Abstract
Abstract 4506
Gene transfer into malignant leukemia cells can be of relevance to overcome conventional therapy-resistance. Either suicide or immune stimulating gene therapy vectors may be a tool for second line treatment of imatinib-resistant chronic myelogenous leukemia (CML). Unfortunately, for gene transfer into CML cells, most current vector systems either lack sufficient transduction efficiency or an acceptable safety profile. Conventional adeno-associated virus (AAV) based vectors have an advantageous safety profile, yet lack the required efficiency.
Pseudotyped recombinant adeno-associated viruses of the serotypes 2/1 to 2/6 (rAAV2/1 to rAAV2/6) were screened on a panel of human CML cell lines and primary CML cells to determine their gene transfer efficacy. Additionally, double-stranded self complementary rAAV (scAAV) were used to determine possible second strand synthesis limitations.
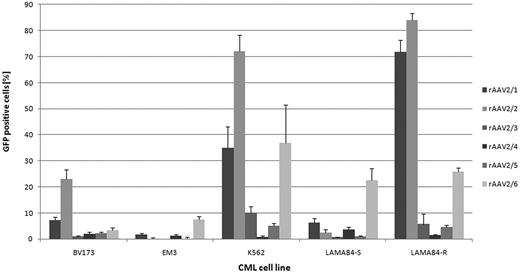
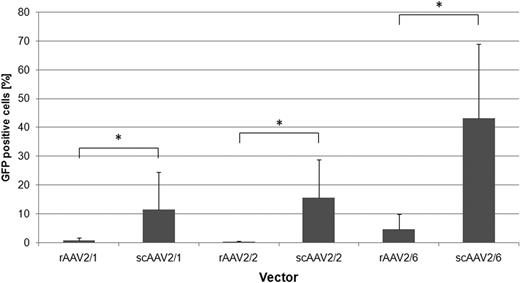
On CML cell lines, generally rAAV2/2 and rAAV2/6 were most efficient (Fig. 1). For both, an interesting difference in transduction efficiency between the imatinib-resistant LAMA84-R and imatinib-sensitive LAMA84-S cells were observed. On primary human CML cells, rAAV2/6 proved to be significantly more efficient than the other tested vectors (4.6% ± 5.3% GFP-positive cells, p=0.011). Additionally, the transduction efficiency could be significantly increased by using scAAV vectors (scAAV2/6: 43.1% ± 25.9% GFP-positive cells, p<0.001 vs rAAV2/6; Fig. 2). Our data suggest that both the conversion of the single stranded AAV genome to double stranded DNA, as well as cell binding/entry are rate limiting steps in efficient transduction of primary human CML cells with AAV vectors. Furthermore, donor-dependent differences in gene transfer efficiency were observed. Of note, data from experiments on CML cell lines seems to provide limited information about the transduction efficiency of rAAV vectors on primary CML cells.
Overall, pseudotyped rAAV and scAAV vectors offer efficient clinically relevant gene transfer into human CML cells with a potential for future clinical application.
Gene transfer efficiency of pseudotyped rAAV vectors on human CML cell lines. A multiplicity of infection of 100 was used (n=3).
Gene transfer efficiency of pseudotyped rAAV vectors on human CML cell lines. A multiplicity of infection of 100 was used (n=3).
Comparison of gene transfer efficiency between scAAV vectors with their single-stranded counterpart on primary human CML cells. At a multiplicity of infection of 100, all tested scAAV vectors were significantly more efficient compared to their single-stranded counterparts (n≥3). *p<0.05
Comparison of gene transfer efficiency between scAAV vectors with their single-stranded counterpart on primary human CML cells. At a multiplicity of infection of 100, all tested scAAV vectors were significantly more efficient compared to their single-stranded counterparts (n≥3). *p<0.05
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal