Abstract
Abstract 3031
Poster Board II-1007
Immunotherapy is a promising therapy for lymphoproliferative malignancies including multiple myeloma (MM). While many phase I and II clinical trials has been conducted using dendritic cells (DC) pulsed with cancer-specific antigen as immunotherapy, none of these studies have shown a significant clinical impact on treatment of patients with cancer, including in MM. The failure of DC-based vaccine in MM has been associated with impaired DC function due to the immunosuppressive environment caused by the cancer cells. This effect may be in part mediated through hyper-activation of STAT3. Using MM as a model, we investigated the effect of MM-derived factors on the function of DC and evaluated the effect on STAT3 hyper-activation in the latter.
We generated DC from monocytes of normal donors using IL-4 and GM-CSF for 48 hours, following maturation using IL-1β, TNF-αa, PGE2 for another 24 hours. MM-derived factors (MM-DF) were obtained by co-culture of the human MM cell line U266 cells with in-vitro generated osteoclasts for 48 hours, and the supernatant was filtered. The filtered supernatant containing MM-DF was used in a ratio of 1:1 with fresh RPMI-1640 in the generation of DC in-vitro. The phenotype of immature and maturated DC was evaluated by flow-cytometry for surface staining of CD40, CD80, CD83, CD86, ILT3, and MHC-II. Lysates of DC were prepared for Western blot to evaluate for phospo (p)-STAT3 and ILT3 expression. The ability of generated DC (in the presence or absence of MM-DF) to stimulate T cells was assessed using 3H-thymidine incorporation.
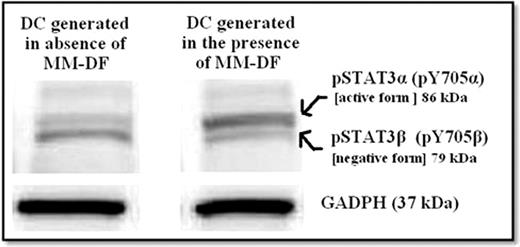
MM-DF did not affect the generation of DC as assessed by CD86 and MHC-II expression, but significantly impaired the maturation of DC with failure of up-regulation of CD40 (64% ±19.3% of control; P=0.075), CD80 (41% ±20.5% of control; P=0.019), and CD83 (35% ±18.0% of control; P=0.0077) in 3 independent experiments. In addition, the surface expression of ILT3 on the generated DC was significantly increased in DC generated in presence of MM-DF (155% increase in mean fluorescence intensity compare to control), furthermore, western blot of DC lysates showed 2.5 fold-increase in the expression of ILT3 in DC generated in presence of MM-DF compare to control. The failure of maturation of DC with higher expression of ILT3 indicates the generation of tolerogenic DC (Transpl Immunol 2003;11:245). The expression of phoso-STAT3αa (pY705αa or the active form of pSTAT3) was also significantly increased in DC generated in the presence of MM-DF compare to control (1.6-2.3 fold-increase), furthermore, higher expression of phoso-STAT3β (negative form of pSTAT3) was found in DC generated in the absence of MM-DF (Figure 1). Finally the function of generated DC in the presence of MM-DF was impaired compare to control (40-60% decrease in ability to stimulate allogeneic T cell) as assessed by 3H-Thymidine incorporaton Assay.
Our results demonstrate a significant effect of MM-DF on the maturation of DC rendering them tolerogenic, and this is associated with hyper-activation of STAT3. RNA silencing experiments to knock down STAT3 and pharmacological inhibition experiments are in progress to assess the ability to abrogate the effect of MM-DC on DC maturation and improve function. This provides a potential therapeutic target in immunotherapy trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal