Abstract
Abstract 304
Carfilzomib (CFZ) is a highly specific and irreversible proteasome inhibitor that has demonstrated promising single-agent activity in relapsed/refractory MM and has a favorable side-effect profile with minimal myelosuppression (Vij et.al., ASCO # 8537, 2009). The purpose of this study is to evaluate the safety and activity of CFZ in combination with lenalidomide (LEN) and low dose dexamethasone (loDex).
This phase Ib trial is evaluating 6 dose levels (≥3 subjects each) to define the maximum tolerated dose (MTD) of CRd in relapsed or refractory MM patients. An additional 30 patients will be enrolled at the highest dose level tolerated. Patients who received 1–3 prior therapies, including prior LEN and/or prior bortezomib (BTZ), were eligible for the study. CFZ was administered intravenously (IV) at 15–27 mg/m2 (days 1, 2, 8, 9, 15, 16), LEN at 10–25 mg po (days 1–21), and loDex at 40 mg po (days 1, 8, 15, 22) in 4-week cycles (C). LoDex was reduced to 40 mg once a month starting with cycle 5. The overall response rate (ORR) (≥Partial Remission) was assessed by the IMWG Criteria, with a secondary assessment of Clinical Benefit Response (CBR: ≥ Minimal Response (MR)) using EBMT Criteria for a MR.
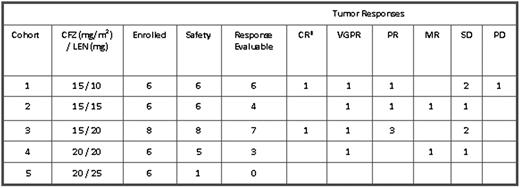
As of 31 July 2009, 32 subjects have been enrolled in the first 5 cohorts: 26 subjects were evaluable for safety and 20 for response (see Table). Prior therapies (median 2.5) in 26 evaluable subjects included steroids (89%), BTZ (77%), alkylating agents (85%), LEN (54%), thalidomide (46%), anthracyclines (31%) and stem cell transplant (81%). Fifty-four percent (14/26) of subjects had received both LEN and BTZ while 50% of patients were refractory or progressed on one or more prior therapies; 73% of all patients had a history of neuropathy, with two-thirds of the cases related to prior bortezomib or immunomodulatory agents (IMiDs). There have been no dose limiting toxicities in the first 5 dose cohorts. No drug-related serious adverse events (SAEs), peripheral neuropathy or renal events were reported. Hematological AEs have been reversible and manageable including Grade (G) ≥3 thrombocytopenia (4/26), anemia (4/26) and neutropenia (2/26). All but 1 of these events was G3; 1 subject had G4 thrombocytopenia lasting less than 7 days. One subject in Cohort 3 had treatment discontinued due to a non-drug-related infection on day 2 of cycle 1. The current ORR is 55% across all cohorts and the CBR is 65%, (see Table). Initial responses were rapid and occurred within the first 2 cycles, and improved with continuing therapy (≥ 3 months). The median number of days on therapy is currently 183 (range 78-367). Five out of 6 subjects have progressed in cohort 1 but 22 out of 26 subjects enrolled in cohorts 2-5 are still on study.
An active dose of CFZ (20mg/m2) in combination with full dose LEN (25 mg) and low-dose dexamethasone (CRd) has been established as a tolerated dose in relapsed MM patients. Dose escalation of CFZ is ongoing in cohort 6 (CFZ 20/27 mg/m2 and LEN 25 mg). The lack of overlapping toxicity of CFZ and LEN allows the use of these agents at full doses and for extended durations >10 months in several cases, despite relapsed or refractory disease. Moreover, prior therapy with BTZ or IMiDs does not preclude a good response to CRd. Based on these data, a Phase 3 trial of CRd vs Rd is planned in relapsed MM in the first half of 2010.
Niesvizky:Millenium: Research Funding, Speakers Bureau; Celgene: Research Funding, Speakers Bureau; Seattle Genetics, Inc: Research Funding; Proteolix: Research Funding, data monitoring committee. Wang:Proteolix, Inc.: Research Funding. Bensinger:Celgene: Speakers Bureau. Gutierrez:Proteolix, Inc.: Employment. Kunkel:Proteolix: Consultancy, Employment. Kauffman:Proteolix, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal