Abstract
Abstract 3676
Poster Board III-612
Regulatory T-cells (Tregs) mediate self-tolerance and moderate the immune response to various antigens. Their frequency and suppressive function are reduced in various autoimmune diseases like type 1 diabetes mellitus, systemic lupus, and rheumatoid arthritis, but may be excessive in certain malignancies. The human T-cell lymphotropic virus type 1 (HTLV-1) is a retrovirus with myriad clinical manifestations, most notably adult T-cell leukemia/lymphoma (ATLL) and HTLV-1 associated myelopathy/ tropical spastic paraparesis (HAM/TSP). We have previously described the decreased frequency and function of HTLV-1 specific CD8+ T-cells in ATLL patients compared to asymptomatic carriers (ACs) and healthy controls (HCs). We have also reported the relative exhaustion of HTLV-1 specific cytotoxic T-lymphocytes (CTLs) compared to cytomegalovirus (CMV)-specific CTLs in ACs, as demonstrated by upregulation of the co-inhibitory molecule, programmed death 1 (PD-1). Recent evidence supports the existence of CD8+ Tregs in humans, defined primarily by forkhead box P3 (FOXP3) transcription factor expression. Although a classic Treg phenotypic marker, FOXP3's aberrant expression makes it a less reliable marker in the setting of ATLL. Low expression or absence of interleukin 7 receptor (IL-7R) (CD127lo/-) on CD25+ T-cells has provided a useful alternative Treg phenotype, allowing preservation of cell viability for functional assays. Existing studies have focused mostly on CD4+ Tregs and a few on CD8+ Tregs in malignancy, but little is known about their involvement in the persistence of chronic viral infections like HTLV-1. In chronic HTLV-1 infection, FOXP3+CD25+ and CD127lo/-CD25+ Tregs comprise 8.3±4.6% and 8.1±4.3% of overall CD8+ T-cells, respectively (n=14). Expression of the FOXP3+CD25+ phenotype in CD8+ T-cells had a tendency towards correlation with the CD127lo/-CD25+ phenotype (r=0.561, p=0.073, n=11). Among ACs, we observed the CD127lo/-CD25+ Treg phenotype in HTLV-1 Tax-specific CD8+ T-cells (45±16.5%, n=8), which was significantly greater than CMV-specific CD8+ Tregs (25±15.8%, n=6) (p<0.05, Figure 1), as characterized by phycoerythrin-labelled HLA-A*2402 Tax301-309 or HLA-A*0201 Tax11-19 and HLA-A*24/ HLA-A*02 CMV tetramers respectively. We have demonstrated that the CD8+CD127lo/-CD25+ phenotype could be an alternative marker of CD8+ Tregs. These observations suggest that HTLV-1 specific CD8+ Tregs could be implicated in the impaired clearance of HTLV-1 infected and transformed cells. This surrogate marker may facilitate further exploration of the immunological significance of antigen specific CD8+ Tregs in chronic viral infection.
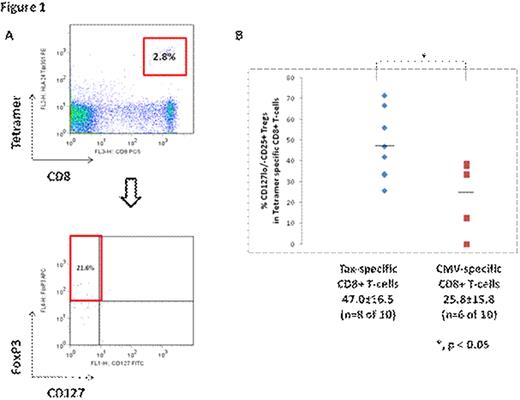
Increased frequency of HTLV-1 tetramer-specific CD8+CD127-/loCD25+ regulatory T-cells. A) Human PBMCs were gated on lymphocytes based on forward and side light scatter, and phycoerythrin-labelled HLA-A*2402 Tax301-309 or HLA-A*0201 Tax11-19 and HLA-A*24 CMV or HLA-A*02 CMV PP65 tetramer (Medical and Biological Laboratorie, Japan) specific CD8+ T-cells analyzed for intracellular FOXP3 and/or surface staining for CD25, CD8, GITR, and CD127 or appropriate isotype controls. The above representative data is shown after subtracting immunoglobulin isotype control background. B) Proportion of cells expressing the regulatory T-cell phenotype CD127-/lo CD25+ was higher for HTLV-1 Tax-specific CD8+ T-cells compared to CMV-specific T-cells (p<0.05) among asymptomatic carriers. Horizontal black bars represent means for the respective tetramer specific cell groups.
Increased frequency of HTLV-1 tetramer-specific CD8+CD127-/loCD25+ regulatory T-cells. A) Human PBMCs were gated on lymphocytes based on forward and side light scatter, and phycoerythrin-labelled HLA-A*2402 Tax301-309 or HLA-A*0201 Tax11-19 and HLA-A*24 CMV or HLA-A*02 CMV PP65 tetramer (Medical and Biological Laboratorie, Japan) specific CD8+ T-cells analyzed for intracellular FOXP3 and/or surface staining for CD25, CD8, GITR, and CD127 or appropriate isotype controls. The above representative data is shown after subtracting immunoglobulin isotype control background. B) Proportion of cells expressing the regulatory T-cell phenotype CD127-/lo CD25+ was higher for HTLV-1 Tax-specific CD8+ T-cells compared to CMV-specific T-cells (p<0.05) among asymptomatic carriers. Horizontal black bars represent means for the respective tetramer specific cell groups.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal