Abstract
Abstract 2626
Poster Board II-602
Smac/DIABLO (second mitochondrial derived activator of caspase/direct IAP binding protein with low pI), an antagonist of inhibitor of apoptosis proteins (IAP) family, was shown to trigger both external and internal apoptosis pathways in acute leukemia cell lines. The role, pathway of action and prognostic significance of Smac/DIABLO protein is not clearly determined in acute myeloid leukemia (AML) patients.
The main objective of this study was to verify whether expression of Smac/DIABLO protein has a prognostic impact on response to induction chemotherapy and overall survival (OS) of adult AML patients. Additionally, we aimed to analyse the apoptotic pathway potentially activated by Smac/DIABLO in AML patients.
Intracellular expression of Smac/DIABLO protein was examined in leukemic blasts isolated from bone marrow or peripheral blood of 89 de novo AML patients with median age 54 (range 23-82). Simultaneously, intracellular expression of active caspase-3 was investigated in 30 de novo AML patients with median age 57 (range 23–79). All measurements were done using multi-colour flow cytometry. In parallel, isotype controls were performed for all measurements. The percentage of Smac/DIABLO- and caspase-3-positive cells was assessed. According to median protein expression the patients were divided into “low-expressers” and “high”-expressers groups.
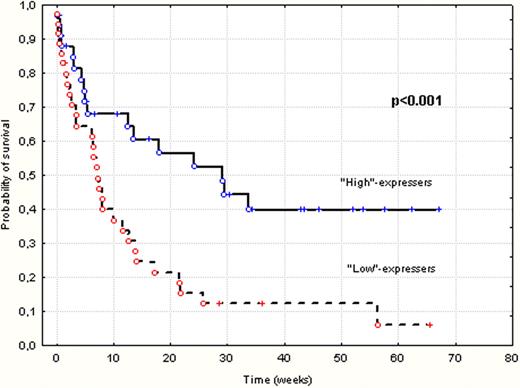
The median of intracellular expression of Smac/DIABLO protein was 65,8% (ranged 0–99,8%) and the median of cleaved caspase-3 was 4,95% (ranged 0,5–40,8) of leukemic blasts. Sixty four out of 89 AML patients received standard induction chemotherapy with daunorubicine and cytarabine (Ara-C) (“3+7”), 24/89 were treated with low dose Ara-C and 2/89 received best supportive care. Forty two (47%) of all patients achieved complete remission (CR). It was found that lower CR rate was associated with poor karyotype and low expression of Smac/DIABLO protein (p<0.01, p<0.01, respectively). The median time of the follow up reached 6 months (range 0.1–68.9). It has been shown that the high expression of Smac/DIABLO (above the median value) and percent of leukemic blast in bone marrow less than 50% were associated with significantly better OS in both univariate (p<0.001, p<0.001, respectively; Figure1) and multivariate (p=0.03, p=0.01, respectively) analyses. However, disease free survival was not influenced by baseline expression of Smac/DIABLO protein in AML cells. Additionally, it has been observed that patients with good and intermediate karyotype according to SWOG classification showed significantly higher expression of Smac/DIABLO protein, compare to poor risk group (median 74,7% vs 37,7% respectively; p< 0.01). Expression of Smac/DIABLO as well as caspase-3 did not correlate with tumour burden-associated risk factors as: number of white blood cells (WBC), percentage of leukemic blasts in bone marrow and serum LDH levels. Furthermore, analyses did not show any correlation between expression of Smac/DIABLO protein and cleaved caspase-3.
These data demonstrate that high Smac/DIABLO protein expression is associated with higher sensitivity to standard chemotherapy, favorable karyotype and longer OS in AML patients. The lack of the correlation between expression of Smac/DIABLO and active caspase-3 may be due to low number of patients with examined expression of caspase-3 or activation different way of cell death by Smac/DIABLO. Further investigations evaluating the relationship between Smac/DIABLO as well as the other pro- and anti-apoptotic proteins should be undertaken to better demonstrate its pathways of action as well as prognostic and potentially therapeutic value in AML.
Kaplan-Meier curves for overall survival in “High”-expressers and “Low”-expressers of Smac/DIABLO protein
Kaplan-Meier curves for overall survival in “High”-expressers and “Low”-expressers of Smac/DIABLO protein
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal