Abstract
Abstract 513
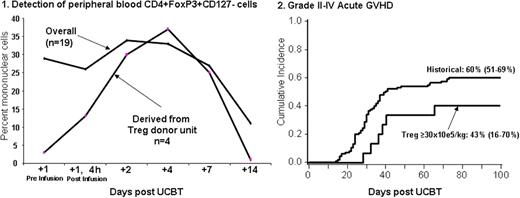
UCB transplantation (UCBT) is associated with greater risk of graft rejection and, in particular in double UCBT, with graft vs. host disease (GVHD). Our group has shown in mouse models that ex vivo activated and expanded Tregs improve engraftment and reduce and treat GVHD. We here report on the first clinical trial to study the safety of the infusion of UCB Tregs in patients receiving a nonmyeloablative double UCB transplant. We enrolled 19 patients (pts) (age 25-65 yrs; weight 52-133; male 9; CMV seropositive 6) with high risk or advanced leukemia (n=12) and lymphoma/CLL (n=7). All patients received the same nonmyeloablative conditioning consisting of cyclophosphamide 50mg/kg/×1day, fludarabine 40 mg/m2/×5d, and total body irradiation 200 cGy/×1d; immunosuppression was cyclosporine A/mycophenolate mofetil (MMF) in the first 17 and sirolimus (Siro)/MMF in the last 2 pts. Tregs were obtained from a 3rd UCB unit and after CD25+ selection (Miltenyi CliniMACS) were activated and expanded in the presence of anti-CD3/CD28 mAb coated beads and IL-2 for 18±1 days. Treg dose escalation levels were 1, 3, 10, 30 × 105/kg on day +1, and 30 × 105/kg on days +1 and +15 after UCBT. After CD25+ selection the median % CD4+/CD25+ was 62% (range , 11-87%) and following culture was 86% (r, 62-97%). Median fold expansion was 198 (r, 13-1169); median mixed lymphocyte reaction suppression at a 1:4 ratio post-culture was 86% (39-95%). All products achieved lot release. Pts were monitored for 48h post-infusion and no dose limiting or serious adverse event was observed. After infusion an increase in the proportion of peripheral blood comprised of CD4+FoxP3+CD127- cells was observed. Donor Tregs were clearly detected in all pts receiving Tregs that were HLA disparate with the pt and graft donor units (Figure 1). Mixed day 21 donor graft UCB chimerism was observed in 59% (10/19) of pts vs. 35% (38/107) in historical controls. The cumulative incidence of grades II-IV acute GVHD was 47% (95%CI, 24-71) and III-IV 16% (95%CI, 0-33). In pts receiving ≥ 30 × 105/kg (n=14), grades II-IV acute GVHD was 43% (95%CI, 16-70) and III-IV 7% (95%CI, 0-21). Compared to historical controls receiving the same conditioning regimen, the median time to the development of acute GVHD was longer (Figures 2), although not statistically significant. Neutrophil recovery was 95% (95%CI, 79-100) at median of 8.5 days (r, 3-36), platelet recovery >50,000/mL was achieved in 74% (95%CI, 53-95) at a median 43 days (r, 27-57), cumulative incidence of opportunistic infections (fungal + viral) at 100 days was 32% (95%CI, 11-53), treatment related mortality at 100 days was 11% (95%CI, 0-25), and disease free-survival was 40% (95%CI, 15-65%). None differed from historical controls. Based on our results, the co-infusion of ex vivo expanded and activated UCB-derived Treg to recipients of nonmyeloablative UCBT 1) is safe at the tested dose levels, 2) leads to detectable increased in Treg-donor unit derived circulating CD4+FoxP3+CD127- cells, and 3) results in an increased the proportion of mixed chimerism at day +21. Identification of the Treg MTD in the context of Siro/MMF immunosuppression will set the stage for the planned definitive studies that will establish the true impact of Tregs on engraftment and GVHD and for future studies in the treatment of autoimmune disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal