Abstract
Abstract 2379
Poster Board II-356
Anti-CD20 (i.e. rituximab) based immuno-chemotherapies are now considered standard of care in CLL. However, addition of rituximab does not appear to decisively alter the dismal clinical outcome for 17p- / TP53 mutated CLL. Relatively little is known about activity of different anti-CD20 antibodies in genetic subgroups of CLL.
In an effort to assess the activity of the next generation mAb GA101 in vitro, we studied B cell depletion / apoptosis in a set of CLL patients. CLL samples were genetically characterized with respect to genetics (genomic aberrations, TP53 mutation, IGHV mutation status), as well as clinical course and immunophenotype. To study the effect on CLL cells, we assessed GA101, Rituximab and Alemtuzumab by in vitro treatment after ficoll separation with FACS (7-AAD) for viability. In addition, in order to maintain ADCC and to mirror the in vivo situation, we studied the effect of GA101 in a whole blood culture (n=10).
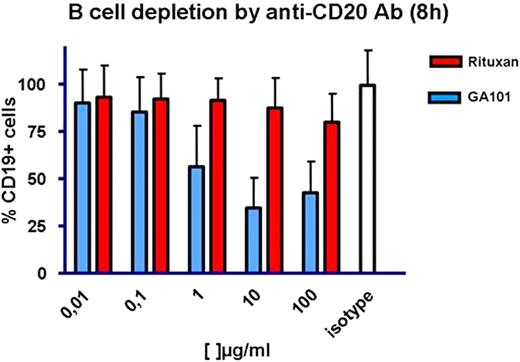
With increasing concentrations (0.01-100mg/ml) of GA101 we observed significant cell death as measured by a B cell depletion assay (whole blood) after GA101 exposure (n=10). After 3 hours the maximum effect was observed at a concentration of 10mg/ml with a reduction of CLL cells to 43% of untreated cells.
The results after 8h were similar, again showing the profound effect in GA101 treated samples (mean B-cell depletion to 34.6% remaining cells). Interestingly, further increasing the dose to 100mg/ml did not increase the B-cell depletion by the antibody, but appeared to decrease the ability to deplete CLL cells (Fig. 1).
In contrast treatment with control antibodies (isotype control, rituximab, alemtuzumab) did not lead to similar B-cell depletion (101%, 69%, and 73% of control cells at 3h). We were particularly interested in any potential effect in cases with TP53 mutation. While the sample number is still low (TP53 mutation / 17p deletion n=3), currently we have no indication of differential response in different genetic subgroups.
Compared to the currently most widely used mAb in CLL (Rituximab), GA101 appears more potent at equivalent concentration in depleting CLL cells. Further in vitro studies are currently ongoing to assess this antibody in particular in genetic high-risk, refractory CLL.
Zenz:Roche: Honoraria. Klein:Roche: Employment, Equity Ownership, Patents & Royalties. Umana:Roche: Employment, Equity Ownership, Patents & Royalties. Döhner:Roche: Research Funding. Stilgenbauer:Roche, Bayer Schering Pharma: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal