Abstract
Abstract 2713
Poster Board II-689
HIF-1 is a transcription factor that serves as a master regulator of cellular responses to hypoxia and regulates genes required for adaptation to hypoxia. Although the expression of HIF-1α subunit is constitutive, HIF-1α protein levels are regulated in response to oxygen tension. Under normoxic conditions, HIF-1α is degraded by the proteasome, and HIF-1 remains inactive. In hypoxia, HIF-1α is stabilized and forms a complex with HIF-1β that allows HIF-1 to function as a transcription factor. Thus, HIF-1α is activated only during hypoxia under normal physiologic conditions. By contrast, HIF-1α is frequently activated in cancer cells, including under normoxic conditions by oncogene products or impaired activity of tumor suppressor genes. We previously reported that there is constitutive stabilization of HIF-1α in many non-Hodgkin lymphoma (NHL) cell lines as well as among a significant fraction of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma patients (Evens et al, BJH, 2008), implicating a potential role of dysregulated HIF activation in NHL. Constitutive expression of HIF-1α enhances vascularization, increases glucose metabolism, and induces the expression of anti-apoptotic proteins in cancer tissues. HIF-1α is thought to be one of the most important molecular targets in the treatment of cancer. PX-478 is a novel small molecule inhibitor of HIF-1α being developed for the treatment of cancer.
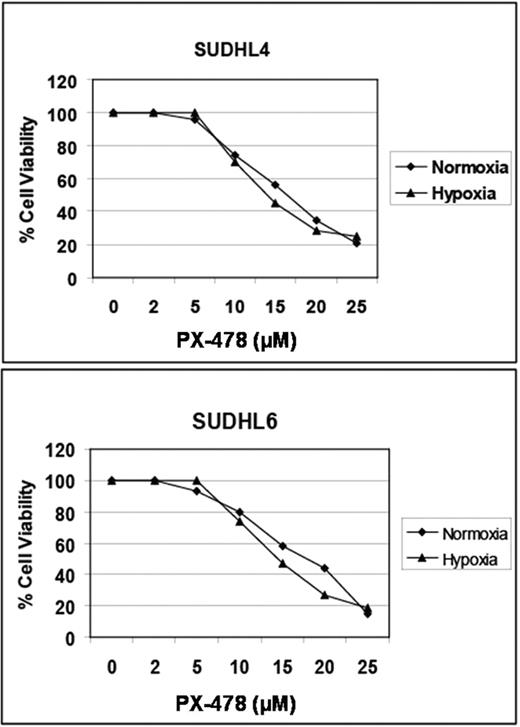
We treated several DLBCL cell lines (SUDHL4, SUDHL6, and SUDHL10) with increasing concentrations (2–25μM) of PX-478 for 4 hours followed by 20-hour incubation under normoxic (5% oxygen) or hypoxic (1.5% oxygen) conditions. Cell viability was assessed by MTT assay. Expression of HIF-1α and HIF-2α were measured by Western blotting after pre-incubation of cells with 5–25μM PX-478 followed by 20-hour incubation under normoxia or hypoxia.
Under hypoxic conditions, dose-dependent downregulation of HIF-1α protein levels were noted with complete absence of HIF-1α by 20μM. Interestingly, lower concentrations of PX-478 were needed for effective HIF-1α downregulation in normoxia. Of note, there was no change in HIF-2α protein levels observed in either normoxic or hypoxic conditions. In cell viability studies, time- and dose-dependent cell death of PX-478 was documented in all cell lines with an associated IC50 of 15–20μM (figure below).
Our observations suggest that the novel small molecule HIF-1α inhibitor, PX-478, effectively downregulates HIF-1α protein at low concentrations and induces cell death in DLBCL cells. Further studies to elucidate the mechanisms of HIF-1α dependent cell death in lymphoma and the associated novel therapeutics to target this pathway are warranted.
Gordon:Cure Tech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal