Abstract
Evidence is accumulating that circulating tissue factor (TF) contributes to the initiation of coagulation and the formation of fibrin. The majority of circulating TF is cryptic, and it has been suggested that close vicinity with anionic phospholipids on the cell surface increases the active conformation of TF. Two recent papers have shown that encryption of TF and initiation of coagulation are facilitated by the enzyme protein disulfide isomerase (PDI), possibly on the surface of activated platelets or endothelial cells. In this brief report, we demonstrate that the majority of PDI in platelets is intracellular where it is exclusively located in the dense tubular system. On activation, PDI remains confined to the intracellular stores of the dense tubular system and is neither released nor targeted to the cell surface. Similar results were obtained in endothelium where PDI remains exclusively localized in the endoplasmic reticulum, both at steady state and after thrombin stimulation.
Introduction
Protein disulfide isomerase (PDI) belongs to a group of proteins that facilitates the formation of disulfide bonds and the proper folding of newly synthesized proteins in the endoplasmic reticulum (ER). Studies have shown that a proportion of PDI is also secreted and can be detected on the cell surface of mammalian cells, including endothelial cells and platelets.1,2 Resting platelets display low levels of isomerase activity on the cell surface, and studies have shown that platelet activation increases the number of cell-surface protein thiol groups by more than 4-fold.3,4 It has been reported that PDI manipulates the redox state of extracellular protein thiols/disulfides, thereby controlling the function of these proteins.4,5 Secretion and targeting of PDI to the surface of activated platelets may contribute to the isomerase activity on the cell surface and regulate the adhesive response of αIIb-β3, α2-β1, and glycoprotein Ib (GPIb).4,5 Released and/or cell surface–expressed PDI activity may also regulate the procoagulant activity of tissue factor (TF).6,7 Two recent papers have suggested a potential role of platelet-derived PDI in the redox modification of TF, thereby regulating the initiation of coagulation in developing thrombi.8,9 In both these studies, it was shown that PDI accumulates in the developing thrombus in vivo and that thrombus growth and fibrin formation could be inhibited by infusion of an anti-PDI antibody, indicating an extracellular PDI expression. The origin of the PDI, however, was not established, and it was argued that targeting of PDI to the platelet plasma membrane and/or activation of the endothelium could be responsible for the encryption of TF. In this brief report, we have addressed these issues and analyzed the subcellular distribution of PDI in nonstimulated and stimulated cells.
Methods
Antibodies
Monoclonal anti-PDI antibodies were from Dr S. Fuller10 (University of Oxford, Oxford, United Kingdom; PDI-1), Stressgen (PDI-2, clone ID3), and Santa Cruz Biotechnology (RL-90). Rabbit polyclonal anti-PDI was a gift from Dr I. Braakman (Utrecht University, Utrecht, The Netherlands).11 Mouse anti–KDEL (10C3) was from Calbiochem. Monoclonal AK2, directed to GPIbα, was from Abcam. Anti–P-selectin was from BD Biosciences PharMingen. Mouse anti–calreticulin was from Affinity Bioreagents. Cy3-conjugated secondary antibody was from Nordic.
Platelet isolation
This study was approved by the Medical Ethical Committee of the University Medical Center Utrecht, with informed consent obtained in accordance with the Declaration of Helsinki. Resting platelets were obtained from blood of healthy volunteers, which was collected directly from the vein into fixative (2.4% paraformaldehyde + 0.24% glutaraldehyde in 0.1M sodium phosphate buffer, pH 7.4, 1:5 volume ratio).12 Blood was also collected into 0.1 vol of 0.13M sodium citrate, and platelet-rich plasma was prepared by centrifugation for 10 minutes at 180g. Platelets were washed with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode (0.145M NaCl, 5mM KCl, 0.5mM Na2HPO4, 1mM MgSO4, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 5mM D-glucose, pH 6.5) containing 10 ng/mL prostacyclin. Washed platelets (2 × 1011 cells/L) were stimulated with 0.5 U/mL thrombin or 20μM thrombin receptor activating peptide at 37°C. Activation was arrested by adding lysis buffer or excess fixative.
Results and discussion
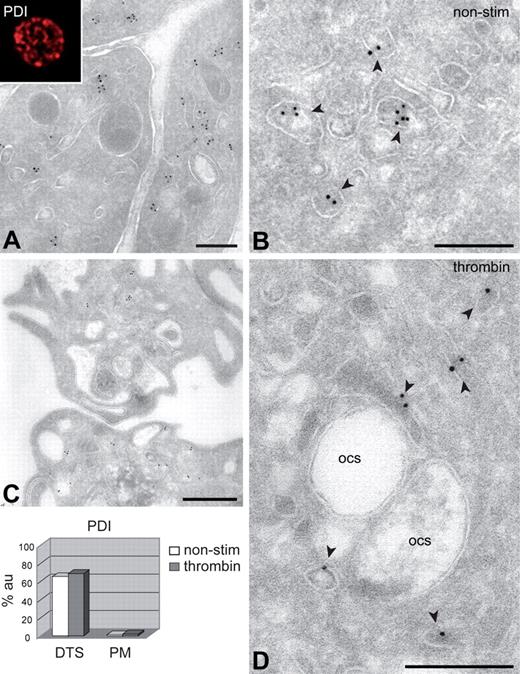
To test whether platelets express and/or secrete PDI, we analyzed the subcellular PDI distribution at steady state and after agonist stimulation. Platelet extracts and releasates obtained after stimulation with thrombin receptor-activating peptide were subjected to Western blot analysis. All 3 monoclonal antibodies to PDI stained prominently a band that corresponds to the molecular mass of PDI (∼ 60 kDa; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). No PDI could be detected in the releasates collected from stimulated cells. To determine the steady-state subcellular location of PDI, fixed nonstimulated platelets were permeabilized and stained with monoclonal anti-PDI antibody and rabbit anti–mouse Cy3. Abundant punctuate staining was detected within the cell (Figure 1). The intracellular distribution of PDI was further analyzed by electron microscopy after immunogold labeling of cryosections (immunoelectron microscopy [IEM]). Gold particles were strictly located within tubule-vesicular membranes and were absent from the cell surface (Figure 1A). The same localization was obtained with another monoclonal antibody directed to PDI (Figure 1B) and with a polyclonal antibody (supplemental Figure 2). To determine whether intracellular PDI becomes associated with the cell surface on stimulation, we analyzed the subcellular distribution after stimulation with thrombin (Figure 1C-D), and after adhesion to collagen under flow (supplemental Figure 3). No redistribution of PDI or increased cell-surface expression could be registered, and the total intracellular PDI remained unchanged (Figure 1 bottom left). To strengthen our observations, we incubated platelet-rich plasma with monoclonal anti-PDI and evaluated the binding by flow cytometry. No cell-surface PDI could be detected, and stimulation with convulxin did not result in cell-surface expression under conditions where P-selectin was translocated to the cell surface (supplemental Figure 1). Pre-embedding IEM using polyclonal anti-PDI revealed no surface labeling, whereas subsequent on-section labeling with the same antibody revealed intracellular localization (supplemental Figure 2).
Subcellular localization of PDI. Fixed nonstimulated and stimulated platelets were prepared for IEM. Immunogold labeling was carried out on frozen thin sections using 10-nm protein A gold conjugates, following standard procedures. Electron micrographs were obtained with a JEOL 1200EX electron microscope. For IF, platelets were allowed to settle on poly-L-lysine–coated coverslips, and PDI was detected after permeabilization with 0.5% Triton X-100. IF images were recorded on a Leitz DMIRB fluorescence microscope, interfaced with a Leica TCSNT confocal laser scanning microscope, using a 63×/1.4 Plan-Apo objective. (A-B) Nonstimulated platelets. Immunogold labeling with 2 different antibodies (panels A and B, respectively) gives the same results.  indicate PDI localization within electron-dense tubular membranes. (Inset) Confocal image of endogenous PDI localization in permeabilized resting platelet. (C-D) Activation with 0.5 U/mL thrombin showing platelet shape change and loss of α-granules. No release of PDI to the OCS or translocation to the cell surface is observed. (Bottom left) Semiquantitative analysis of the endogenous PDI distribution. Secondary markers were rabbit anti–mouse Cy3 (IF) and 10-nm protein A gold (IEM). α indicates α-granules. Scale bars represent 500 nm (A,C) or 200 nm (B,D).
indicate PDI localization within electron-dense tubular membranes. (Inset) Confocal image of endogenous PDI localization in permeabilized resting platelet. (C-D) Activation with 0.5 U/mL thrombin showing platelet shape change and loss of α-granules. No release of PDI to the OCS or translocation to the cell surface is observed. (Bottom left) Semiquantitative analysis of the endogenous PDI distribution. Secondary markers were rabbit anti–mouse Cy3 (IF) and 10-nm protein A gold (IEM). α indicates α-granules. Scale bars represent 500 nm (A,C) or 200 nm (B,D).
Subcellular localization of PDI. Fixed nonstimulated and stimulated platelets were prepared for IEM. Immunogold labeling was carried out on frozen thin sections using 10-nm protein A gold conjugates, following standard procedures. Electron micrographs were obtained with a JEOL 1200EX electron microscope. For IF, platelets were allowed to settle on poly-L-lysine–coated coverslips, and PDI was detected after permeabilization with 0.5% Triton X-100. IF images were recorded on a Leitz DMIRB fluorescence microscope, interfaced with a Leica TCSNT confocal laser scanning microscope, using a 63×/1.4 Plan-Apo objective. (A-B) Nonstimulated platelets. Immunogold labeling with 2 different antibodies (panels A and B, respectively) gives the same results.  indicate PDI localization within electron-dense tubular membranes. (Inset) Confocal image of endogenous PDI localization in permeabilized resting platelet. (C-D) Activation with 0.5 U/mL thrombin showing platelet shape change and loss of α-granules. No release of PDI to the OCS or translocation to the cell surface is observed. (Bottom left) Semiquantitative analysis of the endogenous PDI distribution. Secondary markers were rabbit anti–mouse Cy3 (IF) and 10-nm protein A gold (IEM). α indicates α-granules. Scale bars represent 500 nm (A,C) or 200 nm (B,D).
indicate PDI localization within electron-dense tubular membranes. (Inset) Confocal image of endogenous PDI localization in permeabilized resting platelet. (C-D) Activation with 0.5 U/mL thrombin showing platelet shape change and loss of α-granules. No release of PDI to the OCS or translocation to the cell surface is observed. (Bottom left) Semiquantitative analysis of the endogenous PDI distribution. Secondary markers were rabbit anti–mouse Cy3 (IF) and 10-nm protein A gold (IEM). α indicates α-granules. Scale bars represent 500 nm (A,C) or 200 nm (B,D).
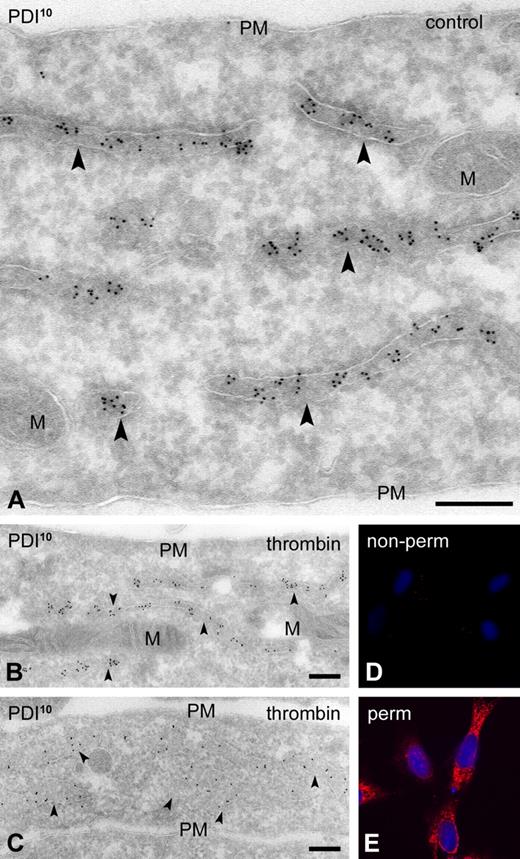
To characterize the intracellular PDI stores, we used monoclonal antibodies directed to the ER-retention peptide KDEL (10C3), the ER resident protein calreticulin, and to GPIbα (AK2). Calreticulin and KDEL were exclusively restricted to the same tubule-vesicular membrane population, whereas GPIbα was abundant on the cell surface and on membranes of the open canalicular system (OCS; supplemental Figure 4). No colocalization was detected with KDEL. These results demonstrate that PDI+ membranes are distinct from the OCS and represent the dense tubular system (DTS). In previous studies, it was suggested that the absence of ER in platelets could have given rise to nonconventional cell-surface localization of PDI.1 Unpublished observations by the same group, however, have recently challenged such predominant cell-surface localization.13 We here demonstrate, using 4 different antibodies, that PDI is strictly localized within the DTS. We obtained the same results in cultured human umbilical vein endothelial cells (HUVECs) and show PDI localized exclusively within the ER, with no expression on the cell surface, both at steady state and after stimulation (Figure 2).
PDI expression in HUVECs is strictly intracellular. HUVECs were grown in 48-well plates. Second- or third-passage cells were fixed with 3% paraformaldehyde (IF) or 2% paraformaldehyde + 0.2% glutaraldehyde (IEM) using standard procedures. Electron micrographs were obtained with a JEOL 1200EX electron microscope. (A) Nonstimulated HUVECs. Immunogold labeling with monoclonal PDI-1. (B-C) HUVECs stimulated with 0.5 U/mL thrombin for 60 minutes. Immunogold labeling with monoclonal anti-PDI-1 (B) and polyclonal anti-PDI (C).  indicate PDI localization within the cisternal lumen of the endoplasmic reticulum. (D-E) IF staining of thrombin-stimulated HUVECs using monoclonal anti–PDI-2. IF images were recorded on a Leitz DMIRB fluorescence microscope, interfaced with a Leica TCSNT confocal laser scanning microscope, using a 63×/1.4 Plan-Apo objective. (D) Nonpermeabilized cells. (E) Permeabilized cells. PM indicates plasma membrane; and M, mitochondria. Scale bars represent 200 nm.
indicate PDI localization within the cisternal lumen of the endoplasmic reticulum. (D-E) IF staining of thrombin-stimulated HUVECs using monoclonal anti–PDI-2. IF images were recorded on a Leitz DMIRB fluorescence microscope, interfaced with a Leica TCSNT confocal laser scanning microscope, using a 63×/1.4 Plan-Apo objective. (D) Nonpermeabilized cells. (E) Permeabilized cells. PM indicates plasma membrane; and M, mitochondria. Scale bars represent 200 nm.
PDI expression in HUVECs is strictly intracellular. HUVECs were grown in 48-well plates. Second- or third-passage cells were fixed with 3% paraformaldehyde (IF) or 2% paraformaldehyde + 0.2% glutaraldehyde (IEM) using standard procedures. Electron micrographs were obtained with a JEOL 1200EX electron microscope. (A) Nonstimulated HUVECs. Immunogold labeling with monoclonal PDI-1. (B-C) HUVECs stimulated with 0.5 U/mL thrombin for 60 minutes. Immunogold labeling with monoclonal anti-PDI-1 (B) and polyclonal anti-PDI (C).  indicate PDI localization within the cisternal lumen of the endoplasmic reticulum. (D-E) IF staining of thrombin-stimulated HUVECs using monoclonal anti–PDI-2. IF images were recorded on a Leitz DMIRB fluorescence microscope, interfaced with a Leica TCSNT confocal laser scanning microscope, using a 63×/1.4 Plan-Apo objective. (D) Nonpermeabilized cells. (E) Permeabilized cells. PM indicates plasma membrane; and M, mitochondria. Scale bars represent 200 nm.
indicate PDI localization within the cisternal lumen of the endoplasmic reticulum. (D-E) IF staining of thrombin-stimulated HUVECs using monoclonal anti–PDI-2. IF images were recorded on a Leitz DMIRB fluorescence microscope, interfaced with a Leica TCSNT confocal laser scanning microscope, using a 63×/1.4 Plan-Apo objective. (D) Nonpermeabilized cells. (E) Permeabilized cells. PM indicates plasma membrane; and M, mitochondria. Scale bars represent 200 nm.
Taken together, our data reveal that in platelets PDI localizes predominantly in DTS. This intracellular pool is neither released nor up-regulated on the cell surface during in vitro activation. Our findings are in disagreement with the studies of Essex et al who used pre-embedding IEM for cell-surface localization and immunofluorescence (IF) to assay intracellular PDI,1,2 but are consistent with reports in other cells14 and with the concept that no secretory properties have been attributed to the DTS. From our ultrastructural experience on platelets, we know that DTS membranes are often positioned close to the plasma membrane. The relative low resolution power of IF used in the initial studies may have led to a misinterpretation of the peripheral staining.
There are several reasons to argue against a role for regulated secretion of PDI in the encryption of TF. First, isomerase activity on the platelet surface may involve other thiol-containing proteins.14 Second, from in vivo studies in platelet-deficient mice, it appeared that a pool of PDI is still exposed at the site of injury, indicating the existence of at least one other origin as well.8 Third, no PDI can be detected on the surface of intact endothelial cells (Figure 2).15 It is, therefore, conceivable that PDI located in the lumen of the ER becomes available extracellularly only after mechanical injury. In a similar fashion, intracellular PDI stores may become available as a consequence of platelet lysis. Taking into account these considerations, our observations reveal that the isomerase-dependent thrombus development and fibrin formation observed in recent in vivo studies are not connected to a regulated secretion of PDI from the DTS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by The Netherlands Organization of Scientific Research (grant ALW 813.08.001).
Authorship
Contribution: H.E.v.N.t.P. analyzed the immuno-EM data and wrote the paper; S.M.v.D. performed the immunofluorescence and immuno-EM experiments; V.D. performed the flow cytometry; and H.F.G.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harry F. G. Heijnen, Department of Clinical Chemistry and Hematology, Cell Microscopy Center, UMCU, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: h.f.g.heijnen@umcutrecht.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal