | 421 (52) | 220 (92) |
| Dasatinib dose received, no. (%) | |||
| 100 mg QD | 147 (14) | 112 (14) | 35 (15) |
| 70 mg BID | 608 (58) | 475 (59) | 133 (56) |
| 140 mg QD | 139 (13) | 105 (13) | 34 (14) |
| 50 mg BID | 149 (14) | 113 (14) | 36 (15) |
| . | Total, n = 1043 . | Imatinib resistant, n = 805 . | Imatinib intolerant, n = 238 . |
|---|---|---|---|
| Male, no. (%) | 516 (49) | 429 (53) | 87 (37) |
| Median age, y (range) | 56 (18-85) | 55 (18-85) | 57 (19-84) |
| Median duration of CML, mo (range) | 58 (1-251) | 68 (3-251) | 24 (1-183) |
| Highest imatinib dose, no. (%) | |||
| Less than 400 mg/day | 1 (< 1) | 1 (< 1) | 0 (0) |
| 400 to 600 mg/day | 643 (62) | 422 (52) | 221 (93) |
| More than 600 mg/day | 398 (38) | 382 (48) | 16 (7) |
| Prior imatinib treatment, % | |||
| Less than 1 y | 213 (20) | 72 (9) | 141 (59) |
| 1 to 3 y | 356 (34) | 291 (36) | 65 (27) |
| More than 3 y | 473 (45) | 442 (55) | 31 (13) |
| Other prior therapy, no. (%) | |||
| Interferon-α | 614 (59) | 508 (63) | 106 (45) |
| Stem cell transplantation | 72 (7) | 62 (8) | 10 (4) |
| Response achieved prior to imatinib failure, no. (%) | |||
| CHR | 882 (85) | 709 (88) | 173 (73) |
| MCyR | 387 (37) | 289 (36) | 98 (41) |
| CCyR | 192 (18) | 135 (17) | 57 (24) |
| Baseline BCR-ABL mutation, no. (%) | |||
| Yes | 402 (39) | 384 (48) | 18 (8) |
| No | 641 (61) | 421 (52) | 220 (92) |
| Dasatinib dose received, no. (%) | |||
| 100 mg QD | 147 (14) | 112 (14) | 35 (15) |
| 70 mg BID | 608 (58) | 475 (59) | 133 (56) |
| 140 mg QD | 139 (13) | 105 (13) | 34 (14) |
| 50 mg BID | 149 (14) | 113 (14) | 36 (15) |
CML indicates chronic myeloid leukemia; CHR, complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; BID, twice daily; and QD, once daily.
Baseline BCR-ABL mutation characteristics
Excluding known single nucleotide polymorphisms, a baseline mutation was detected in 402 patients (39%), comprising 384 patients with imatinib resistance/suboptimal response (48% of resistant patients) and 18 patients with imatinib intolerance (8% of intolerant patients). In the 3 clinical studies included in the analysis, a baseline BCR-ABL mutation was detected in 40% of patients from Study 013, 44% of patients from Study 017, and 36% of patients from Study 034. A total of 63 different BCR-ABL mutations were detected affecting 49 amino acids (Table 2). Amino acids most frequently mutated were G250 (n = 61), M351 (n = 54), M244 (n = 46), F359 (n = 42), H396 (n = 37), Y253 (n = 26), and E255 (n = 25). A broad range of mutations were represented in each of the 3 clinical studies, with some sampling differences observed in the frequencies of individual mutations (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Overall, a T315I mutation was detected in 21 patients (5% of patients with a mutation), which represents a lower frequency than would be expected in the clinical population; this likely reflects a lack of recruitment by investigators based on the known unresponsiveness of T315I to dasatinib. More than 1 concurrent BCR-ABL mutation was detected in 64 patients (2 mutations in 50 patients and 3 mutations in 14 patients). Known single nucleotide polymorphisms detected comprised T240T (n = 1), K247R (n = 6), F311V (n = 1), and E499E (n = 19).
Frequencies of individual baseline BCR-ABL mutations and polymorphisms within the total analysis population
| . | No. . |
|---|---|
| Mutation | |
| H201L | 1 |
| Y232S | 1 |
| M237V | 1 |
| I242T | 1 |
| M244V | 46 |
| L248V | 15 |
| del248-274 | 3 |
| G250E | 60 |
| G250V | 1 |
| Q252H | 6 |
| Y253F | 3 |
| Y253H* | 23 |
| E255K | 16 |
| E255V | 11 |
| E258D | 1 |
| L273M | 3 |
| D276G | 8 |
| E279K | 8 |
| E281X† | 1 |
| V289I | 1 |
| E292V | 1 |
| L298V | 1 |
| V299L | 1 |
| F311I | 4 |
| F311L | 2 |
| T315I | 21 |
| F317L | 14 |
| Y342H | 1 |
| M351T | 54 |
| E355G | 19 |
| F359C | 5 |
| F359I | 12 |
| F359V | 27 |
| D363Y | 1 |
| L364I | 1 |
| A365V | 1 |
| A366G | 1 |
| V379I | 2 |
| L384M | 2 |
| L387M | 5 |
| M388L | 4 |
| Y393C | 1 |
| H396P | 4 |
| H396R | 33 |
| A397P | 2 |
| S417Y | 1 |
| I418S | 1 |
| I418V | 2 |
| S438C | 3 |
| P441L | 1 |
| E450A | 2 |
| E450G | 2 |
| E450K | 2 |
| E450V | 1 |
| E453K | 4 |
| E453V | 1 |
| E459G | 1 |
| E459K | 12 |
| M472I | 1 |
| P480L | 2 |
| F486S | 13 |
| D504D | 1 |
| G514S | 1 |
| Polymorphism | |
| T240T | 1 |
| K247R | 6 |
| F311V | 1 |
| E499E | 19 |
| . | No. . |
|---|---|
| Mutation | |
| H201L | 1 |
| Y232S | 1 |
| M237V | 1 |
| I242T | 1 |
| M244V | 46 |
| L248V | 15 |
| del248-274 | 3 |
| G250E | 60 |
| G250V | 1 |
| Q252H | 6 |
| Y253F | 3 |
| Y253H* | 23 |
| E255K | 16 |
| E255V | 11 |
| E258D | 1 |
| L273M | 3 |
| D276G | 8 |
| E279K | 8 |
| E281X† | 1 |
| V289I | 1 |
| E292V | 1 |
| L298V | 1 |
| V299L | 1 |
| F311I | 4 |
| F311L | 2 |
| T315I | 21 |
| F317L | 14 |
| Y342H | 1 |
| M351T | 54 |
| E355G | 19 |
| F359C | 5 |
| F359I | 12 |
| F359V | 27 |
| D363Y | 1 |
| L364I | 1 |
| A365V | 1 |
| A366G | 1 |
| V379I | 2 |
| L384M | 2 |
| L387M | 5 |
| M388L | 4 |
| Y393C | 1 |
| H396P | 4 |
| H396R | 33 |
| A397P | 2 |
| S417Y | 1 |
| I418S | 1 |
| I418V | 2 |
| S438C | 3 |
| P441L | 1 |
| E450A | 2 |
| E450G | 2 |
| E450K | 2 |
| E450V | 1 |
| E453K | 4 |
| E453V | 1 |
| E459G | 1 |
| E459K | 12 |
| M472I | 1 |
| P480L | 2 |
| F486S | 13 |
| D504D | 1 |
| G514S | 1 |
| Polymorphism | |
| T240T | 1 |
| K247R | 6 |
| F311V | 1 |
| E499E | 19 |
Excluding known polymorphisms, a total of 480 BCR-ABL mutations, comprising 63 different types of mutation, were detected in 402 patients.
Includes 1 patient whose mutation was incorrectly listed in the Study 013 database as Y253K.
X indicates a stop codon.16
Efficacy according to BCR-ABL mutation status
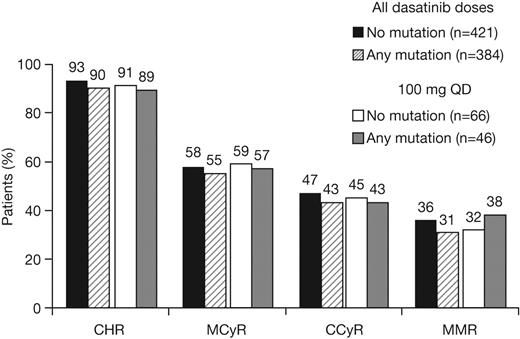
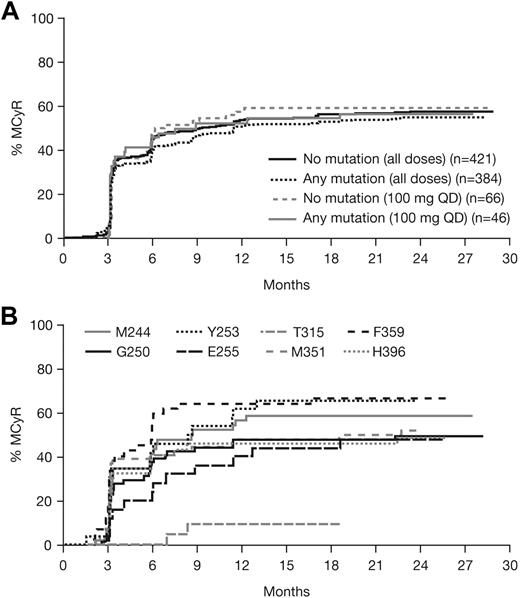
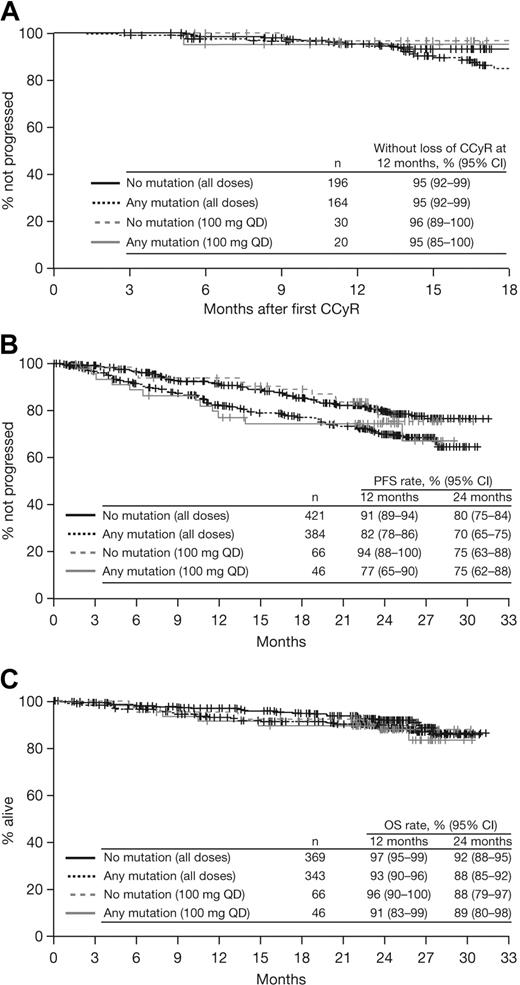
Patients with or without a baseline BCR-ABL mutation achieved notable rates of response with dasatinib treatment (Table 3; Figure 1). Among patients with imatinib resistance or suboptimal response, cytogenetic response rates in patients with or without a BCR-ABL mutation were MCyR in 55% and 58%, respectively, and CCyR in 43% and 47%, respectively. Rates were similar after 1 year of follow-up (MCyR: 53% and 55%, respectively; CCyR: 40% and 41%, respectively), indicating that most responses were achieved within the first year of treatment. Among patients who achieved a MCyR, median times to response for those with or without a mutation, respectively, were 3.0 (95% CI: 2.8-3.1) versus 2.9 (95% CI: 2.8-3.0) months (Figure 2A), and 91% versus 92% had maintained their MCyR at 12 months. Among patients who achieved a CCyR, median times to CCyR in patients with or without a mutation, respectively, were 5.4 (95% CI: 3.1-5.6) versus 3.1 (95% CI: 2.9-5.5) months, and CCyRs were maintained at 12 months by 95% versus 95% of patients (Figure 3A). Among the total group of patients with imatinib resistance or suboptimal response, rates at 24 months in patients with or without a BCR-ABL mutation, respectively, were 70% versus 80% for PFS, 86% versus 90% for TFS, and 88% versus 92% for OS (Figure 3B-C).
Cumulative response rates with dasatinib treatment according to BCR-ABL mutation status
| . | n . | n (%) or n/N (%) . | |||
|---|---|---|---|---|---|
| CHR . | MCyR . | CCyR . | MMR* . | ||
| All eligible patients | 1043 | 955 (92) | 639 (61) | 533 (51) | 399/992 (40) |
| Any BCR-ABL mutation | 402 | 362 (90) | 225 (56) | 175 (44) | 125/383 (33) |
| No BCR-ABL mutation | 641 | 593 (93) | 414 (65) | 358 (56) | 274/609 (45) |
| Patients with resistance or suboptimal response to imatinib | 805 | 735 (91) | 454 (56) | 360 (45) | 260/771 (34) |
| Any BCR-ABL mutation | 384 | 345 (90) | 211 (55) | 164 (43) | 115/367 (31) |
| No BCR-ABL mutation | 421 | 390 (93) | 243 (58) | 196 (47) | 145/404 (36) |
| Patients with intolerance to imatinib | 238 | 220 (92) | 185 (78) | 173 (73) | 139/221 (63) |
| Any BCR-ABL mutation | 18 | 17 (94) | 14 (78) | 11 (61) | 10/16 (63) |
| No BCR-ABL mutation | 220 | 203 (92) | 171 (78) | 162 (74) | 129/205 (63) |
| Patients with any BCR-ABL mutation | |||||
| Grouping by dasatinib IC50 | |||||
| IC50 greater than 3nM (excluding T315I)† | 121 | 110 (91) | 56 (46) | 39 (32) | 26/114 (23) |
| IC50 3nM or less‡ | 176 | 167 (95) | 107 (61) | 93 (53) | 65/171 (38) |
| IC50 unknown§ | 84 | 79 (94) | 60 (71) | 43 (51) | 34/78 (44) |
| Grouping by dose schedule | |||||
| 100 mg QD | 49 | 44 (90) | 27 (55) | 20 (41) | 17/47 (36) |
| 70 mg BID | 240 | 219 (91) | 140 (58) | 115 (48) | 79/231 (34) |
| 140 mg QD | 50 | 42 (84) | 28 (56) | 17 (34) | 11/46 (24) |
| 50 mg BID | 63 | 57 (90) | 30 (48) | 23 (37) | 18/59 (31) |
| Patients with 2 or more concurrent BCR-ABL mutations at baseline | 64 | 58 (91) | 33 (52) | 23 (36) | 16/61 (26) |
| 2 mutations | 50 | 47 (94) | 29 (58) | 21 (42) | 15/48 (31) |
| 3 mutations | 14 | 11 (79) | 4 (29) | 2 (14) | 1/13 (8) |
| . | n . | n (%) or n/N (%) . | |||
|---|---|---|---|---|---|
| CHR . | MCyR . | CCyR . | MMR* . | ||
| All eligible patients | 1043 | 955 (92) | 639 (61) | 533 (51) | 399/992 (40) |
| Any BCR-ABL mutation | 402 | 362 (90) | 225 (56) | 175 (44) | 125/383 (33) |
| No BCR-ABL mutation | 641 | 593 (93) | 414 (65) | 358 (56) | 274/609 (45) |
| Patients with resistance or suboptimal response to imatinib | 805 | 735 (91) | 454 (56) | 360 (45) | 260/771 (34) |
| Any BCR-ABL mutation | 384 | 345 (90) | 211 (55) | 164 (43) | 115/367 (31) |
| No BCR-ABL mutation | 421 | 390 (93) | 243 (58) | 196 (47) | 145/404 (36) |
| Patients with intolerance to imatinib | 238 | 220 (92) | 185 (78) | 173 (73) | 139/221 (63) |
| Any BCR-ABL mutation | 18 | 17 (94) | 14 (78) | 11 (61) | 10/16 (63) |
| No BCR-ABL mutation | 220 | 203 (92) | 171 (78) | 162 (74) | 129/205 (63) |
| Patients with any BCR-ABL mutation | |||||
| Grouping by dasatinib IC50 | |||||
| IC50 greater than 3nM (excluding T315I)† | 121 | 110 (91) | 56 (46) | 39 (32) | 26/114 (23) |
| IC50 3nM or less‡ | 176 | 167 (95) | 107 (61) | 93 (53) | 65/171 (38) |
| IC50 unknown§ | 84 | 79 (94) | 60 (71) | 43 (51) | 34/78 (44) |
| Grouping by dose schedule | |||||
| 100 mg QD | 49 | 44 (90) | 27 (55) | 20 (41) | 17/47 (36) |
| 70 mg BID | 240 | 219 (91) | 140 (58) | 115 (48) | 79/231 (34) |
| 140 mg QD | 50 | 42 (84) | 28 (56) | 17 (34) | 11/46 (24) |
| 50 mg BID | 63 | 57 (90) | 30 (48) | 23 (37) | 18/59 (31) |
| Patients with 2 or more concurrent BCR-ABL mutations at baseline | 64 | 58 (91) | 33 (52) | 23 (36) | 16/61 (26) |
| 2 mutations | 50 | 47 (94) | 29 (58) | 21 (42) | 15/48 (31) |
| 3 mutations | 14 | 11 (79) | 4 (29) | 2 (14) | 1/13 (8) |
CHR indicates complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; MMR, major molecular response; IC50, median inhibition concentration; QD, once daily; and BID, twice daily.
MMR rates are calculated in patients who had BCR-ABL transcript levels assessed.
L248V, G250E, Q252H, E255K/V, V299L, F317L, L384M, and F486S.
M244V, Y253F/H, D276G, E279K, F311L, M351T, F359V, V379I, L387M, and H396P/R. Patients were excluded if they had a concurrent mutation with a dasatinib IC50 higher than 3 nM.
Patients were excluded if they had concurrent mutations with a known dasatinib IC50 (as listed in † and ‡).
Cumulative response rates in patients with or without a BCR-ABL mutation who received dasatinib treatment at any dose or 100 mg once daily (QD) after resistance or suboptimal response to imatinib. CHR indicates complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; and MMR, major molecular response.
Cumulative response rates in patients with or without a BCR-ABL mutation who received dasatinib treatment at any dose or 100 mg once daily (QD) after resistance or suboptimal response to imatinib. CHR indicates complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; and MMR, major molecular response.
Time to first major cytogenetic response (MCyR). (A) Patients with or without a BCR-ABL mutation who received dasatinib treatment at any dose or 100 mg once daily (QD) after resistance or suboptimal response to imatinib. (B) Patients within the total analysis population with commonly mutated amino acids (n ≥ 20).
Time to first major cytogenetic response (MCyR). (A) Patients with or without a BCR-ABL mutation who received dasatinib treatment at any dose or 100 mg once daily (QD) after resistance or suboptimal response to imatinib. (B) Patients within the total analysis population with commonly mutated amino acids (n ≥ 20).
Kaplan-Meier analyses of patients with or without a BCR-ABL mutation who received dasatinib treatment at any dose or 100 mg once daily (QD) after resistance or suboptimal response to imatinib. (A) Duration of complete cytogenetic response (CCyR). (B) Progression-free survival (PFS). (C) Overall survival (OS).
Kaplan-Meier analyses of patients with or without a BCR-ABL mutation who received dasatinib treatment at any dose or 100 mg once daily (QD) after resistance or suboptimal response to imatinib. (A) Duration of complete cytogenetic response (CCyR). (B) Progression-free survival (PFS). (C) Overall survival (OS).
Among patients who were treated with dasatinib 100 mg once daily, time to response, response rates, duration of response, PFS, TFS, and OS in patients with or without BCR-ABL mutations were similar to those observed in the total group (Figures 1,Figure 2–3).
Sixty-four patients had 2 or more BCR-ABL mutations at baseline, including 5 patients who had T315I plus another mutation (Table 3). Excluding patients with T315I, response rates in patients with 2 or more mutations (CHR in 97%, MCyR in 54%, CCyR in 39%, and MMR in 29%) appeared comparable with the total group of patients with any mutation at baseline. PFS rates at 12 and 24 months in patients with 2 or more mutations were 77% and 55%, respectively (81% and 57%, respectively, excluding patients with T315I).
Efficacy according to individual BCR-ABL mutations
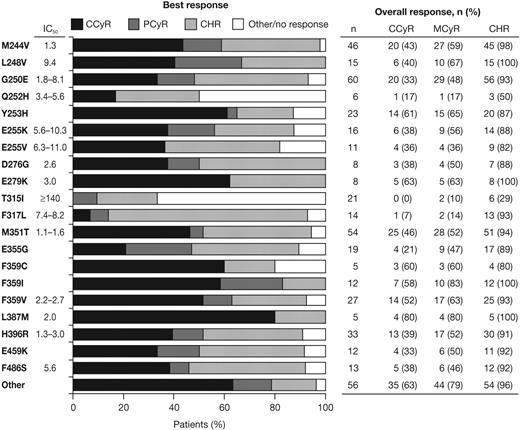
High response rates were observed in patients with the majority of different BCR-ABL mutation types, including highly imatinib-resistant mutations in L248, Y253, E255, F359, and H396, in addition to other common mutations in G250 and M351 (Figure 4). MCyRs were rapidly achieved in patients with the most commonly mutated amino acids, except T315I (Figure 2B). During dasatinib treatment, patients with an F317L mutation had a high rate of CHR (13/14, 93%), but low rates of MCyR (2/14, 14%) and CCyR (1/14, 7%). Among responding patients with F317L, 5 of 13 had a CHR at baseline (data not available for 4 patients) and 1 patient had a MCyR at baseline (data not available for 1 patient). For the 2 patients with a baseline F317L mutation who had a MCyR recorded on study, durations of MCyR during dasatinib treatment were 85 and 507 days (the latter patient maintained a CCyR for 416 days).
Best response achieved in each patient and overall rates of hematologic or cytogenetic response after dasatinib treatment of patients with different BCR-ABL mutations. Individual mutations occurring in ≥ 5 patients are listed with dasatinib IC50 values (in nanomolar), as reported by O'Hare et al3 and Redaelli et al.19 Other indicates total of all patients with other mutations that are not listed.
Best response achieved in each patient and overall rates of hematologic or cytogenetic response after dasatinib treatment of patients with different BCR-ABL mutations. Individual mutations occurring in ≥ 5 patients are listed with dasatinib IC50 values (in nanomolar), as reported by O'Hare et al3 and Redaelli et al.19 Other indicates total of all patients with other mutations that are not listed.
As expected, few responses were observed in the 21 patients who had a baseline T315I mutation: CHR in 6 patients, MCyR in 2 patients (maintained for 381 and 485 days), and no CCyRs. Among responding patients with T315I, 4 of 6 had a CHR at baseline (data not available for 1 patient) and 1 patient had a MCyR at baseline (data not available for 1 patient). Of the 21 patients with T315I at baseline, 16 had T315I alone and responses on study were CHR in 5 and MCyR in 1 patient. Of the 5 patients with a T315I mutation who had a different concurrent mutation, 1 patient had a CHR and 1 had a MCyR on study.
Analysis according to dasatinib IC50
To investigate the efficacy of dasatinib against mutations associated with an increased level of in vitro insensitivity, patients with mutations were grouped according to the dasatinib IC50 value for mutations reported by O'Hare et al3 and Redaelli et al.19 Consistent with previous suggestions,20 an IC50 value greater than 3nM was used to classify mutations as having an intermediate in vitro sensitivity to dasatinib. Other subgroups comprised patients with mutations with low IC50 values (≤ 3nM; ie, high sensitivity to dasatinib) or unknown IC50. Because the clinical resistance and high IC50 of the T315I mutation is well established, patients with this mutation were excluded from the IC50-based analysis.
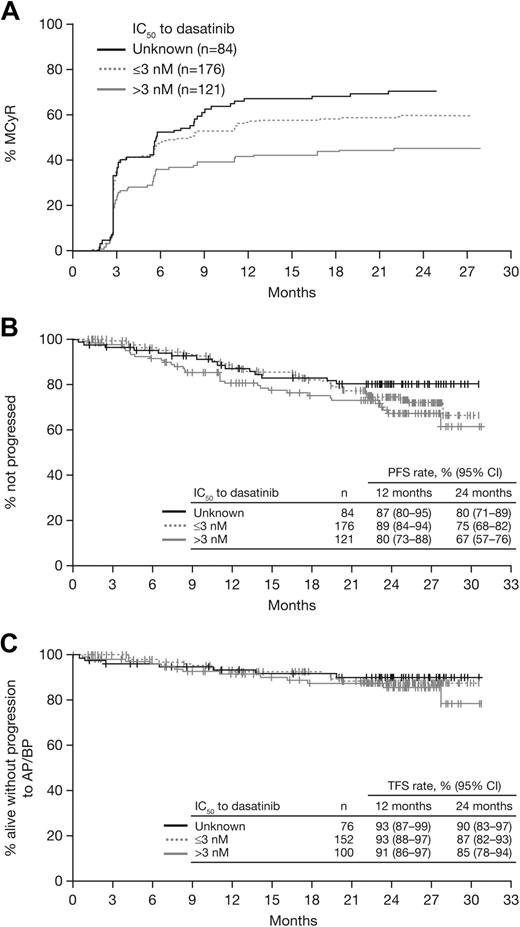
In 121 patients who had a BCR-ABL mutation with an intermediate IC50 (> 3nM), response rates were MCyR in 46%, CCyR in 32%, and MMR in 23% (Table 3). In patients who had a mutation with a low IC50 (n = 176) or unknown IC50 (n = 84), respectively, response rates were MCyR in 61% and 71%, CCyR in 53% and 51%, and MMR in 38% and 44%. Among responding patients in each subgroup, median time to MCyR was similar (2.8-3.0 months; Figure 5A), and MCyR was maintained at 12 months by 87% of responders in the intermediate IC50 subgroup and 93% in both the low and unknown IC50 subgroups. At 24 months, PFS rates were 67% to 80%, TFS rates were 85% to 90%, and OS rates were 89% to 91% (Figure 5B-C).
Analysis of responses and outcomes according to dasatinib IC50, based on cellular IC50 values reported by O'Hare et al3 and Redaelli et al.19 . (A) Time to major cytogenetic response (MCyR). (B) Progression-free survival (PFS). (C) Transformation-free survival (TFS).
Mutational assessment at the end of dasatinib treatment
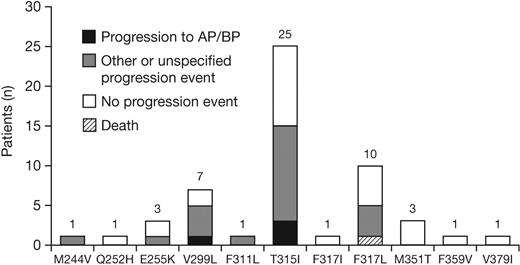
Of the 1043 patients analyzed, 536 (51%) progressed or discontinued dasatinib therapy, and 174 (17%) had a mutational assessment near to or after the time of progression/discontinuation. Among these 174 patients, 54 new mutations were detected in 47 patients (7 patients developed more than 1 new mutation), including 19 patients who developed a new mutation with a dasatinib IC50 greater than 3nM. The most commonly detected new mutations were T315I, V299L, and F317L (Figure 6). Among patients with or without a new mutation, respectively, 70% versus 37% had a preexisting mutation after prior imatinib treatment and 62% versus 32% had received a prior imatinib dose greater than 600 mg/day; other baseline characteristics were similar (Table 4). Response rates to dasatinib in the 174 patients among those who did eventually develop a new mutation compared with those who did not, respectively, were CHR in 85% versus 79%, MCyR in 34% versus 20%, and CCyR in 23% versus 13%. Among the 47 patients who had developed a new mutation, 24 had experienced a progression event, including 4 who progressed to AP or BP CML at the time of the assessment.
New BCR-ABL mutations detected at the time of progression or dasatinib discontinuation (n = 174). Other progression event indicates loss of complete hematologic response, loss of major cytogenetic response, or increasing white cell count. The progression event was not specified by the investigator for 3 patients who developed M244V, T315I, or V299L. AP indicates accelerated phase; BP, blast phase.
New BCR-ABL mutations detected at the time of progression or dasatinib discontinuation (n = 174). Other progression event indicates loss of complete hematologic response, loss of major cytogenetic response, or increasing white cell count. The progression event was not specified by the investigator for 3 patients who developed M244V, T315I, or V299L. AP indicates accelerated phase; BP, blast phase.
Baseline characteristics and treatment responses in patients who had a mutational assessment close to or following progression or discontinuation of dasatinib therapy (n = 174) who did or did not develop a new BCR-ABL mutation
| . | With new mutation, n = 47 . | Without new mutation, n = 127 . |
|---|---|---|
| Male, no. (%) | 26 (55) | 57 (45) |
| Median age, y (range) | 60 (32-82) | 57 (20-85) |
| Median duration of CML, mo (range) | 72 (4-246) | 61 (4-163) |
| Highest imatinib dose, no. (%) | ||
| Less than 400 mg/d | 0 (0) | 1 (1) |
| 400 to 600 mg/d | 18 (38) | 85 (67) |
| More than 600 mg/d | 29 (62) | 41 (32) |
| Prior imatinib treatment, % | ||
| Less than 1 y | 5 (11) | 21 (17) |
| 1 to 3 y | 16 (34) | 51 (40) |
| More than 3 y | 26 (55) | 55 (43) |
| Other prior therapy, no. (%) | ||
| Interferon-α | 25 (53) | 79 (62) |
| Stem cell transplantation | 2 (4) | 7 (6) |
| Response achieved prior to imatinib failure, no. (%) | ||
| CHR | 34 (72) | 103 (81) |
| MCyR | 11 (23) | 32 (25) |
| CCyR | 8 (17) | 12 (9) |
| MMR | 4 (9) | 8 (6) |
| Baseline BCR-ABL mutation, no. (%) | ||
| Yes | 33 (70) | 47 (37) |
| No | 14 (30) | 80 (63) |
| Response achieved on dasatinib therapy, no. (%) | ||
| CHR | 40 (85) | 100 (79) |
| MCyR | 16 (34) | 26 (20) |
| CCyR | 11 (23) | 16 (13) |
| . | With new mutation, n = 47 . | Without new mutation, n = 127 . |
|---|---|---|
| Male, no. (%) | 26 (55) | 57 (45) |
| Median age, y (range) | 60 (32-82) | 57 (20-85) |
| Median duration of CML, mo (range) | 72 (4-246) | 61 (4-163) |
| Highest imatinib dose, no. (%) | ||
| Less than 400 mg/d | 0 (0) | 1 (1) |
| 400 to 600 mg/d | 18 (38) | 85 (67) |
| More than 600 mg/d | 29 (62) | 41 (32) |
| Prior imatinib treatment, % | ||
| Less than 1 y | 5 (11) | 21 (17) |
| 1 to 3 y | 16 (34) | 51 (40) |
| More than 3 y | 26 (55) | 55 (43) |
| Other prior therapy, no. (%) | ||
| Interferon-α | 25 (53) | 79 (62) |
| Stem cell transplantation | 2 (4) | 7 (6) |
| Response achieved prior to imatinib failure, no. (%) | ||
| CHR | 34 (72) | 103 (81) |
| MCyR | 11 (23) | 32 (25) |
| CCyR | 8 (17) | 12 (9) |
| MMR | 4 (9) | 8 (6) |
| Baseline BCR-ABL mutation, no. (%) | ||
| Yes | 33 (70) | 47 (37) |
| No | 14 (30) | 80 (63) |
| Response achieved on dasatinib therapy, no. (%) | ||
| CHR | 40 (85) | 100 (79) |
| MCyR | 16 (34) | 26 (20) |
| CCyR | 11 (23) | 16 (13) |
CML indicates chronic myeloid leukemia; CHR, complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; and MMR, major molecular response.
Among the 174 patients who had a mutational assessment close to or after progression/discontinuation, 45 patients had a baseline BCR-ABL mutation that persisted until the end of dasatinib treatment (16 with dasatinib IC50 > 3nM), including 11 patients who both retained a mutation and developed a new mutation. In addition, 42 patients had lost a BCR-ABL mutation (19 with dasatinib IC50 > 3nM), including 24 patients who both lost a mutation and gained a new mutation.
Discussion
This report of more than 1000 patients, representing one of the largest analyses of BCR-ABL mutations to date, demonstrates that dasatinib is associated with favorable response rates in patients with or without BCR-ABL mutations after prior imatinib treatment, and that responses were durable throughout more than 2 years of follow-up. Among patients from the subgroup with resistance/suboptimal response to prior imatinib, low rates of progression events (PFS 70% to 80%) and high rates of survival (88% to 92%) were observed in patients with or without a baseline mutation. Across various measures, the efficacy achieved with dasatinib 100 mg once daily, which is associated with optimal tolerability, was similar to other doses including the previously approved 70-mg twice-daily dose.
Notable rates of hematologic, cytogenetic, and molecular responses were observed across almost all individual mutation types. As observed previously, patients with T315I responded poorly to dasatinib. Among patients who had other baseline mutations with a dasatinib IC50 greater than 3nM, that is, an intermediate IC50 value, response rates appeared to be slightly lower than those observed in patients with mutations having a low dasatinib IC50 (≤ 3nM), or unknown IC50. When mutations in the intermediate IC50 subgroup were examined individually, a favorable response rate was achieved with several mutations, including patients with highly imatinib-resistant E255K/V mutations (CCyR in 38%/36%, maintained for at least 450 days in 7 of these 9 patients), and also patients with L248V (CCyR in 40%) or G250E (CCyR in 33%). CCyR rates appeared to be lower in patients with F317L (1/14, 7%), Q252H (1/6, 17%), L384M (0/2, 0%), or V299L (0/1, 0%), although because of the small numbers of patients in some cases, caution is advised when interpreting these response rates. These data indicate that the IC50 cutoff of 3nM, as derived from in vitro studies, is only partially predictive of a decreased level of clinical responses to dasatinib, and several mutations with an IC50 greater than 3nM are associated with favorable responses to dasatinib. In groups of patients with mutations having a low or unknown dasatinib IC50, response rates were comparable with those observed in patients with no mutations. Newly emerging mutations associated with clinical resistance to dasatinib have been reported in amino acids T315, F317, and V299, which all have a low in vitro sensitivity to dasatinib.21-24 Overall, these data indicate that dasatinib has a similar potential for efficacy in the majority of patients with imatinib resistance (with or without a BCR-ABL mutation). Alternative treatment options should be considered for patients with baseline mutations that are clearly associated with resistance to dasatinib or lower levels of response, that is, T315I, F317L, and V299L. In the current analysis, these mutations represent less than 5% of imatinib-resistant patients, although the overall incidence may be higher in the clinical population based on the likely underrepresentation of T315I.
In addition to dasatinib, nilotinib (Tasigna; Novartis) is an alternative second-generation BCR-ABL inhibitor that is approved for the treatment of patients with CML-CP with resistance or intolerance to imatinib. Dasatinib and nilotinib have distinct effects on different types of BCR-ABL mutants, although T315I mutants are highly resistant to both agents (and imatinib). In patients who have developed nilotinib resistance, frequently detected mutations include T315I, some P-loop mutations (Y253H and E255K/V), and F359C/V.23,25 Unlike dasatinib, F317L has not been commonly associated with nilotinib resistance. In a recent analysis of nilotinib clinical study data in CML-CP, the CCyR rate after 12 months of nilotinib treatment in patients with a mutation in amino acids Y253H, E255K/V, or F359C/V was 0%.25 In the current analysis, dasatinib treatment was associated with favorable CCyR rates in patients with these mutations (61% for Y253H, 38%/36% for E255K/V, and 60%/52% for F359C/V). Response rates to nilotinib were not reported for other individual mutations, preventing further comparisons.
A recent manuscript has reported an IC50-based mutational analysis of patients who were treated at a single center (M. D. Anderson Cancer Center) with either dasatinib or nilotinib after prior imatinib failure.26 Within the study group, 30 patients had CML-CP and a BCR-ABL mutation; approximately one-half were treated with dasatinib. Compared with the subgroup of patients with CML-CP whose mutations had low IC50 values for dasatinib or nilotinib (n = 15), patients with mutations with intermediate IC50 values (n = 8) had a substantially lower rate of PFS at 24 months (22% vs 78%) and decreased survival (70% vs 100%). In the current analysis of dasatinib-treated patients only, this was not observed. Reasons for the divergent findings include the possible selection bias due to the small number of patients with CML-CP included in the M. D. Anderson analysis and the effects of nilotinib treatment.
In previous reports, detection of a BCR-ABL mutation has been associated with disease relapse or progression during imatinib treatment.12,27-30 The current analysis suggests that, with the exception of a small number of well-characterized mutations, dasatinib treatment can overcome this negative prognosis. This is consistent with the conclusions of other analyses where patients with mutations received a second-line TKI after imatinib resistance.11,31,32 Dasatinib has several attributes that may explain its ability to overcome the majority of imatinib-resistant mutations. BCR-ABL mutations arising during imatinib treatment mostly cluster into the imatinib binding site and other regions in ABL involved in conformational changes required for imatinib binding.1,33 Compared with imatinib, dasatinib has a distinct chemistry and a different (although overlapping) binding site within ABL, requiring fewer critical binding residues. In particular, interaction with the P-loop region appears less important for dasatinib than imatinib.34 In addition, in vitro modeling indicates that dasatinib has less stringent conformational requirements for ABL binding and predicts that dasatinib can bind both the catalytically active and inactive conformations of ABL, whereas imatinib can bind only the inactive conformation.34 Furthermore, dasatinib has more than 300-fold higher potency than imatinib against unmutated BCR-ABL, and this increased potency is maintained against most mutated BCR-ABL proteins tested.3
In addition to patients with a BCR-ABL mutation at baseline, response rates and durability of responses were also notable in imatinib-resistant patients without a mutation. This suggests the high potency and distinct chemistry of dasatinib compared with imatinib can overcome other mechanisms of imatinib resistance, such as BCR-ABL overexpression. In addition, low activity of the human OCT-1 influx protein is associated with lower clinical response rates to imatinib,35 and recent data have shown that unlike imatinib, dasatinib influx into cells is not dependent on OCT-1.36 Furthermore, dasatinib inhibits the activity of SRC-family kinases, which have been associated with imatinib resistance.37,38 It should also be noted that a lack of normal hematopoietic reserve in patients with prolonged disease can result in failure to achieve a cytogenetic response, and that this might explain the lack of response observed in some patients without a mutation or with a dasatinib-sensitive mutation.
Overall, the results of this analysis demonstrate that dasatinib is an effective treatment for the majority of patients with CML-CP who have developed an imatinib-resistant BCR-ABL mutation and is associated with durable responses and favorable long-term outcomes. In vitro sensitivity of different BCR-ABL mutants only partially predicts clinical outcome. Patients who fail imatinib therapy should undergo mutational analysis to facilitate rational selection of second-line therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Funding for clinical trials and statistical analysis was provided by Bristol-Myers Squibb (BMS). Professional medical writing assistance was provided by Jeremy Gardner of StemScientific, funded by Bristol-Myers Squibb. A.H. was supported by the German José-Carreras Foundation (DJCLS H 03/01). Clinical trial numbers: CA180-034 (NCT00123474), CA180-013 (NCT00101660), CA180-017 (NCT00103844).
Authorship
Contribution: M.C.M. designed and performed research, collected, analyzed, and interpreted data, and participated in writing the manuscript; J.E.C. collected and interpreted data, performed research, and participated in writing the manuscript; D.-W.K. and B.J.D. collected data; P.E. performed research, collected, analyzed, and interpreted data, and participated in writing the manuscript; R.P. collected data; S.B. and T.P.H. performed research and interpreted data; J.P.R. performed research, interpreted data, and participated in writing the manuscript; L.P. and J.M. collected data, performed statistical analysis, and interpreted data; and A.H. designed and performed research, collected, analyzed, and interpreted data, and participated in writing the manuscript.
Conflict-of-interest disclosure: J.E.C. and A.H. have received research funding from BMS, Novartis, and Wyeth. D.-W.K. has received research funding from BMS, Novartis, Wyeth, and Merck, and has acted in a consultant/speaker role for Novartis and Wyeth. Oregon Health & Science University and B.J.D. have a financial interest in MolecularMD; B.J.D. receives clinical trial funding from Novartis and BMS. S.B. has received research funding and honoraria from BMS and Novartis. T.P.H. has received research funding and has acted in a consultant/speaker role for BMS and Novartis. J.P.R. has acted in a consultant role for BMS and Novartis and has received research funding and honoraria from BMS and Novartis. L.P. and J.M. are employees of BMS. The remaining authors declare no competing financial interests.
Correspondence: Andreas Hochhaus, Abt Hämatologie und Internistische Onkologie, Universitätsklinikum Jena, Erlanger Allee 101, 07740 Jena, Germany; e-mail: andreas.hochhaus@med.uni-jena.de.