Abstract
Abstract 2316
Poster Board II-293
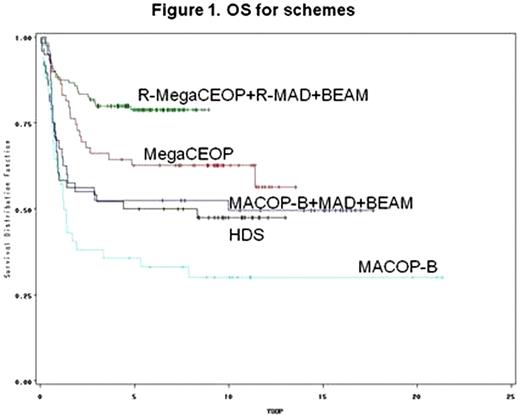
DLBCL at diagnosis with an intermediate/high or high-risk score according to age-adjusted International Prognostic Index (aa-IPI) had a dismal prognosis. High-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) was showed an effective salvage treatment for chemo-sensitive relapsed patients, but conflicting results were observed as first line treatment in randomized trials. The addition of Rituximab to standard chemotherapy significantly improved the outcome, but so far less data are available in young DLBCL patients with a poor prognosis. Aim of the analysis was to test the role of four important steps in first line treatment in DLBCL at poor prognosis: HDC and ASCT, Rituximab, new regimens as dose-dense chemotherapy MegaCEOP versus third generation MACOP-B chemotherapy, involved-field radiotherapy (IF-RT), in 309 untreated patients & 61 years enrolled from 1986 to 2006 into four GIMURELL and IIL consecutive trials. Patients and methods: 42 patients were enrolled into a phase II study and treatead with: 12 weekly infusion of MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin) chemotherapy (Vitolo U, J Clin Oncol 1992); 40 patients into a phase II trial with 8 weekly MACOP-B as induction therapy, followed by HDC with 2 courses of MAD (mitoxantrone, high-dose cytarabine, dexametasone) and BEAM and ASCT (Vitolo U, J Clin Oncol 1997); 107 patients into a phase III randomized trial, 48 treated with high dose sequential (HDS) chemotherapy with ASCT and 59 with 6 beweekly infusions of MegaCEOP (epirubicin, cyclophosphamide, vincristine, prednisone), respectively (Vitolo U, Haematol 2005); 120 patients into a phase II trial with an induction phase with dose-dense chemoimmunotherapy Rituximab-MegaCEOP for 4 cycles followed by HDC with 2 courses of Rituximab-MAD and BEAM plus ASCT (Vitolo U, Haematol 2009). IF-RT was performed at the end of treatment as consolidation of bulky disease or residual disease. A Cox proportional hazard model was performed for Overall Survival (OS) and Progression-Free Survival (PFS) to estimate the HRs of the 4 treatment variables (with/without Rituximab, HDC + ASCT, new regimens vs third generation regimen MACOP-B, radiotherapy yes/not), adjusted by aa-IPI, age and sex. Results: All 309 patients were evaluable for clinical characteristics and for response evaluation: median age 44 years (15-60); 178 males and 131 females; 10% were at Low-Intermediate, 51% at Intermediate-High and 39% at High risk according to aa-IPI score; PS >2 59%, 96 31% had BM involvement, 53% bulky disease, 84% LDH >normal, 34% >2 extranodal sites and 12/19/69% stage II/III/IV respectively. Rituximab was performed in 120 patients; IF-RT in 108 patients. New generation regimens were performed in 227 patients, MACOP-B in 82. As intention to treat, ASCT was scheduled for 208 patients. ASCT was performed in 171 patients (82%), 39 did not because of: progression disease in 22, toxicity in 9 and poor mobilization in 6. Complete response at the end of treatment was achieved in 212 pts (69%), PR in 23 (7%), 57 (18%) did not respond and 17 (6%) died of toxicity. Secondary haematological malignancies or solid tumour were observed in only 3 patients. With a median follow-up of 10 years, 10-yr OS and 10-yr PFS rates were: 59% (95%CI: 53-65) and 48% (95%CI: 41-55). The Cox's multivariable model showed that long term survival was significantly improved in patients treated with Rituximab compared to those treated without immunotherapy (HR=0.36, 95% CI=0.21-0.63, p .0003) and in patients that underwent IF-RT (HR=0.42, 95% CI: 0.27-0.66, p .0002), while no clear benefit could be detected for new regimens vs MACOP-B (HR=0.86, 95%CI: 0.57-1.31, p .482) and for ASCT (HR=0.94, 95%CI: 0.62-1.41, p .751). No important differences were observed after stratification by IPI. Similar results were obtained for PFS. Discussion: the introduction of immunotherapy and the irradiation of involved fields have played a major role in the improvement of long term survival of young patients with untreated DLBCL at poor prognosis, while little, if any, benefit has derived from newer dose-dense regimens and from ASCT. Randomized trials are ongoing to evaluate the impact of HDC and ASCT supplemented with Rituximab in poor-prognosis DLBCL compared to standard or dose-dense chemoimmunotherapy in the Rituximab era.
Vitolo:Roche:.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal