Abstract
One of the most unexpected and fascinating discoveries in oncology over the past few years is the interplay between abnormalities in protein-coding genes and noncoding RNAs (ncRNAs) that is causally involved in cancer initiation, progression, and dissemination. MicroRNAs (miRNAs), small regulatory ncRNAs, are involved in the pathogenesis of all types of human cancers, including leukemias, mainly via dysregulation of expression of cancer genes. Increasing evidence shows that miRNAs can work as tumor suppressors (inhibiting malignant potential) or oncogenes (activating malignant potential). Researchers first identified this new paradigm of molecular oncology in patients with chronic lymphocytic leukemia (CLL). Understanding the roles of miRNAs and other ncRNAs in leukemic cells is not only uncovering a new layer of gene regulation but also providing new markers for improved diagnosis and prognosis, as well as novel therapeutic options for CLL patients. Herein we focus on the roles of miRNAs and ultraconserved ncRNA genes in CLL, highlighting what is already known about their function, proposing a novel model of CLL predisposition and progression, and describing the challenges for the near future.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world, accounting for approximately 30% of all cases of leukemia in the United States with an annual incidence of approximately 10 000 new cases per year and a median age at diagnosis of 65 years.1-3 Discoveries over the past 7 years have shown that CLL, considered the Cinderella of hematologic malignancies because of poor understanding of it at the molecular level despite decades of research, has a quite interesting molecular pathogenesis because of unexpected connections with noncoding RNAs (ncRNAs), which are RNAs that do not code for a protein4 and include microRNAs (miRNAs)5 and noncoding ultraconserved genes (UCGs).6
Structurally, miRNAs are short 19- to 25-nucleotide (nt) RNAs that are processed from much longer primary transcripts (pri-miRNAs, which are hundreds to thousands of nucleotides) and arise from hairpin loop structures (pre-miRNAs, 60-110 nt) after successive enzymatic maturation steps involving the RNAses III Drosha in the nucleus and Dicer in the cytoplasm. After incorporation into the ribonucleoprotein RNA-induced silencing complex, comprising proteins such as Dicer and members of the Argonaute family, miRNAs bind to messenger RNAs (mRNAs) primarily at their 3′ untranslated regions (UTRs) via partial complementarity with their “seed” sequences (the first 2-8 nt at the miRNA's 5′ end proved to be important for proper target recognition) and “seedless” complementary sequences. Consequently, mRNA translation and/or stability are impaired, ultimately resulting in a reduction in protein expression levels. MiRNAs are strongly conserved among distantly related organisms (including invertebrates, vertebrates, and plants) and are involved in various critical biologic processes, including development, differentiation, metabolism, immunity, neuronal patterning, cell-cycle regulation, apoptosis, stress response, and aging.7 Researchers have estimated that the human genome contains more than 1000 miRNAs and that miRNAs negatively regulate the expression of the majority of protein-coding genes (PCGs). Emerging evidence indicates that the effects of miRNAs on gene expression may be more varied than initially proposed.8 MiRNAs can activate mRNA expression via AU-rich elements inside mRNA 3′UTRs and initiate the translation of proteins whose expression they normally repress during cell proliferation (miR-363-3 and let-7).9 Furthermore, authors reported that miRNA-dependent mRNA repression occurred via binding sites located inside mRNA-coding sequences as shown for miRNAs regulating fundamental processes such as embryonic stem cell differentiation (miR-134, miR-296, and miR-470).10 In addition, studies showed that specific miRNAs carrying distinct hexanucleotide terminal motifs, such as miR-29b, were enriched in the nucleus, suggesting that miRNAs have extra functions in different subcellular compartments.11 In addition, miRNAs in the nucleus may act at the promoter level, affecting transcription of protein-coding genes (miR-373).12 Finally, recent evidence suggests that miRNAs can regulate ncRNAs from the category of long UCGs.6
In this review, we present evidence indicating that ncRNAs, especially miRNAs, are causally involved in cancer initiation, progression, and dissemination of malignant CLL cells and show that knowledge of their roles can be exploited for the diagnosis, prognosis, and treatment of CLL.
The anatomy of the CLL genome: hot spots and small noncoding genes
For decades investigators have observed recurrent chromosomal abnormalities without known PCGs in patients with CLL, suggesting the involvement of yet-to-be-deciphered common pathogenetic pathways. Chromosomal alterations occur in approximately 80% of CLL cases; these alterations include the 13q deletion (13q−; > 50%), the 11q deletion (11q−; 18%), trisomy of chromosome 12 (12%), and the 17p deletion (17p−; 7%). Thus, CLL is characterized by a high rate of recurrence of the same chromosomal abnormalities (with 13q−, 11q−, and 17p− being among the most common), suggesting that these abnormalities may affect a common pathway(s) important to the pathogenesis of CLL that has yet to be identified.
Description of the miRNA alterations in cancer cells (detailed in more than 1500 publications) started in 2002 with the finding that the genes in the 13q14 deletion locus involved in CLL pathogenesis were actually 2 miRNAs, miR-15a and miR-16-1, located in the fourth intron of the DLEU2 gene.13 Over the previous decade, researchers cloned DLEU1 and DLEU2, 2 long noncoding genes residing in the proximal (centromeric) region of deletion, but their pathogenic roles have yet to be established, and studies actually found that DLEU2 is the host gene for the miRNA cluster.13 At that time, miRNA involvement in CLL appeared to be either a characteristic finding for this type of leukemia, representing an isolated mechanism for human disease (such as the trinucleotide repeat expansions in specific and rare neurologic diseases),14 or the initial finding of a new, more general mechanism responsible for human tumorigenesis. The first clue indicating the general involvement of miRNAs in cancer was the identification of a genome-wide correlation between genomic localization of miRNAs and cancer-associated genomic regions (for an extensive review see Calin and Croce15 ). Overall, many of the miRNAs mapped at fragile sites (FRAs), minimal regions of amplification, sites of loss of heterozygosity, and common breakpoint regions in or near oncogenes and/or tumor-suppressor genes (TSGs) involved in many leukemias and lymphomas and carcinomas (Table 1). Interestingly, the majority of miRNAs are mapped at “orphan” FRAs, which are FRAs that are frequently deleted from cancer cells with no well-identified TSGs (eg, miR-29a and miR-29b-1, which are located in FRA7H; Table 1, Figure 1).15 Further confirming this, authors reported that orthologous miRNA genes are located in cancer-associated genomic regions in humans and mice and potentially are involved in the development of hematologic cancers and brain tumors.18
MiRNAs and CLL: genomics, pathways, and clinical correlations
| Human miRNA (location)/cancer function . | Cancer-associated genomic region . | Deregulation in tumors, including CLL . | Putative functions and targets . | Diagnostic and prognostic markers . |
|---|---|---|---|---|
| Let-7 family (various)/antitumorigenic | Urothelial, bladder, esophageal, ovarian, cervical, and breast cancers and lung adenocarcinoma; FRA9D and FRA11B | (1) Down-regulation in CLL; lung, breast, gastric, ovarian, prostate, and colon cancers; and leiomyomas; (2) point mutation in the let-7e precursor sequence, which affects maturation | Molecular mechanism: MYCN positively regulates let-7b transcription, and LIN-28 regulates the maturation of let-7a, represses cell proliferation/growth, and promotes angiogenesis. Targets: CCND1, CDC25a, CDK6, CRD-BP, HOXA9, IMP-1, MYC, and RAS | Poor prognosis: low let-7a-2 expression (lung and ovarian cancer); drug resistance: let-7i affects chemotherapy potency; therapy: intranasal delivery of let-7a adenovirus reduces growth of Ras-induced lung tumors in mice |
| MiR-16-1/miR-15a cluster (13q14.3, intron 4 ncRNA DLEU2)/antitumorigenic | B-CLL, adult lymphoblastic leukemia, head and neck squamous cell carcinoma, oral cancers, and lipoma | (1) Down-regulation in CLL, DLBCLs, multiple myeloma, pituitary adenoma, and prostate and pancreatic cancers; (2) germline mutations in patients with B-CLL and the NZB mouse strain | Molecular mechanism: induce apoptosis in leukemia cells, miR-16 regulates the cell cycle via down-regulation of expression of G0/G1 proteins. Targets: BCL2, CARD10, CCND1, CDK6, CDC27, DMTF1, MCL1, NGN2, and VEGF | Poor prognosis: higher miR-15a and miR-16 expression in de novo aggressive CLL; drug resistance: miR-16 affects chemotherapy potency and modulates sensitivity to vincristine in gastric cancer cell lines |
| miR-21 (17q23.1, 3′ UTR TMEM49)/oncogenic | Neuroblastoma and breast cancer; FRA17B | Overexpression in CLL; glioblastomas; breast, lung, prostate, colon, stomach, esophageal, and cervical carcinomas; uterine leiomyosarcoma; and DLBCL | Molecular mechanism: STAT3 regulates miR-21 at the transcriptional level; miR-21 knockdown induces apoptosis in glioblastoma cells; miR-21 induces invasion and metastasis in colorectal cancers. Targets BCL2, MASPIN, PDCD4, PTEN, TPM1, MCL1, TCL1 | Poor prognosis: high miR-21 expression (in colon cancer); good prognosis: high miR-21 expression in de novo DLBCL; drug resistance: miR-21 affects chemotherapy potency in NCI60 cells |
| miR-29 family (various)/antitumorigenic | Prostate cancer aggressiveness locus; FRA7H | Down-regulation in CLL and acute myelogenous leukemia; colon, breast, and lung cancer; and cholangiocarcinoma tumor models (KMCH) | Molecular mechanism: miR-29 family reverses aberrant methylation in lung cancer and acute myelogenous leukemia. Targets: DNMT3A, DNMT3B | Poor prognosis: low miR-29c expression correlates with short intervals from diagnosis of to therapy for CLL |
| miR-34 family (1p36.23 and 11q23.1, intergenic)/antitumorigenic | CLL, lung and breast cancer; t(3;11) (B-cell leukemia line) | (1) Down-regulation in CLL and pancreatic cancer cell lines; (2) hypermethylation of miR-34b/miR-34c in colon cancer | Molecular mechanism: P53 transactivates miR-34a and the miR-34b/miR-34c cluster; miR-34a induces up-regulation of the P53 pathway and down-regulation of the E2F pathway in colon cancer cell lines. Targets: BCL2, CCND1, CCNE2, CDK4/6, DLL1, E23, Notch1, MYCN, MET | Therapy: low levels of miR-34a expression are associated with impaired DNA damage response and fludarabine-refractory CLL |
| miR-143/145 cluster (intergenic, 5q32)/antitumorigenic | Prostate cancer aggressiveness, myelodysplastic syndrome | Down-regulation in colon adenomas and carcinomas, breast and lung cancer, and B-cell malignancies | Molecular mechanism: miR-143 and miR-145 precursors are abnormally processed in colon cancer. Targets: ERK5, HOXA9, PARP8 | |
| miR-155 (21q21.3)/oncogenic | Colon cancer | Molecular mechanism: pre-B-cell proliferation and lymphoblastic leukemia/high-grade lymphoma in miR-155 transgenic mice. Targets: uc.346A and uc.160 | Poor prognosis: high miR-155 expression (in lung cancer, DLBCL, and aggressive CLL) | |
| miR-181 family (various)/oncogenic and antitumorigenic | FRA1K, FRA9E, and FRA19B | Overexpression in breast, pancreatic, and prostate cancer | Molecular mechanism: MYCN regulates the transcription of the miR-181 cluster. Targets: HOXA11, TCL1 | Poor prognosis: low miR-181 expression in aggressive CLL with 11q−; high miR-181a expression correlates with short intervals from diagnosis of to therapy for CLL |
| Human miRNA (location)/cancer function . | Cancer-associated genomic region . | Deregulation in tumors, including CLL . | Putative functions and targets . | Diagnostic and prognostic markers . |
|---|---|---|---|---|
| Let-7 family (various)/antitumorigenic | Urothelial, bladder, esophageal, ovarian, cervical, and breast cancers and lung adenocarcinoma; FRA9D and FRA11B | (1) Down-regulation in CLL; lung, breast, gastric, ovarian, prostate, and colon cancers; and leiomyomas; (2) point mutation in the let-7e precursor sequence, which affects maturation | Molecular mechanism: MYCN positively regulates let-7b transcription, and LIN-28 regulates the maturation of let-7a, represses cell proliferation/growth, and promotes angiogenesis. Targets: CCND1, CDC25a, CDK6, CRD-BP, HOXA9, IMP-1, MYC, and RAS | Poor prognosis: low let-7a-2 expression (lung and ovarian cancer); drug resistance: let-7i affects chemotherapy potency; therapy: intranasal delivery of let-7a adenovirus reduces growth of Ras-induced lung tumors in mice |
| MiR-16-1/miR-15a cluster (13q14.3, intron 4 ncRNA DLEU2)/antitumorigenic | B-CLL, adult lymphoblastic leukemia, head and neck squamous cell carcinoma, oral cancers, and lipoma | (1) Down-regulation in CLL, DLBCLs, multiple myeloma, pituitary adenoma, and prostate and pancreatic cancers; (2) germline mutations in patients with B-CLL and the NZB mouse strain | Molecular mechanism: induce apoptosis in leukemia cells, miR-16 regulates the cell cycle via down-regulation of expression of G0/G1 proteins. Targets: BCL2, CARD10, CCND1, CDK6, CDC27, DMTF1, MCL1, NGN2, and VEGF | Poor prognosis: higher miR-15a and miR-16 expression in de novo aggressive CLL; drug resistance: miR-16 affects chemotherapy potency and modulates sensitivity to vincristine in gastric cancer cell lines |
| miR-21 (17q23.1, 3′ UTR TMEM49)/oncogenic | Neuroblastoma and breast cancer; FRA17B | Overexpression in CLL; glioblastomas; breast, lung, prostate, colon, stomach, esophageal, and cervical carcinomas; uterine leiomyosarcoma; and DLBCL | Molecular mechanism: STAT3 regulates miR-21 at the transcriptional level; miR-21 knockdown induces apoptosis in glioblastoma cells; miR-21 induces invasion and metastasis in colorectal cancers. Targets BCL2, MASPIN, PDCD4, PTEN, TPM1, MCL1, TCL1 | Poor prognosis: high miR-21 expression (in colon cancer); good prognosis: high miR-21 expression in de novo DLBCL; drug resistance: miR-21 affects chemotherapy potency in NCI60 cells |
| miR-29 family (various)/antitumorigenic | Prostate cancer aggressiveness locus; FRA7H | Down-regulation in CLL and acute myelogenous leukemia; colon, breast, and lung cancer; and cholangiocarcinoma tumor models (KMCH) | Molecular mechanism: miR-29 family reverses aberrant methylation in lung cancer and acute myelogenous leukemia. Targets: DNMT3A, DNMT3B | Poor prognosis: low miR-29c expression correlates with short intervals from diagnosis of to therapy for CLL |
| miR-34 family (1p36.23 and 11q23.1, intergenic)/antitumorigenic | CLL, lung and breast cancer; t(3;11) (B-cell leukemia line) | (1) Down-regulation in CLL and pancreatic cancer cell lines; (2) hypermethylation of miR-34b/miR-34c in colon cancer | Molecular mechanism: P53 transactivates miR-34a and the miR-34b/miR-34c cluster; miR-34a induces up-regulation of the P53 pathway and down-regulation of the E2F pathway in colon cancer cell lines. Targets: BCL2, CCND1, CCNE2, CDK4/6, DLL1, E23, Notch1, MYCN, MET | Therapy: low levels of miR-34a expression are associated with impaired DNA damage response and fludarabine-refractory CLL |
| miR-143/145 cluster (intergenic, 5q32)/antitumorigenic | Prostate cancer aggressiveness, myelodysplastic syndrome | Down-regulation in colon adenomas and carcinomas, breast and lung cancer, and B-cell malignancies | Molecular mechanism: miR-143 and miR-145 precursors are abnormally processed in colon cancer. Targets: ERK5, HOXA9, PARP8 | |
| miR-155 (21q21.3)/oncogenic | Colon cancer | Molecular mechanism: pre-B-cell proliferation and lymphoblastic leukemia/high-grade lymphoma in miR-155 transgenic mice. Targets: uc.346A and uc.160 | Poor prognosis: high miR-155 expression (in lung cancer, DLBCL, and aggressive CLL) | |
| miR-181 family (various)/oncogenic and antitumorigenic | FRA1K, FRA9E, and FRA19B | Overexpression in breast, pancreatic, and prostate cancer | Molecular mechanism: MYCN regulates the transcription of the miR-181 cluster. Targets: HOXA11, TCL1 | Poor prognosis: low miR-181 expression in aggressive CLL with 11q−; high miR-181a expression correlates with short intervals from diagnosis of to therapy for CLL |
For more details, see Spizzo et al.16 Gene symbols appear as indicated in the National Center for Biotechnology Information PubMed database (http://www.ncbi.nlm.nih.gov/sites/entrez).17
DLBCL indicates diffuse large-B cell lymphoma; and STAT3, signal transducer and activator of transcription 3.
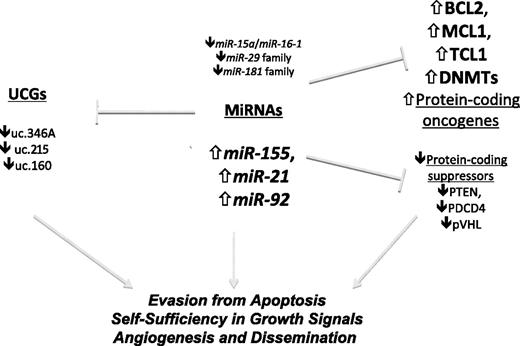
Involvement of ncRNAs in human leukemias.miRNAs regulate the expression of protein-coding genes and can act as oncogenes, tumor suppressors, or both. UCGs, which are regulated by miRNAs, can act as oncogenes, whereas their role as tumor suppressors has been hypothesized but has not been experimentally proven until now. The main examples of protein coding targeted by miRNAs are presented.
Involvement of ncRNAs in human leukemias.miRNAs regulate the expression of protein-coding genes and can act as oncogenes, tumor suppressors, or both. UCGs, which are regulated by miRNAs, can act as oncogenes, whereas their role as tumor suppressors has been hypothesized but has not been experimentally proven until now. The main examples of protein coding targeted by miRNAs are presented.
miR-15a/miR-16-1 cluster
For years, hemizygous and/or homozygous deletions have been observed in the 13q14.3 chromosomal region in more than 50% of CLL cases, making these deletions the most frequently observed chromosomal abnormalities in CLL.19,20 13q14 deletions also occur in approximately 50% of mantle cell lymphoma cases, 16% to 40% of multiple myeloma cases, and 60% of prostate cancer cases, suggesting that one or more TSGs at 13q14 are involved in the pathogenesis of these tumors in humans. Decades of genomic studies failed to identify any suppressor genes that may be involved in the pathogenesis of CLL. However, the cluster encoding miR-15a and miR-16-1 was identified by mapping the involved CLL gene to a 30-kb region between exons 2 and 5 of the LEU2 gene in 2 patients with CLL: one carrying a t(2;13)(q32;q14) translocation and the other having bilateral retinoblastoma and ulcerative colitis.13 The miR-15a/miR-16-1 cluster was initially found deleted or down-regulated in approximately 70% of CLL cases (compared with CD5+ lymphocytes obtained from healthy donors).13 Moreover, a mutation that considerably reduces the expression of this cluster has been found in association with loss of the normal allele in leukemic cells obtained from 2 patients with CLL (1 of whom had a family history of CLL and breast cancer), representing the classical Knudson mechanism of suppressor gene inactivation.21 The roles of miR-15a and miR-16-1 as TSGs in CLL were reinforced by a study showing an inverse correlation between their expression and that of the antiapoptotic gene BCL2 and that the cluster directly targets and represses expression of BCL2 in leukemic cells (Figure 1).22 These findings identified the miR-15a/miR-16-1 cluster as the candidate tumor suppressor located at 13q14 in CLL cells. Consistent with the oncosuppressive model, cell growth and cell-cycle progression were negatively regulated by miR-16-1.23 Further supporting the extended roles of 13q14 deletion and the miR-15a/miR-16-1 cluster in human cancer, this cluster of miRNAs reportedly has a tumor-suppressor function in prostate cancers by targeting cyclin D1 and WNT3A oncogenes,24 and this could also be true for multiple myeloma or mantle cell lymphoma. The existence of a homologous cluster of miRNAs (miR-15b/miR-16-2) in a region that is not commonly deleted from CLL cells (3q25) raises a question about the relative contribution of these paralogous clusters in the pathogenesis of CLL. A report indicating that patients with CLL having a monoallelic 13q14 deletion have slower lymphocyte growth kinetics than those with a biallelic 13q14 deletion25 suggests that residual expression of miR-15/miR-16 contributes to improved prognosis for this malignancy. Because most patients with indolent CLL carry deletions of the miR-15a/miR-16-1 cluster at 13q14, losses of these miRNAs likely are the initiating events for CLL.
miR-34 family
The miR-34 family contains 3 members, consisting of miR-34a, which is located in cancer-associated genomic region 1p36 and is frequently lost or rearranged in many cancers, including neuroblastoma, chronic myelogenous leukemia, acute myelogenous leukemia, and other hematologic malignancies26 ; and the miR-34b/miR-34c cluster, which is located at CLL deleted region 11q23 centromeric to the ATM suppressor gene that is infrequently mutated in CLL except for cases carrying 11q−.27 Loss of the long arms of chromosomes 11 and 13 is a common cytogenetic abnormality in patients with CLL; however, 11q− is found only in patients with aggressive disease. miR-34a is transcriptionally induced by p53 and has been proven to directly target CDK6, CCND1, CDK4, CCNE2, and MET.28 Supporting these findings, researchers showed that deletion of 17p13/P53 was associated with down-regulation of miR-34a expression in patients with CLL.29 Interestingly, a critical synthenic region on zebrafish chromosome 9 maps to the minimal deleted region in CLL patients on both human chromosomes, suggesting a common ancestry for the 2 clusters of miRNAs miR-15a/miR-16-1 and miR-34b/miR-34c.30 Investigators have delineated the pathogenic role played by the miR-34 family in tumorigenesis in general and CLL in particular by analyzing the transcriptome induced by overexpression of miR-34, which is highly similar to that observed with expression of the P53 tumor suppressor. In fact, the transcriptome is highly enriched for genes that regulate cell-cycle progression, apoptosis, DNA repair, and angiogenesis.31,32 Further proving its tumor-suppressive role, studies have shown miR-34a to be proficient in inducing cell-cycle arrest and subsequent caspase-dependent apoptosis via BCL233 and E2F transcription factor 334 repression. The miR-34b/miR-34c cluster carries a third miRNA, miR-34b-5p, that has sites of complementarity in the 3′UTR of the oncogene TCL1,35 which is overexpressed in CLL cases with 11q−.36
miR-29b and miR-181b
Researchers have demonstrated a tumor-suppressor function in CLL for miR-29a and miR-29b (located at the cancer deleted 7q32 region)36,37 as well as miR-181a and miR-181b (located in 2 clusters at the cancer deleted regions, 1 each at 1q31.3 and at 9q33).36,38 Indeed, they can directly target TCL1,36 an oncogene that coactivates AKT and regulates several pathways (such as the nuclear factor κB, murine double minute, and cyclin D1 pathways) involved in cell survival, proliferation, and death (Figure 1). Studies have widely proven TCL1's relevance to CLL: (1) TCL1 is a marker of aggressive CLL as evidenced by its direct correlation with 70-kDa zeta-associated protein 70 (ZAP-70) expression levels and unmutated immunoglobulin heavy-chain variable-region gene (IgVH) status (both markers of a poor prognosis for CLL)39 ; (2) TCL-1 overexpression correlates with 11q−,36 another common chromosomal abnormality in CLL; and (3) TCL1 transgenic mice models have shown that TCL1-driven CLLs have all the characteristics of aggressive CLLs.40,41 On the other hand, ectopic expression of miR-181 in murine hematopoietic progenitors increases the fraction of B cells both in vitro and in vivo, suggesting a B-cell proliferation role for miR-181.42
Signal transduction in CLL: the interplay between miRNAs and coding genes
CLL is characterized by the progressive accumulation of morphologically mature CD5+ B lymphocytes in the blood, marrow, and lymphatic tissues. The great majority of CLL cells (> 90%) are nondividing and arrested at G0 or G1 phase of the cell cycle. CLL cells are also quite resistant to apoptosis, and researchers have suggested that an excess of B cells is more likely a result of decreased apoptosis and deregulation of cell-cycle control than of an increased proliferation rate. MiRNA alterations affect all of the 6 hallmarks of malignant cells43 ; in CLL, the main effects are evasion of apoptosis, self-sufficiency in growth, and as recent studies suggest, stimulation of angiogenesis and dissemination (Table 1, Figure 1).
Evasion of apoptosis
Apoptosis is a physiologic cellular self-destruction mechanism essential for a variety of biologic events (eg, development, tissue homeostasis) that leads to removal of unwanted cells. The hallmark of the malignant, mostly nondividing, B cells in CLL cases is overexpression of the antiapoptotic protein BCL2.44 In normal tissues, BCL2 is responsible for maintaining the delicate homeostasis between proliferation and apoptosis and promotes cell survival by inhibiting cell death.45 In follicular lymphomas and a fraction of diffuse large B-cell lymphomas, the mechanism of BCL2 activation is the translocation t(14;18)(q32;q21), which places the BCL2 gene under the control of immunoglobulin heavy chain enhancers, resulting in deregulated expression of the gene.46 BCL2 is juxtaposed to immunoglobulin loci in less than 5% of all CLL cases.47 In the remaining 95% of cases, until recently, no one had discovered a mechanism explaining BCL2 dysregulation in CLL. However, investigators have shown that miR-15a and miR-16-1 are major direct negative regulators of the BCL2 antiapoptotic protein22 and indirect activators of the intrinsic apoptotic program leading to apoptotic peptidase activating factor 1/caspase-9/poly(adenosine diphosphate–ribose) polymerase pathway activation. These 2 miRNAs are also capable of suppressing the tumorigenicity of leukemic cells with deletion of both miR-15a and miR-16 loci at 13q14 in a murine xenograft tumor model.48 The down-modulation of these 2 miRNAs in leukemias has also been correlated with altered expression of other antiapoptotic proteins (eg, MCL1).48 miR-29b, another miRNA whose expression is known to be down-regulated in patients with CLL having poor prognosis, directly targets MCL1, which belongs to the BCL2 family of antiapoptotic factors.49
Recently, researchers identified a novel regulatory mechanism by which miRNAs regulate apoptosis by repressing the itchy E3 ubiquitin protein ligase homolog (ITCH) and consequently inducing expression of the proapoptotic regulator p73 in CLL. Transcriptional activation of miR-106b, mediated by E2F1 and MYC after treatment of primary CLL cells with deacetylase inhibitors, led to down-regulation of E3-ubiquitin ligase ITCH expression and reciprocal accumulation of the proapoptotic substrate, p73. miR-106b–dependent repression of ITCH is accompanied by mitochondrial dysfunction, processing of caspase-9, and apoptosis of CLL cells.50 This mechanism may be CLL specific, as it was not reproducible in sarcoma or fetal kidney cells.51,52
miR-21 overexpression is a common trait of most malignancies, including CLL.53 This miRNA acts prevalently as an antiapoptotic protein by blocking the expression of critical apoptosis-related genes. Among the apoptotic targets of miR-21 are the tumor-suppressor PDCD454 and the tumor-suppressor PTEN,55 the silencing of which favors the transmission of antiapoptotic survival signals through the phosphoinositide 3-kinase/AKT pathway. Further confirming its antiapoptotic activities, miR-21 expression is induced by the cell survival pathway downstream of interleukin-6 via direct transcriptional activation of signal transducer and activator of transcription 3 and ectopic up-regulation of miR-21 expression in myeloma cells, which reduces their apoptosis levels.56
Self-sufficiency in growth signals
Normal cells require growth stimuli, whereas cancer cells are capable of generating their own growth signals without having to rely on mitogens in the surrounding environment to actively proliferate. RAS activation is a common way by which tumor cells escape from growth factor dependency and become oncogene addicted. Let-7, which was one of the first identified miRNAs and whose expression is consistently down-regulated in carcinomas, lymphomas, and leukemias,57 is a now well-documented posttranscriptional regulator of RAS.58 The relevance of this mechanism is indicated by an inverse correlation between let-7 and RAS expression levels and, more recently, by the fact that therapeutic approaches targeting let-7 in lung cancer models can influence tumor regression by affecting cell proliferation.59 In the leukemogenic process, RAS signaling is increased, and in CLL in particular, it is linked with concomitant loss of let-7 expression.60,61
miR-143 and miR-145 cluster on chromosome 5q, and their expression is commonly down-regulated in different tumor types, such as solid tumors (breast, colon, and ovarian tumors) and most B-cell malignancies, including CLL, Burkitt lymphoma, and Epstein-Barr virus–transformed B-cell lymphomas.62 Loss of these miRNAs has been shown to accelerate cell proliferation by deregulating the extracellular signal-related kinase 5 (ERK5) signaling pathway because they directly target ERK5 mRNA. Constitutive activation of the ERK5 pathway is known to play a role in the pathogenesis of Hodgkin lymphoma, inducing high levels of expression of the HOXB9 gene together with the neighboring miR-196a-1 gene.63 This finding supports the existence of indirect regulatory loops between miRNAs (in this case, between miR-143/miR-145 and miR-196a). A recent study showed that miR-145 exerts its antiproliferative effects on tumor cells by suppressing insulin receptor substrate 1 expression levels in addition to acting on ERK5.64 Insulin receptor substrate 1 is a docking protein for the insulin-like growth factor type 1 receptor and the insulin receptor, which sends mitogenic and antiapoptotic signals as part of an oncogenic pathway, and is differentially expressed in B cells and myeloid leukemia cells.65 Furthermore, miR-145 participates in p53-dependent regulation of the oncogene c-Myc, as expression of this miRNA is directly induced at the transcriptional level by p53 and directly repressed at the posttranscriptional level by c-Myc, consequently inhibiting tumor cell growth.66 This specific function may explain why B cells in CLL can acquire additional growth advantages by loss of expression of this miRNA.
Hypoxia and angiogenesis
Rapid growth of tumor cells usually creates a hypoxic environment, which induces cell-adaptation responses, such as hypoxia-inducible factor (HIF)–dependent survival pathways and angiogenesis. CLL B cells have high endogenous levels of vascular endothelial growth factor (VEGF) mRNA expression and are able to spontaneously secrete VEGF. Possibly related to these high VEGF levels, the marrow and lymph nodes of patients with CLL show high degrees of tissue neovascularization. In tumor progression, hypoxia can contribute to modulation of miRNA expression, partly via direct HIF-1 transcriptional activation of specific miRNAs.67 Elevated plasma VEGF levels in CLL cases are associated with advanced disease, even in early-stage cases.68 These miRNAs have dual functions: (1) they aid cells in engaging antiapoptotic programs, sustaining cell survival (eg, miR-26, miR-107, and miR-210), and (2) they participate in the angiogenic process. The initial evidence that the von Hippel-Lindau gene product (pVHL), without any genetic alterations, can be deregulated in CLL by aberrantly expressed miRNAs uncovered the molecular mechanism of abnormal autocrine VEGF secretion in CLL cases.68 In fact, pVHL, which is responsible for HIF-1α degradation, is expressed at notably lower levels in CLL B cells than in normal B cells. Concomitantly, miR-92-1, which is overexpressed in CLL B cells, targets the pVHL transcript and represses expression of it. Thus, overexpressed miR-92-1 is indirectly responsible for stabilization of HIF-1α, which in turn can form a transcriptionally active complex (with p300 and phosphorylated signal transducer and activator of transcription 3) at the VEGF promoter, upholding its sustained overexpression.68
NcRNA networks in CLL: interaction of long UCGs with miRNAs
Because studies have clearly proven that miRNAs are involved in CLL, we asked whether other ncRNAs play roles in this disease. We focused our attention on the ultraconserved regions (UCRs) of the human genome that are highly conserved among various species exactly as miRNAs are important for CLL. For example, the active molecules in the miR-15a/miR-16-1 cluster are completely conserved in humans, mice, and rats and highly conserved in 9 of the 10 sequenced primate species. Bejerano et al69,70 first discovered UCRs in 2004 in a bioinformatic comparison of the mouse, rat, and human genomes; phylogenetically, UCRs date from a very early period in vertebrate evolution, as no researchers have described any orthologous counterparts in sea squirts, flies, or worms.71 We proved that UCRs are not silent relicts of evolution, but are highly transcribed. By profiling the expression of 481 UCRs longer than 200 bp in CLL cells using an array, we found a signature consisting of 19 differentially expressed UCGs (8 up-regulated and 11 down-regulated).6 Overall, these data indicated that UCRs are differentially expressed in normal and neoplastic tissues in humans.
The regulatory mechanisms underlying UCR expression are still poorly understood. Nevertheless, an intriguing interaction between UCRs and miRNAs was demonstrated, suggesting the existence of a network of ncRNAs with reciprocal controlling functions (Figure 1). Similar to that described for miRNAs, a specific signature composed of 5 UCGs (uc.269A, uc.160, uc.215, uc.346A, and uc.348) differentiates between CLLs with poor (high levels of ZAP-70 expression) and good (low levels of ZAP-70 expression) prognosis. We reported a negative correlation between these 5 UCGs and a previously reported miRNA expression signature in CLL cases.21 Consistently, complementary matching sites for miRNAs in these UCG sequences were found; in particular, miR-155 (the expression of which is frequently up-regulated in several human malignancies, including CLL),53 directly targets uc.346A and uc.160, and miR-24-1 directly targets uc.160 (Figure 1).6 This interaction between miRNAs and UCGs adds a further level of complexity to the regulation of ncRNA expression and may have biologic and clinical significance for patients with CLL.
A shift in the cancer-predisposition paradigm: germline mutations in miRNAs
Despite decades of research, the molecular basis for the majority of familial cancers is unknown. CLL is one of the primary examples of these cancers, as a consistent proportion (10%-20%) of patients have a family history of CLL or other hematologic or solid cancers, whereas researchers have not found clear culprit(s) by scanning for alterations in PCGs.72 A multitude of scientific groups have studied CLL predisposition over the past 3 decades, but the genetic basis for the majority of familial cases as well as the hereditary contribution to CLL remain largely unknown. To date, no studies have identified common predisposing loci for CLL, although recent linkage and association studies are providing new leads for susceptibility loci.73 MiRNAs are ideal cancer-predisposing gene candidates, and abnormal expression of miRNAs because of sequence variations may represent a new form of cancer predisposition. Because each miRNA has numerous targets, inherited minor variations in miRNA expression may have important consequences for the expression of various protein-coding oncogenes and tumor suppressors involved in malignant transformation. Accumulation of additional somatic abnormalities in PCGs or ncRNAs, including miRNAs, is necessary for the full development of the malignant phenotype (Figure 2).74,75 The first report of sequence variations in miRNAs in cancer cases identified a C-to-T homozygous substitution in the pri-miR-16-1 located 7 nt's in the 3′ direction after the end of the pre-miRNA in 2 patients with CLL, one of whom had a family history of CLL and breast cancer. This substitution was associated with reduced production of both mature miR-15a and miR-16-1, revealing a functional impact on the processing of this miRNA.21 Globally, germline and somatic mutations in miRNA were identified in approximately 10% of patients with CLL.21 Although these mutations are rare,76 researchers observed a similar mutation in the New Zealand black (NZB) strain of mice susceptible to the development of CLL late in life; specifically, they identified a mutation 6-nt downstream from the murine miR-16 gene.25 Levels of miR-16 expression were decreased in NZB lymphoid tissue, and functionally, exogenous miR-16 delivered to an NZB malignant B-1 cell line resulted in cell-cycle alterations and increased apoptosis.25 Taken together, these 2 studies—1 of human CLL and the other of a murine model of human indolent CLL—indicate that miR-16 is the first miRNA proven to be involved in CLL predisposition and cancer predisposition in general.
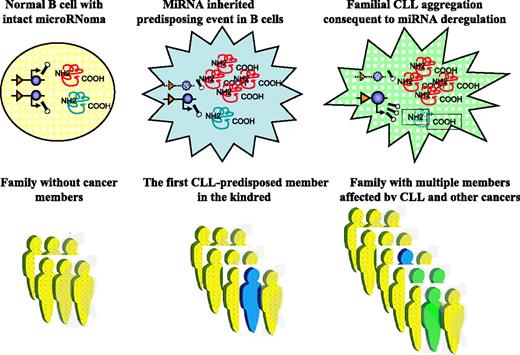
MiRNA involvement in CLL predisposition. MiRNA alterations can predispose people to CLL development. This proposed model shows structural and/or expression abnormalities of miRNAs in the germline that may represent inherited predisposing events. For simplicity, only gene deletion is shown, but all other types of loss-of-function and gain-of-function mutations described for PCGs can be involved in miRNA disruption (blue color). For CLL to develop, a second genetic event in addition to the predisposing one must occur in a somatic cell (green color). This can be a PCG alteration or a “hit” in another miRNA (presented here as amplification of expression). The consequences of these abnormalities are reflected by the levels of expression of various target mRNAs: overexpression of target oncogenes in the case of miRNA deletion and down-regulation of expression of target TSGs in the case of miRNA amplification.
MiRNA involvement in CLL predisposition. MiRNA alterations can predispose people to CLL development. This proposed model shows structural and/or expression abnormalities of miRNAs in the germline that may represent inherited predisposing events. For simplicity, only gene deletion is shown, but all other types of loss-of-function and gain-of-function mutations described for PCGs can be involved in miRNA disruption (blue color). For CLL to develop, a second genetic event in addition to the predisposing one must occur in a somatic cell (green color). This can be a PCG alteration or a “hit” in another miRNA (presented here as amplification of expression). The consequences of these abnormalities are reflected by the levels of expression of various target mRNAs: overexpression of target oncogenes in the case of miRNA deletion and down-regulation of expression of target TSGs in the case of miRNA amplification.
Because the thermodynamics of RNA-RNA binding plays essential roles in miRNA interaction with the target mRNA, it follows that sequence variations influencing this interaction will be identified in CLL cases. Recently, investigators identified a new single nucleotide polymorphism (SNP) in the let-7 complementarity site77 on the v-ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) 3′UTR (LCS6).78 Functionally, this SNP induces KRAS overexpression in vitro and is associated with a significantly increased risk of non–small cell lung cancer in moderate smokers. Moreover, in patients harboring this SNP, let-7 expression levels were lower in lung tumors than in nonvariant allele tumors, suggesting that this SNP is associated with non–small cell lung cancer with severe clinical outcomes.78
Using miRNAs as CLL markers
Researchers have defined several factors predicting the clinical course of CLL, and a lack of somatic mutations of IgVH and high-level expression of ZAP-70 or β2-microglobulin are associated with an aggressive clinical course of CLL.2,3 Studies have also found that genomic aberrations in CLL are important independent predictors of progression and survival: patients with 17p− and 11q− have more aggressive disease than do patients without these deletions, whereas patients with 13q− and normal cytogenetics have indolent clinical disease courses.79 However, the molecular basis for these correlations was largely unknown until recent evidence clearly indicated that these regions contain miRNA loci and that, in addition to being causal in the pathogenesis of CLL, the levels of expression of miRNAs are predictors of clinical behavior.
MiRNAs as diagnostic and prognostic markers for CLL
The miRNA profiling in patients with CLL identified a unique miRNA signature that was differentially expressed in patients with various IgVH and ZAP-70 kinase statuses.21 In that study, 94 samples of blood obtained from patients with CLL for which the level of expression of ZAP-70 and the mutated/unmutated IgVH status were known identified a unique signature of 13 miRNAs (Table 2). This signature includes some of the most frequently deregulated miRNAs in different types of hematologic malignancies (such as miR-15/miR-16, the miR-29 family, miR-155, and miR-221) and has important prognostic implications, as the levels of expression of the members of the signature correlate with prognostic factors (ZAP-70 expression and IgVH mutations). This study was followed by 2 others that generated largely nonoverlapping miRNA signatures compared with those in the initial study.60,80 The disparity in these results probably reflected the different methodologies used to generate the miRNA profiles in each case and the use of different sample storage methods. Nevertheless, each of these 3 studies consistently found that expression of miR-223 and members of the miR-29 family was down-regulated in CLL cases and associated with selected prognostic factors for aggressive disease (Table 2). This systematic evaluation of the expression of miR-223 and miR-29c in relation to all clinically relevant prognostic factors for CLL found that the expression of these miRNAs was decreased in patients who had a poor prognosis and that low miR-223 and miR-29c expression was associated with inferior treatment-free survival as well as reduced overall survival durations. In addition, investigators developed a quantitative method of simultaneously determining the levels of expression of miR-223, miR-29c, ZAP-70, and lipoprotein lipase in samples by devising a polymerase chain reaction score based on the expression of none to all of these 4 adverse prognostic markers within each sample.81
MiRNA profiling studies of CLL
| MiRNA signatures . | Prognostic marker . | Study . | Year . |
|---|---|---|---|
| miR-15a, miR-195, miR-221, miR-23b, miR-155, miR-223, miR-29a-2, miR-24-1, miR-29b-2, miR-146, miR-16-1, miR-16-2, and miR-29c | Unmutated IgVH, high ZAP-70 | Calin et al21 | 2005 |
| miR-29c and miR-223 | Unmutated IgVH | Fulci et al80 | 2007 |
| miR-181a, let-7a, miR-30d, miR-155, and miR-29 | Unmutated IgVH | Marton et al60 | 2008 |
| miR-223 and miR-29c | ZAP-70 and LPL | Stamatopoulos et al81 | 2009 |
| MiRNA signatures . | Prognostic marker . | Study . | Year . |
|---|---|---|---|
| miR-15a, miR-195, miR-221, miR-23b, miR-155, miR-223, miR-29a-2, miR-24-1, miR-29b-2, miR-146, miR-16-1, miR-16-2, and miR-29c | Unmutated IgVH, high ZAP-70 | Calin et al21 | 2005 |
| miR-29c and miR-223 | Unmutated IgVH | Fulci et al80 | 2007 |
| miR-181a, let-7a, miR-30d, miR-155, and miR-29 | Unmutated IgVH | Marton et al60 | 2008 |
| miR-223 and miR-29c | ZAP-70 and LPL | Stamatopoulos et al81 | 2009 |
LPL indicates lipoprotein lipase.
Expanding the significance of miR-29 family in human cancers, Fabbri et al showed that these genes target the de novo DNA methyltransferases (DNMTs) and can reactivate silenced tumor-suppressor genes.82 Thus, loss of miR-29 family members may play a role in causing epigenetic changes associated with cancer in general and CLL also. More recently, Garzon et al has shown loss of miR-29b in acute myelogenous leukemia and that miR-29b can also regulate indirectly the expression of the maintenance DNMT (DNMT1). Introduction of miR-29b in acute myelogenous leukemia cells resulted in the reactivation of p16 tumor-suppressor gene, in growth arrest, and in apoptosis.83 Thus dysregulation of miRNA expression can contribute to the epigenetic changes observed in CLL too and may provide alternative ways for patient stratification and therapeutic intervention. Several other miRNAs may be tested as markers for CLL in the near future. Underexpression of miR-181a, let-7a, and miR-30d and overexpression of miR-155 are characteristics of patients with CLL with different clinical outcomes,60 whereas miRNA analysis of patients with CLL revealed in bone marrow and lymphoid tissues low miR-150 and high miR-155 expression levels.84
MiRNA-sensing chemotherapy resistance
The oncosuppressor gene P53 maps to 17p in the region deleted in patients with CLL, and researchers have found p53 mutations in approximately 10% of CLL cases, indicating a possible role for P53 in CLL pathogenesis.85 P53 deletion identifies patients with CLL that is resistant to chemotherapy. Recently, investigators observed a link in cytogenetically well-defined CLL samples between the levels of expression of miR-34a, a member of an miRNA family positively regulated by P53, and response to DNA damage, P53 status, and significantly, response to fludarabine-based treatment.86 Low miR-34a expression levels were statistically significantly associated with impaired DNA damage response, P53 mutations, and fludarabine-refractory CLL either with or without P53 deletion. Up-regulation of miR-34a expression after irradiation was associated with induction of Bax and p21, but not Puma expression. These are straightforward findings providing a new piece to the recently identified puzzle of miRNA involvement in drug resistance and sensitivity in patients with CLL. An 829C>T polymorphism in the dihydrofolate reductase binding site for miR-24 led to loss of function and resulted in dihydrofolate reductase overexpression and methotrexate resistance in cancer cells.87 Furthermore, a study showed that by regulating BCL2 expression, miR-15b and miR-16 may modulate the sensitivity of cancer cells to some anticancer drugs.88 Determining whether these genes are also involved in chemotherapy resistance in patients with CLL would be interesting.
MiRNAs and RNA-inhibition therapy for CLL: new hope
One of the most exciting avenues of research related to miRNAs is their possible use in therapy for CLL. Overall survival durations in patients with CLL vary widely. Whereas some patients may experience rapid progression and/or need therapy, others may enjoy a very long period without any leukemia-associated symptoms or complications. In the future, patient-specific therapeutic drugs may be designed for people with CLL harboring abnormalities in miRNA expression in their malignant cells. MiRNAs are potential targets for therapy as well, as knocking down overexpression of miRNAs and inducing expression of silenced miRNAs in cancer cells may contribute to selective tumor killing (Table 3). Loss of miRNA expression in patients with CLL may selectively suppress proapoptotic pathways, providing such malignancies with a survival advantage. For example, chemotherapeutic drugs that activate miR-106b may initiate a p53-independent mechanism that targets CLL cells,50 whereas restoration of miR-34a expression can overcome P53-dependent resistance to chemotherapy.86 One significant question is why miRNA-related therapy could be more efficient than previously tested RNA inhibition therapies such as the use of antisense oligonucleotides, ribozymes, or siRNAs. Differently from all these agents that are working on a one-to-one basis with their targets, the microRNAs are targeting several members of pathways important for a disease. For example, we proved recently that the cluster miR-15a/miR-16-1 is targeting not only BCL2 but and also MCL1, both important antiapoptotic oncogenes, as well as other significant cancer-related genes, such as Jun, MSH2, or WT1 (Wilms tumor 1).48
MiRNA-targeted CLL therapies
| Therapy . | Comments . |
|---|---|
| Mimic miRNA | A mimic miRNA represents a partially double-stranded RNA that mimics endogenous precursor miRNA and is processed to form the active miRNA molecule that targets specific mRNAs. |
| Antagomir | An antagomir is a single-stranded 23-nt RNA molecule complementary to the targeted miRNA that has been modified to increase the stability of the RNA and protect it against degradation by a partial phosphorothioate backbone in addition to 2′-O-methoxyethyl. miRNA/antagomir duplexes induce degradation of the miRNA and recycling of the antagomir.89 |
| Anti-miRNA oligonucleotide | An anti-miRNA oligonucleotide is a single-stranded, chemically modified (usually with a 2′-O-methyl group) 17- to 22-nt nucleic acid molecule that is directed against a specific miRNA. It functions as an ASO via Watson-Crick binding pairs with the miRNA of interest.90 |
| Locked nucleic acid (LNA) | A LNA is an ASO modified using LNA technology, and LNA anti-miRNAs have the same mechanism of action that ASOs/anti-miRNA oligonucleotides do but with the advantages of increased stability and strength of target binding.91 |
| Therapy . | Comments . |
|---|---|
| Mimic miRNA | A mimic miRNA represents a partially double-stranded RNA that mimics endogenous precursor miRNA and is processed to form the active miRNA molecule that targets specific mRNAs. |
| Antagomir | An antagomir is a single-stranded 23-nt RNA molecule complementary to the targeted miRNA that has been modified to increase the stability of the RNA and protect it against degradation by a partial phosphorothioate backbone in addition to 2′-O-methoxyethyl. miRNA/antagomir duplexes induce degradation of the miRNA and recycling of the antagomir.89 |
| Anti-miRNA oligonucleotide | An anti-miRNA oligonucleotide is a single-stranded, chemically modified (usually with a 2′-O-methyl group) 17- to 22-nt nucleic acid molecule that is directed against a specific miRNA. It functions as an ASO via Watson-Crick binding pairs with the miRNA of interest.90 |
| Locked nucleic acid (LNA) | A LNA is an ASO modified using LNA technology, and LNA anti-miRNAs have the same mechanism of action that ASOs/anti-miRNA oligonucleotides do but with the advantages of increased stability and strength of target binding.91 |
RNA inhibition can be used to treat CLL in different ways: directly targeting miRNAs that participate in CLL initiation and progression and using miRNA molecules as therapeutic agents against mRNA in genes involved in CLL. The miRNAs and anti-miRNA agents are still in preclinical studies, and in vitro toxicity studies are underway.
ASO indicates antisense oligonucleotide.
Two strategies for inhibition of RNA expression to treat CLL can be implemented. First, “the sandwich RNA inhibition strategy” focuses on a major molecular alteration clearly linked with CLL pathogenesis via the use of multiple agents. Given recently published studies showing the relative efficacy of oblimersen sodium in treating relapsed or refractory CLL,92 designing regimens using a cocktail of anti-BCL2 ASOs and miRNAs targeting BCL2, such as miR-15 and miR-16, for indolent CLL would be feasible. Second, “the multiplex RNA-inhibition strategy” targets various molecular defects in the same pathway, such as apoptosis. With this strategy, multiple synthetic miRNAs targeting the overexpressed apoptosis regulators BCL2 (miR-15 and miR-16) and MCL1 (the miR-29 family) may have a better chance of consistently and robustly reducing these proteins' expression levels than single-agent therapy. The potential use of miRNAs and/or their antisense inhibitors in cancer treatment has only recently been envisioned, and clinical trials of their use in this manner certainly will be scheduled soon.
Conclusions and future directions
MiRNAs and possibly other ncRNA alterations are involved in the initiation and progression of CLL. The causes of the more widespread differential expression of miRNA genes in malignant B cells versus their normal counterpart can be explained by the locations of these genes in cancer-associated genomic regions, epigenetic mechanisms, and alterations in the miRNA processing machinery (for review see Calin and Croce15 ). MiRNA-expression profiling of human CLLs has identified signatures associated with diagnosis, staging, progression, prognosis, and response to treatment. In addition, this profiling has been used to identify miRNA genes that may be downstream targets of activated oncogenic pathways or to target PCGs involved in cancer. Although they may lead to a new understanding of CLL in particular and cancer pathogenesis in general, explaining the involvement of miRNAs and ncRNAs in CLL and using this knowledge for the patient's benefit are still challenging (Table 4), but certainly, the time for the “ncRNA revolution” in CLL is here.
Noncoding RNAs and CLL: challenges for future investigations
| Challenge . | Comments . |
|---|---|
| Does CLL develop in knockout mouse models of miR-15/miR-16, and is it indolent or aggressive? | If yes, this will be the ultimate confirmation that this cluster is causally involved in the initiation of CLL. Presumably the miR-15a/miR-16-1 knockout will develop the indolent form of CLL like the NZB mice. The presence of various long ncRNAs in the same deleted chromosomal region as well as an independent, highly similar cluster on chromosome 3 that is rarely deleted in patients with CLL might be a challenge to this in vivo approach. In addition, does aggressive CLL develop in other mouse models, such as knockout models for the miR-29 or miR-181 family? |
| Does CLL predisposition result from sequence variations in miRNA interactor sites? | The high number of germline DNA sequence variations linked with low levels of predisposition to CLL offer the basis for this investigation. |
| MiRNAs as plasma/serum markers for diagnosis, prognosis, and response to therapy in patients with CLL. | Although technically feasible, such studies would involve thousands of patients and, more important, equal numbers of controls. The levels of expression of various miRNAs in the normal population are largely unknown. For example, are the miRNA expression levels in serum and/or plasma in a 20-year-old white man the same as those in a 60-year-old African American woman? |
| Should miR-15/miR-16 expression levels be increased in patients with aggressive CLL? | Most aggressive CLLs are derived from indolent cases. Thus it is possible that loss of miR-15a/miR-16-1 cooperates with TCL1 overexpression in causing or contributing to the aggressive form of CLL. Thus it seems possible that introduction of miR-15/miR-16 into aggressive CLLs will be beneficial. |
| Because studies have already found that networks of interacting ncRNAs function in CLL pathogenesis, should we try to identify the ncRNAs involved in response of CLL to therapy? | The possibility that aberrations in these ncRNAs together with aberrations in PCGs can explain the failure of current chemotherapy regimens to cure CLL should be considered. |
| Challenge . | Comments . |
|---|---|
| Does CLL develop in knockout mouse models of miR-15/miR-16, and is it indolent or aggressive? | If yes, this will be the ultimate confirmation that this cluster is causally involved in the initiation of CLL. Presumably the miR-15a/miR-16-1 knockout will develop the indolent form of CLL like the NZB mice. The presence of various long ncRNAs in the same deleted chromosomal region as well as an independent, highly similar cluster on chromosome 3 that is rarely deleted in patients with CLL might be a challenge to this in vivo approach. In addition, does aggressive CLL develop in other mouse models, such as knockout models for the miR-29 or miR-181 family? |
| Does CLL predisposition result from sequence variations in miRNA interactor sites? | The high number of germline DNA sequence variations linked with low levels of predisposition to CLL offer the basis for this investigation. |
| MiRNAs as plasma/serum markers for diagnosis, prognosis, and response to therapy in patients with CLL. | Although technically feasible, such studies would involve thousands of patients and, more important, equal numbers of controls. The levels of expression of various miRNAs in the normal population are largely unknown. For example, are the miRNA expression levels in serum and/or plasma in a 20-year-old white man the same as those in a 60-year-old African American woman? |
| Should miR-15/miR-16 expression levels be increased in patients with aggressive CLL? | Most aggressive CLLs are derived from indolent cases. Thus it is possible that loss of miR-15a/miR-16-1 cooperates with TCL1 overexpression in causing or contributing to the aggressive form of CLL. Thus it seems possible that introduction of miR-15/miR-16 into aggressive CLLs will be beneficial. |
| Because studies have already found that networks of interacting ncRNAs function in CLL pathogenesis, should we try to identify the ncRNAs involved in response of CLL to therapy? | The possibility that aberrations in these ncRNAs together with aberrations in PCGs can explain the failure of current chemotherapy regimens to cure CLL should be considered. |
Acknowledgments
We thank Milena Nicoloso for critical reading of this review and Don Norwood for expert editorial assistance. We apologize to the many colleagues whose work we did not cite because of space limitations.
C.M.C. is supported by program project grants from the National Cancer Institute, and G.A.C. is supported by the National Cancer Institute, the CLL Global Research Foundation, as a Fellow at The University of Texas M. D. Anderson Research Trust, as a Fellow of The University of Texas System Regents Research Scholar program, and by the Ladjevardian Regents Research Scholar Fund.
National Institutes of Health
Authorship
Contribution: G.A.C and C.M.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlo M. Croce, Human Cancer Genetics Program and Department of Molecular Virology, Immunology and Medical Genetics, The Ohio State University, 460 W 12th Ave, Rm 1082, Columbus, OH 43210; e-mail: carlo.croce@osumc.edu.