Abstract
The role of the Wiskott-Aldrich syndrome protein (WASp) in platelet function is unclear because platelets that lack WASp function normally. WASp constitutively associates with WASp-interacting protein (WIP) in resting and activated platelets. The role of WIP in platelet function was investigated using mice that lack WIP or WASp. WIP knockout (KO) platelets lack WASp and thus are double deficient. WIP KO mice have a thrombocytopenia, similar to WASp KO mice, resulting in part from enhanced platelet clearance. Most WIP KO, but not WASp KO, mice evolved platelet-associated immunoglobulins (Ig) of the IgA class, which normalize their platelet survival but diminish their glycoprotein VI (GPVI) responses. Protein tyrosine phosphorylation, including that of phospholipase C-γ2, and calcium mobilization are impaired in IgA-presenting WIP KO platelets stimulated through GPVI, resulting in defects in α-granule secretion, integrin αIIbβ3 activation, and actin assembly. The anti-GPVI antibody JAQ1 induces the irreversible loss of GPVI from circulating platelets in wild-type mice, but not in WIP KO mice that bear high levels of platelet-associated IgAs. Together, the data indicate that platelet-associated IgAs negatively modulate GPVI signaling and function in WIP KO mice.
Introduction
Wiskott-Aldrich syndrome (WAS) is a recessive hematopoietic disorder that is characterized by immunodeficiency, eczema, and severe microthrombocytopenia.1 The WASP gene implicated in WAS or X-linked thrombocytopenia (XLT) is located on the Xp11.22-p11.23 locus of the X chromosome and encodes a protein of 502 amino acids and 64 kDa, called WAS protein (WASp).2,3 WASp expression is restricted to nonerythroid hematopoietic cells, notably lymphocytes and granulocytes, in which its deficiency results in impaired cell polarization, motility, podosome formation, and phagocytosis.1 WASp regulates actin assembly by activating the actin filament barbed-end amplifier Arp2/3 complex in vitro and in cultured cells.4-6 In T cells, WASp redistributes to the plasma membrane and stimulates the Arp2/3 complex after CD3 ligation, a prerequisite for immunologic synapse formation.7,8
The mechanisms of WAS-associated thrombocytopenia or XLT remain poorly understood, although increased platelet destruction by the spleen is thought to play a major role. Clearance of WAS platelets is accelerated because platelets isolated from WAS patients are cleared rapidly from the circulation when transfused autologously.9 Platelet-associated antibodies could be responsible for the fast clearance of WAS platelets because antibodies are no longer detectable and circulating platelet number and size increase after WAS patients undergo splenectomy.10-12 Megakaryocytes isolated from WAS patients form proplatelets normally and produce platelets of normal size in vitro.13
WASp knockout (KO) mice have a moderate thrombocytopenia.14,15 The reason for the difference between the human and mouse platelet phenotypes is unclear. The higher turnover (3-4 vs 7-8 days) and smaller size (5-7 vs 7-10 fL) of mouse versus human platelets may contribute. The clearance system of the mouse may also be more adversely affected by WASp deficiency, compared with human, diminishing the clearance capacity in the mouse. Premature proplatelet formation and platelet production are observed in the bone marrow compartment of WASp KO mice.16
In human platelets, WASp is phosphorylated on tyrosine and associates with the tyrosine kinase Syk through the adaptor protein CrkL after stimulation by the collagen receptor glycoprotein VI (GPVI) and the low affinity IgG Fc receptor, FcγRIIA.17-19 Furthermore, WASp immunoprecipitates and localizes with the adaptor proteins SLP-76, ADAP, Nck, and VASP during platelet spreading on fibrinogen.20,21 Together, these studies suggest that WASp may play a role in platelet signaling downstream of the immunoreceptor tyrosine-based activation motif-containing receptors, GPVI and FcγRIIA, or in shape change downstream of the fibrinogen receptor, the integrin αIIbβ3. However, platelets isolated from WAS patients or from WASp KO mice function normally: they change shape, assemble actin, and activate and redistribute their Arp2/3 complex normally when activated through GPVI or their thrombin receptor, suggesting that WASp may have more specialized functions in platelets.18,22
WASp contains numerous protein-interacting domains. Its N-terminus has a WASp homology 1 (WH1) domain that binds to the C-terminus of WASp-interacting protein (WIP).23,24 WIP is a protein of 503 amino acids and 63 kDa that is ubiquitously expressed and binds the WASp homolog N-WASP in nonhematopoietic cells.25,26 WIP regulates actin polymerization induced by the Arp2/3 complex downstream of N-WASP and cortactin in vitro,26-28 and its overexpression leads to increase in F-actin clustering in B cells23 and elaboration of filopodia in fibrobasts.25,26 Importantly, most missense mutations in WAS patients map to the WH1 domain, suggesting that WIP is a biologically important partner of WASp.29-31
Here we show that WASp constitutively complexes with WIP in resting and activated platelets. WIP KO platelets lack WASp, and WIP expression is reduced in WASp KO platelets, indicating that protein stability requires complex formation. Furthermore, WIP KO mice evolve platelet-associated immunoglobulins of the IgA class and have impaired functional responses to stimulation via GPVI.
Methods
Mice
Wild-type (WT), WASp KO, and WIP KO mice were maintained on a 129Sv background.14,32 Mice were treated as approved by the Harvard Medical Area Standing Committee on Animals according to National Institutes of Health standards as set forth in the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals.
Reagents
The collagen-related peptide (CRP; amino acids GCO(GPP)10GCOG) was synthesized by the Tufts University Core Facility and cross-linked as previously described.33 Mouse antibody B9 directed against the 250 N-terminal amino acids of human WASp and anti-Syk antibody N19 and rabbit anti–phospholipase C (PLC)–γ2 antibody Q20 were obtained from Santa Cruz Biotechnology. Rabbit antibody B4 directed against WASp GTPase-binding domain and mouse anti-phosphotyrosine antibody 4G10 were obtained from Upstate Biotechnology. Rabbit antibody K374 directed against the 8 C-terminal amino acids of human WASp was a gift from Dr Ignacio Molina (University of Granada School of Medicine). Rabbit antibody C45 directed against the 45 C-terminal amino acids of human WIP was previously described.7 Fluorescein isothiocyanate (FITC)–labeled rat anti–mouse GPIbα (CD42b), GPXI (CD42a), GPV (CD42d), and GPVI antibodies were obtained from Emfret Analytics. FITC-labeled rat antimouse P-selectin (CD62P) and phycoerythrin (PE)–labeled Armenian hamster anti–mouse GPIIIa (CD61, integrin β3) antibodies were obtained from BD Biosciences. Unlabeled goat antibodies directed against mouse immunoglobulin classes were obtained from Southern Biotechnology. 5-Chloromethylfluorescein diacetate (CMFDA), Oregon Green 488–labeled fibrinogen, and Alexa Fluor 488–labeled donkey anti–mouse, anti–rabbit, and anti–goat IgG antibodies were obtained from Invitrogen. Protein G–conjugated Sepharose beads were obtained from GE Healthcare. All other reagents were of the highest purity available (Sigma-Aldrich).
Blood platelet count
Blood was collected from mice by retro-orbital plexus bleeding in ethylenediaminetetraacetic acid. Peripheral blood platelet count was performed using an ADVIA 120/2120 automated hematology analyzer (Bayer HealthCare, Diagnostics Division).
Platelet preparation
Blood was collected from mice by retro-orbital plexus bleeding in ACD. Mouse platelet-rich plasma was obtained by centrifugation of the blood at 100g for 8 minutes, followed by centrifugation of the supernatant and the buffy coat at 100g for 6 minutes. Mouse platelets were isolated by 2 sequential centrifugations of the platelet-rich plasma at 1200g for 5 minutes in 140mM NaCl, 5mM KCl, 12mM trisodium citrate, 10mM glucose, 12.5mM sucrose, pH 6 (washing buffer), and resuspended in 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 140mM NaCl, 3mM KCl, 0.5mM MgCl2, 5mM NaHCO3, 10mM glucose, pH 7.4 (resuspension buffer) as described.34 Platelet concentration was determined by flow cytometry using 5.5-μm diameter SPHERO rainbow beads (Spherotech) as a reference,34 and was adjusted to 2 to 4 × 108/mL depending on the assay performed. Platelets were allowed to rest for 30 minutes before use.
Platelet survival
Platelets were labeled with 1.8μM CMFDA for 15 minutes at 37°C in resuspension buffer.34 Unincorporated dye was removed by centrifugation at 100g for 5 minutes. CMFDA-labeled platelets were injected into WT recipient mice via the retro-orbital plexus.35 Blood samples were collected immediately (< 5 minutes) and 2, 24, 48, and 72 hours after transfusion into 0.1 volume of ACD anticoagulant. Whole blood analysis was performed by flow cytometry using a BD Biosciences FACSCalibur flow cytometer, and the percentage of CMFDA+ platelets determined. A total of 50 000 events were collected in each sample. CMFDA+ platelets measured at time less than 5 minutes were set as 100%.
Flow cytometry
To determine whether immunoglobulins bind to platelets, WT, WIP KO, and WASp KO platelets were incubated with a nonimmune goat IgG or goat antibodies directed against mouse immunoglobulin classes, followed by Alexa Fluor 488–labeled donkey anti–goat IgG antibody. Fluorescence was quantified using a BD Biosciences FACSCalibur flow cytometer. A total of 20 000 events were analyzed for each sample. For the functional assays, resting and activated platelets were incubated with a FITC-labeled anti–P-selectin antibody or Oregon Green 488–labeled fibrinogen for 30 minutes at room temperature,34 or were fixed and permeabilized in BD Cytofix/Cytoperm solution, washed in BD Perm/Wash solution, and incubated with PE-labeled mouse anti-WASp antibody B9 or 2μM tetramethylrhodamine isothiocyanate (TRITC)–phalloidin.22
Mouse IgA ELISA
Nunc-Immuno plates (R&D Systems) were coated overnight with goat antimouse IgA antibody (Southern Biotechnology) and blocked with 3% bovine serum albumin in phosphate-buffered saline. Lysates from 5 × 107 mouse platelets or mouse plasma were added to the wells. IgAs were probed with alkaline phosphatase-conjugated goat anti–mouse IgA (Southern Biotechnology), and p-nitrophenyl phosphate was used as substrate. Optical density was read at 405 nm in a ThermoMax kinetic microplate reader (Molecular Devices). Purified mouse IgA (Southern Biotechnology) was used as standard. All samples were analyzed in triplicate.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblot analysis
Platelets were lysed at 4°C in 1% Nonidet P-40, 150mM NaCl, 50mM Tris, pH 7.4, containing 1mM ethyleneglycoltetraacetic acid, 1mM Na3VO4, and Complete protease inhibitor cocktail (Roche Diagnostics). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 5% β-mercaptoethanol was added. After boiling for 5 minutes, platelet proteins were separated on 8% polyacrylamide gels and transferred onto an Immobilon-P membrane (Millipore). Membranes were incubated overnight in 0.2% Tween-20, 100mM NaCl, and 20mM Tris, pH 7.4, containing 1% bovine serum albumin, then probed with antibodies. Detection was performed with an enhanced chemiluminescence system (Pierce Chemical).
For the immunoprecipitation studies, Nonidet P-40 lysates were centrifuged at 14 000g for 10 minutes at 4°C. Soluble fractions were incubated with antibodies directed against proteins of interest for 2 hours at 4°C, followed by incubation with protein G-conjugated Sepharose beads (GE Healthcare) for 1 hour at 4°C. After washing, immune complexes were resolved by SDS-PAGE.
Measurement of intracellular calcium
Mouse platelets (2 × 108/mL) were loaded with 10μM Indo 1-AM in washing buffer for 45 minutes at room temperature. Platelets were washed and resuspended to their original concentration in resuspension buffer containing 1mM CaCl2. Intracellular calcium variations were recorded using a spectrofluorimeter LS50 (PerkinElmer Life and Analytical Sciences). Excitation wavelength was 354 nm and emission wavelengths were 405 and 485 nm.
Statistical analysis
Comparison was performed using the unpaired Student t test or the Bonferroni test following 1-way analysis of variance. A P value less than .05 was considered statistically significant.
Results
WIP associates with WASp in platelets
WIP expression and association with WASp were examined in resting and activated WT platelets (Figure 1A). WASp immunoprecipitates were prepared from mouse platelet lysates using the mouse anti-WASp antibody B9 and were probed with rabbit anti-WIP antibody C45 and rabbit anti-WASp antibody B4. Immunoprecipitated WASp was detected as a single band of 64 kDa.22 WIP, which was detected as a double band of 60/63 kDa, associated with WASp in both resting and activated platelets. The WIP-WASp complex was not disrupted when platelets were stimulated with either 3 μg/mL CRP, specific for the collagen receptor GPVI, or 0.5 U/mL thrombin for 2 minutes. Platelet activation for longer times and under stirring conditions to induce engagement of the fibrinogen receptor, the integrin αIIbβ3, and subsequent outside-in signaling did not disrupt the WIP-WASp complex (data not shown).
WIP-WASp complex in platelets. (A) WT mouse platelets were activated with 3 μg/mL CRP or 0.5 U/mL thrombin for 0, 0.5, 1, and 2 minutes at 37°C as indicated. WASp was immunoprecipitated with mouse antibody B9. Immunoprecipitates were subjected to SDS-PAGE and probed with rabbit anti-WIP antibody C45 or anti-WASp antibody B4, as indicated. A control mouse IgG was used as negative control for specificity. (B) Platelet lysates before and after immunoprecipitation with anti-WASp antibody B9 or an isotype control were probed for WASp and WIP, as indicated. (C) Lysates corresponding to 4 × 106 WT, WASp KO, and WIP KO platelets were subjected to SDS-PAGE and probed with anti-WIP antibody C45 and anti-WASp antibody B4, as indicated. Anti-Syk probe was used as a control for loading. (D) WT and WIP KO platelets were probed with PE-labeled mouse anti-WASp antibody B9 (open) or an isotype control (shaded).
WIP-WASp complex in platelets. (A) WT mouse platelets were activated with 3 μg/mL CRP or 0.5 U/mL thrombin for 0, 0.5, 1, and 2 minutes at 37°C as indicated. WASp was immunoprecipitated with mouse antibody B9. Immunoprecipitates were subjected to SDS-PAGE and probed with rabbit anti-WIP antibody C45 or anti-WASp antibody B4, as indicated. A control mouse IgG was used as negative control for specificity. (B) Platelet lysates before and after immunoprecipitation with anti-WASp antibody B9 or an isotype control were probed for WASp and WIP, as indicated. (C) Lysates corresponding to 4 × 106 WT, WASp KO, and WIP KO platelets were subjected to SDS-PAGE and probed with anti-WIP antibody C45 and anti-WASp antibody B4, as indicated. Anti-Syk probe was used as a control for loading. (D) WT and WIP KO platelets were probed with PE-labeled mouse anti-WASp antibody B9 (open) or an isotype control (shaded).
WASp immunoprecipitation resulted in more than 95% depletion of both WIP and WASp from platelet lysates, as evidenced by densitometric analysis of the immunoblots (Figure 1B), suggesting that both proteins associate with a 1:1 ratio in platelets, as in T cells.7 Similar results were obtained with human platelets and/or when WIP was immunoprecipitated (data not shown).
WIP KO platelets lack WASp
Platelets isolated from WASp KO and WIP KO mice were examined for WIP and WASp expression (Figure 1C). Expression of both the 60- and 63-kDa bands corresponding to WIP was decreased to 57% plus or minus 20% (mean ± SD; n = 11) in WASp KO platelets, compared with WT platelets, as evidenced by densitometric analysis of the immunoblots. Anti-WASp antibody B4 did not detect WASp in WIP KO platelets, nor did rabbit anti-WASp antibody K374 directed against WASp C-terminus (data not shown).
Intracellular flow cytometry using fluorescently labeled anti-WASp antibody B9 confirmed no detectable expression of WASp in WIP KO platelets (Figure 1D). Together, the data show that formation of the WIP-WASp complex is required for the integrity of both components in platelets.
Normal expression of platelet surface glycoproteins in WIP KO mice
Expression of major platelet surface glycoproteins on WASp KO and WIP KO platelets was examined by flow cytometry (Table 1). WASp or WIP deficiency did not result in any effect on the expression of the von Willebrand factor receptor subunits GPIbα (CD42b), GPIX (CD42a), and GPV (CD42d), the fibrinogen receptor subunit GPIIIa (CD61, integrin β3), or the collagen receptor GPVI.
Expression of major surface glycoproteins on WASp KO and WIP KO platelets
| Platelet glycoprotein . | WASp KO platelets . | WIP KO platelets . |
|---|---|---|
| GPIbα (CD42b) | 1.01 ± 0.24 | 0.99 ± 0.01 |
| GPIX (CD42a) | 0.95 ± 0.05 | 1.04 ± 0.26 |
| GPV (CD42d) | 1.00 ± 0.08 | 1.01 ± 0.14 |
| GPIIIa (CD61, integrin β3) | 0.97 ± 0.07 | 1.07 ± 0.16 |
| GPVI | 0.98 ± 0.16 | 1.05 ± 0.32 |
| Platelet glycoprotein . | WASp KO platelets . | WIP KO platelets . |
|---|---|---|
| GPIbα (CD42b) | 1.01 ± 0.24 | 0.99 ± 0.01 |
| GPIX (CD42a) | 0.95 ± 0.05 | 1.04 ± 0.26 |
| GPV (CD42d) | 1.00 ± 0.08 | 1.01 ± 0.14 |
| GPIIIa (CD61, integrin β3) | 0.97 ± 0.07 | 1.07 ± 0.16 |
| GPVI | 0.98 ± 0.16 | 1.05 ± 0.32 |
The binding of fluorescently labeled antibodies directed against the platelet glycoproteins was measured by flow cytometry. Data are expressed as the ratio of fluorescence bound to the surface of WASp KO or WIP KO versus WT platelets and represent mean ± SD (n = 13-15 for each independent group).
WIP KO mice evolve platelet-associated IgAs
Because platelets isolated from WAS patients are often coated with antibodies,10-12 this possibility was investigated in WASp KO and WIP KO mice. Platelets isolated from WIP KO, but not WASp KO, mice showed an increase of platelet-associated immunoglobulins by flow cytometry, compared with WT mice (Figure 2A). A positive signal for mouse immunoglobulins could be detected on the surface of platelets isolated from 16 of 21 WIP KO mice (76.2%), with a prevalence in mice of ages more than 12 weeks (data not shown), compared with 2 of 16 (12.5%) in both WT and WASp KO mice. In the rest of the study, WIP KO platelets are separated into 2 groups, WIP KOlow and WIP KOhigh, based on their levels of associated immunoglobulins.
WIP KO mice evolve platelet-associated IgAs. (A) WT, WASp KO, and WIP KO platelets were incubated with Alexa Fluor 488–labeled donkey anti–mouse immunoglobulin antibody (open) or anti–rabbit immunoglobulin antibody (shaded) as negative control. (B) WT and WIP KO platelets were incubated with a nonimmune goat IgG or goat antibodies directed against mouse Ig classes as described, followed by Alexa Fluor 488–labeled donkey anti–goat IgG antibody. Results are ratio between mean fluorescence obtained with specific and unspecific antibodies and represent the mean ± SE of 4 to 6 mice per group. Box plot of platelet-associated (C) and plasma (D) IgA levels from WT and WIP KO littermates, as determined by isotype-specific ELISA. NS indicates not significant.
WIP KO mice evolve platelet-associated IgAs. (A) WT, WASp KO, and WIP KO platelets were incubated with Alexa Fluor 488–labeled donkey anti–mouse immunoglobulin antibody (open) or anti–rabbit immunoglobulin antibody (shaded) as negative control. (B) WT and WIP KO platelets were incubated with a nonimmune goat IgG or goat antibodies directed against mouse Ig classes as described, followed by Alexa Fluor 488–labeled donkey anti–goat IgG antibody. Results are ratio between mean fluorescence obtained with specific and unspecific antibodies and represent the mean ± SE of 4 to 6 mice per group. Box plot of platelet-associated (C) and plasma (D) IgA levels from WT and WIP KO littermates, as determined by isotype-specific ELISA. NS indicates not significant.
Antibodies directed against various immunoglobulin isotypes were used to determine the class of the platelet-associated immunoglobulins observed in WIP KOhigh mice (Figure 2B). Staining for mouse IgA induced a 2.66- plus or minus 1.38-fold (range, 1.43--5.16-fold; n = 6) increase in the mean fluorescence in WIP KOhigh platelets, compared with a nonimmune antibody (P < .05). None of the Ig specific antibodies bound to WT platelets (n = 4).
The number of platelet-associated IgAs in WIP KO mice was further determined by ELISA. WIP KO mice had 2222 plus or minus 3162 IgAs/platelet (mean ± SD; n = 14), compared with 215 plus or minus 199 (n = 7; P < .05) in WT mice (Figure 2C). However, plasma IgA levels in WIP KO mice were normal (Figure 2D), as described previously.32 Together, the data show that WT and WASp KO mouse platelets minimally associate with immunoglobulins, whereas WIP KO mice evolve high levels of platelet-associated IgAs. It is noteworthy that WASp was not detectable in WIP KOlow or WIP KOhigh platelets (data not shown), indicating that platelet-associated IgAs do not influence WASp stability.
Thrombocytopenia in WIP KO mice
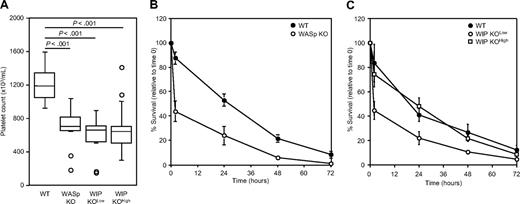
The peripheral blood platelet counts of WASp KO and WIP KO mice were determined (Figure 3A). WASp KO mice had a moderate thrombocytopenia with 697 plus or minus 241 × 103 platelets/μL (mean ± SD; n = 12), compared with 1197 plus or minus 176 × 103 platelets/μL in WT mice (n = 24; P < .001), as described previously.14,15 WIP KOlow and WIP KOhigh mice had low blood platelet counts compared with WT mice, with 593 plus or minus 243 × 103 platelets/μL (n = 11; P < .001) and 676 plus or minus 254 × 103 platelets/μL (n = 20; P < .001), respectively. No statistical difference was observed between the platelet counts of WASp KO, WIP KOlow, and WIP KOhigh mice. None of these mice had any obvious bleeding tendencies.
Platelet count and survival. (A) Box plot of platelet count in peripheral blood of WT, WASp KO, WIP KOlow, and WIP KOhigh mice. Circles represent outliers. (B) Platelets were isolated from WT and WASp KO mice, labeled with CMFDA, and injected into 129Sv WT recipients. (C) Platelets were isolated from WT, WIP KOlow, and WIP KOhigh mice, labeled with CMFDA, and injected into 129Sv WT recipients. Results are percentage of positive cells relative to time less than 5 minutes and represent the mean ± SD of 4 transfused mice.
Platelet count and survival. (A) Box plot of platelet count in peripheral blood of WT, WASp KO, WIP KOlow, and WIP KOhigh mice. Circles represent outliers. (B) Platelets were isolated from WT and WASp KO mice, labeled with CMFDA, and injected into 129Sv WT recipients. (C) Platelets were isolated from WT, WIP KOlow, and WIP KOhigh mice, labeled with CMFDA, and injected into 129Sv WT recipients. Results are percentage of positive cells relative to time less than 5 minutes and represent the mean ± SD of 4 transfused mice.
To determine whether the cause of the thrombocytopenia observed in WASp KO and WIP KO mice was the result of increased platelet clearance or decreased platelet production, CMFDA-labeled platelets isolated from each KO mouse strain were infused into WT recipients (Figure 3B-C). The initial recoveries (< 5 minutes) of WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were similar when transfused into WT recipients. WT platelets disappeared at a constant rate over time for approximately 72 hours with a half-time life of 36 hours. In contrast, approximately 60% of both WASp KO (P < .01) and WIP KOlow (P < .001) platelets disappeared from the circulation by 2 hours after injection. The remaining circulating platelets disappeared at the same rate as WT platelets. Approximately 25% of WIP KOhigh platelets are cleared within the first 2 hours after transfusion, and the remaining platelet population circulates longer than WIP KOlow platelets. CMFDA-labeled platelets did not show any sign of activation, as evidenced by lack of P-selectin expression (data not shown).
Impaired platelet functions in WIP KO mice
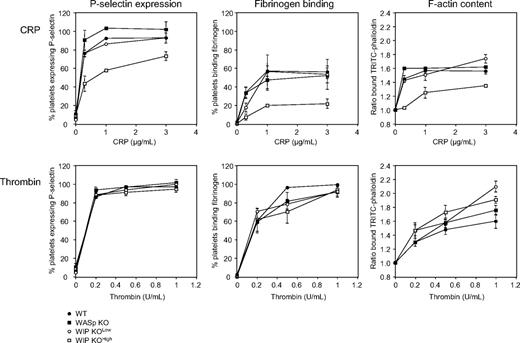
The significance of WASp and WIP expression in platelet function was tested (Figure 4). Platelets isolated from WT, WASp KO, WIP KOlow, and WIP KOhigh mice were treated with CRP, specific for the collagen receptor GPVI36 or thrombin and analyzed by flow cytometry for P-selectin expression as a marker for α-granule secretion. CRP induced a concentration-dependent increase of P-selectin expression in platelets isolated from WT, WASp KO, and WIP KOlow mice. However, P-selectin expression induced by CRP was substantially reduced in WIP KOhigh platelets. P-selectin expression in response to thrombin stimulation, which activates platelets through G-protein–coupled receptors, was not affected by WIP or WASp deficiency.
Functional defects in WIP KO platelets. WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were activated with increasing concentrations of CRP or thrombin for 2 minutes at 37°C, as indicated. Platelets were then incubated with an FITC-labeled anti–mouse P-selectin antibody or with Oregon Green 488–labeled fibrinogen, and analyzed by flow cytometry. Results are percentage of positive platelets compared with rest and represent the mean ± SE of 4 independent experiments. Resting and activated platelets were fixed, permeabilized, stained with TRITC-labeled phalloidin, and analyzed by flow cytometry. Results are the ratio between the fluorescence of activated versus resting platelets and represent the mean ± SE of 3 independent experiments.
Functional defects in WIP KO platelets. WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were activated with increasing concentrations of CRP or thrombin for 2 minutes at 37°C, as indicated. Platelets were then incubated with an FITC-labeled anti–mouse P-selectin antibody or with Oregon Green 488–labeled fibrinogen, and analyzed by flow cytometry. Results are percentage of positive platelets compared with rest and represent the mean ± SE of 4 independent experiments. Resting and activated platelets were fixed, permeabilized, stained with TRITC-labeled phalloidin, and analyzed by flow cytometry. Results are the ratio between the fluorescence of activated versus resting platelets and represent the mean ± SE of 3 independent experiments.
Fibrinogen binding was measured by flow cytometry as a marker for integrin αIIbβ3 activation. Both CRP and thrombin stimulated an increase of fibrinogen binding to WT, WASp KO, and WIP KOlow platelets. Fibrinogen binding was reduced in WIP KOhigh platelets in response to CRP. Integrin αIIbβ3 activation induced by thrombin was normal.
We next addressed shape change in WASp KO and WIP KO platelets by measuring the F-actin content of resting and activated cells. Mouse platelets were stimulated with CRP and thrombin, and their F-actin content was determined by intracellular flow cytometry using TRITC-labeled phallacidin binding.22 Actin assembly stimulated with 0.3, 1, or 3 μg/mL CRP was reduced in WIP KOhigh platelets. In agreement, these platelets exhibited only a small increase of their actin filament barbed-end numbers after CRP stimulation and failed to spread when exposed to CRP-coated surface (data not shown). WASp KO platelets assembled actin normally, as described previously.22 Platelet stimulation with thrombin resulted in a significant increase in the F-actin content of WT, WASp KO, WIP KOlow, and WIP KOhigh platelets. Together, the data suggest that platelet-associated IgAs in WIP KOhigh mice modulate platelet collagen receptor GPVI, but not G-protein–coupled thrombin receptor signaling.
Impaired GPVI signaling in WIP KO platelets
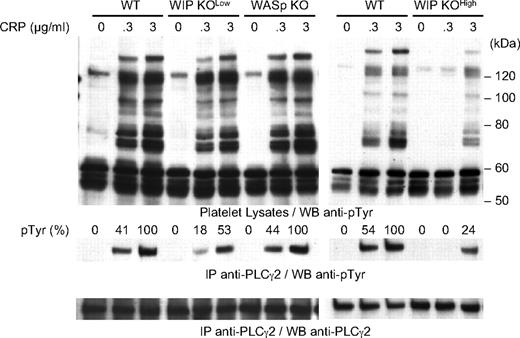
Signaling downstream of GPVI is initiated by tyrosine phosphorylation and activation of signaling proteins that include PLC-γ2.36,37 Hence, the ability of WASp KO and WIP KO platelets to phosphorylate proteins on tyrosine in response to CRP was investigated (Figure 5). CRP induced equivalent protein tyrosine phosphorylation in WT and WASp KO platelets, including a 130-kDa protein that comigrates with PLC-γ2. Immunoprecipitation confirmed that PLC-γ2 phosphorylation was comparable in CRP-stimulated WT and WASp KO platelets, as evidenced by densitometric analysis of the immunoblots. On the other hand, WIP KOlow platelets had a 56% and a 47% reduction in PLC-γ2 phosphorylation at 0.3 and 3 μg/mL CRP, respectively. This defect was more severe in WIP KOhigh platelets, resulting in absent PLC-γ2 phosphorylation at 0.3 μg/mL CRP and a 76% reduction in PLC-γ2 phoshorylation at 3 μg/mL CRP.
Protein tyrosine phosphorylation in CRP-stimulated platelets. WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were activated with 0.3 or 3 μg/mL CRP for 2 minutes at 37°C as indicated. Platelet lysates and anti–PLC-γ2 immunoprecipitates were subjected to SDS-PAGE and probed with the anti-phosphotyrosine antibody 4G10. The membrane was stripped and probed with anti–PLC-γ2 antibody as loading control. Results are percentage of PLC-γ2 tyrosine phosphorylation compared with WT platelets stimulated with 3 μg/mL CRP and are representative of 3 independent experiments.
Protein tyrosine phosphorylation in CRP-stimulated platelets. WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were activated with 0.3 or 3 μg/mL CRP for 2 minutes at 37°C as indicated. Platelet lysates and anti–PLC-γ2 immunoprecipitates were subjected to SDS-PAGE and probed with the anti-phosphotyrosine antibody 4G10. The membrane was stripped and probed with anti–PLC-γ2 antibody as loading control. Results are percentage of PLC-γ2 tyrosine phosphorylation compared with WT platelets stimulated with 3 μg/mL CRP and are representative of 3 independent experiments.
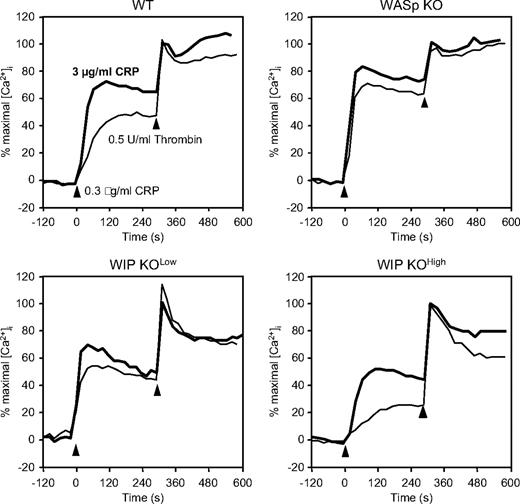
The functional consequence of PLC-γ2 phosphorylation was evaluated by measurements of intracellular calcium in Indo1-loaded platelets (Figure 6). Normal maximal calcium mobilization in response to thrombin was observed in WASp KO, WIP KOlow, and WIP KOhigh platelets, compared with WT. In contrast, differential calcium mobilization was observed in the KO platelets after stimulation with a low or high dose of CRP. WT platelets stimulated with 0.3 and 3 μg/mL CRP reached 50% and 75% of maximal calcium mobilization after 175 and 105 seconds, respectively. WASp KO platelets had enhanced calcium mobilization, both in peak intensity (70% and 85%) and time to peak (80 and 60 seconds). WIP KOlow platelets had normal calcium mobilization but reached their peak intensity after a shorter time interval (70 and 40 seconds) than WT platelets. The calcium response of WIP KOhigh platelets was poor, as the peak intensity only reached 25% and 50% of maximal response after stimulation with a low and high dose of CRP, respectively, consistent with their diminished PLC-γ2 phosphorylation.
Calcium mobilization in CRP-stimulated platelets. Indo1-AM-loaded WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were activated with 0.3 (normal) or 3 μg/mL CRP (bold) for 5 minutes followed by 0.5 U/mL thrombin for 5 minutes. Results are plotted as percentage of thrombin-induced calcium mobilization and are representative of 3 independent experiments.
Calcium mobilization in CRP-stimulated platelets. Indo1-AM-loaded WT, WASp KO, WIP KOlow, and WIP KOhigh platelets were activated with 0.3 (normal) or 3 μg/mL CRP (bold) for 5 minutes followed by 0.5 U/mL thrombin for 5 minutes. Results are plotted as percentage of thrombin-induced calcium mobilization and are representative of 3 independent experiments.
Limited GPVI shedding in WIP KO mice
Because anti-GPVI antibodies, such as JAQ1, can induce GPVI shedding from the surface of circulating platelets in mice and humans,38-40 the ability of JAQ1 to cause GPVI shedding in WIP KO mice was investigated (Figure 7). In vivo treatment of WT mice with JAQ1 resulted in the loss of 88% of surface GPVI on WT platelets after 48 hours, as reported previously.38,39 JAQ1 induced an intermediate GPVI shedding in WIP KOlow mice but had no effect on the level of GPVI expression in WIP KOhigh mice.
Limited GPVI shedding in WIP KO mice. WT, WIP KOlow, and WIP KOhigh mice were injected intraperitoneally (▩) or not (□) with 10 μg JAQ1, and platelets were analyzed by flow cytometry for GPVI expression after 48 hours. Results are plotted as mean fluorescence intensity and represent the mean of 2 independent mice.
Limited GPVI shedding in WIP KO mice. WT, WIP KOlow, and WIP KOhigh mice were injected intraperitoneally (▩) or not (□) with 10 μg JAQ1, and platelets were analyzed by flow cytometry for GPVI expression after 48 hours. Results are plotted as mean fluorescence intensity and represent the mean of 2 independent mice.
Discussion
Our results show that WASp and WIP constitutively associate in resting and in activated platelets. WIP KO platelets lack both WIP and WASp, whereas attenuated WIP expression is observed in WASp KO platelets. WASp KO and WIP KO mice have a moderate thrombocytopenia resulting in part from enhanced platelet clearance. WIP KO mice evolve platelet-associated IgAs, which normalize their platelet survival but diminish their platelet responses through the collagen receptor GPVI.
WASp immunoprecipitation depletes more than 95% of both WASp and WIP from mouse platelet lysates (Figure 1). The data are consistent with observations in T cells, in which WASp and WIP form a tight complex with 1:1 stoichiometry.7 Platelet activation with either CRP or thrombin did not disrupt the WIP-WASp complex, despite WIP phosphorylation on Ser488 by protein kinase C-θ.41 It was initially thought that WASp and WIP dissociate after WIP phosphorylation in T cells.7 Evidence has since shown that the apparent disruption of the complex was caused by the phosphorylation-mediated loss of binding to the specific anti-WIP antibody used, and no dissociation was observed with other antibodies directed against WIP N-terminus.42 In activated natural killer cells, WIP phosphorylation on Ser488 by protein kinase C-θ leads to formation of a multiprotein complex containing WIP, WASp, actin, and myosin IIA, rather than disruption of the WIP-WASp complex.43 Similarly, WASp immunoprecipitates and localizes with the adaptor proteins SLP-76, ADAP, Nck, and VASP during platelet spreading on fibrinogen.20,21
WASp could not be detected in WIP KO platelets using either immunoblot or intracellular flow cytometry analysis (Figure 1), consistent with observations in T cells.44,45 WIP expression was also reduced by approximately 45% in WASp KO platelets. Hence, expression of both proteins is required for each other's integrity. This observation is the first to show that WASp regulates WIP integrity in a cell, as WIP is normally expressed in WASp KO T cells.44 The limited protein synthetic capacity of platelets may contribute to the loss of WIP relative to T cells. The pathway mediating WIP and WASp degradation in platelets is unknown, although both WIP and WASp are substrates of the protease calpain in human and WT mouse platelets (data not shown).17
High levels of platelet-associated IgAs were detected in most but not all WIP KO mice (Figure 2). Thus, WIP KO platelets separate into 2 groups, WIP KOlow and WIP KOhigh, based on their levels of IgAs. Importantly, WASp was not detectable in WIP KOlow or WIP KOhigh platelets, showing that WASp degradation is the result of the lack of WIP, but not to the presence of low or high levels of platelet-associated IgAs. IgA binding to platelets did not correlate with increased plasma IgA levels because those were found to be normal in WIP KO mice, as described previously.32 IgAs are the principal immunoglobulin class in external secretions where they protect mucosal surfaces against inhaled and ingested pathogens.46 The reason for IgA binding to WIP KO platelets is unclear.
Although WIP KO platelets lack both WIP and WASp, independently of the presence of IgAs, WIP KOlow and WIP KOhigh mice had a moderate thrombocytopenia, similar to that of WASp KO mice14,15 (Figure 3). Our results contrast with those of Curcio et al who reported normal platelet counts in WIP KO mice.47 We do not have any explanation for the discrepancy, as mouse strain and gene deletion are the same in both studies. Both increased platelet clearance and decreased platelet production contribute to the lower blood platelet counts observed in WIP KO mice. Transfused WIP KOlow platelets cleared rapidly from the circulation (Figure 3), similar to WASp KO platelets and consistent with clinical observations in autologously transfused WAS patients.9 Surprisingly, WIP KOhigh platelets were cleared initially with a much slower rate than that of WIP KOlow platelets. It is tempting to speculate that (1) platelet-associated IgAs may protect against increased clearance by directly blocking binding sites for immune cells on platelets, and/or (2) the clearance system becomes initially saturated and therefore inhibited by high levels of IgAs bound to WIP KOhigh platelets. On the other hand, the platelet count is reduced in WIP KOhigh platelets, which stands in contrast to the clearance results of transfused platelets into WT mice. It is possible that IgAs negatively modulate platelet production by interfering with platelet precursors, megakaryocytes. Because platelets from WIP KO mice are deficient in both WIP and WASp, the role of WASp deficiency in their platelet abnormalities remains to be defined. The mechanisms responsible for the clearance of WASp KO and WIP KO platelets remain to be determined.
WIP KOhigh mice had GPVI signaling and functional defects (Figures 4,Figure 5–6). PLC-γ2 phosphorylation and calcium mobilization were severely impaired in CRP-stimulated WIP KOhigh platelets, correlating with decreased P-selectin expression, integrin αIIbβ3 activation, and actin assembly (Table 2). Furthermore, GPVI shedding induced by JAQ1 was inhibited by the presence of platelet-associated IgAs in WIP KOhigh mice (Figure 7). Together, the data indicate that IgAs target GPVI or a nearby receptor and negatively modulate GPVI signaling, either by blocking its interaction with its ligands, CRP and JAQ1, or changing its surface architecture and associated scaffolding or signaling proteins. There are 2 mechanisms by which IgAs could bind to GPVI: WIP KO mice may produce anti-GPVI IgAs or may have circulating IgA complexes that bind to GPVI. Normal GPVI expression levels in WIP KO platelets make the presence of anti-GPVI antibodies unlikely (Table 1), as it is generally accepted that anti-GPVI antibodies induce GPVI shedding in vivo.38-40 On the other hand, IgA deposits in the kidney of older WIP KO mice suggest the presence of circulating IgA complexes that could bind to GPVI.47 IgA-mediated nephropathy has been reported for WAS/XLT patients48,49 and WIP KO mice,47 but not for WASp KO mice. This difference may explain why WIP KO, but not WASp KO, mice have high levels of IgAs bound to their platelets and supports the hypothesis by Curcio et al that WIP KO mice have a phenotype closer to that of WAS than WASp KO mice.47
Summary of protein expression and CRP-mediated events in WASp KO, WIP KOlow, and WIP KOhigh platelets
| Protein expression/event . | WASp KO platelets . | WIP KOlow platelets . | WIP KOhigh platelets . |
|---|---|---|---|
| WASp expression | 0 | 0 | 0 |
| WIP expression | 55 | 0 | 0 |
| PLC-γ2 phosphorylation | 100 | 45-55 | 0-25 |
| Calcium mobilization | 115-140 | 100 | 50-70 |
| P-selectin expression | 110-120 | 100 | 60-80 |
| αIIbβ3 activation | 100-110 | 55-100 | 25-45 |
| Actin assembly | 110-125 | 95-125 | 35-75 |
| Protein expression/event . | WASp KO platelets . | WIP KOlow platelets . | WIP KOhigh platelets . |
|---|---|---|---|
| WASp expression | 0 | 0 | 0 |
| WIP expression | 55 | 0 | 0 |
| PLC-γ2 phosphorylation | 100 | 45-55 | 0-25 |
| Calcium mobilization | 115-140 | 100 | 50-70 |
| P-selectin expression | 110-120 | 100 | 60-80 |
| αIIbβ3 activation | 100-110 | 55-100 | 25-45 |
| Actin assembly | 110-125 | 95-125 | 35-75 |
Values are percentages relative to protein expression and CRP-mediated events in WT platelets.
PLC-γ2 phosphorylation mediated by CRP was diminished in WIP KOlow platelets (Figure 5), although to a lesser degree than that found for WIP KOhigh platelets (Table 2). This indicates that WIP positively modulates PLC-γ2 phosphorylation in platelets, even in the absence of high levels of platelet-associated IgAs. PLC-γ2 phosphorylation is reduced in WIP KO mast cells, resulting however in decreased calcium mobilization and impaired functional responses after FcϵRI ligation.50 By contrast, WIP KOlow platelets had normal functional responses downstream of GPVI (Figure 4). Partial PLC-γ2 phosphorylation could be sufficient for normal platelet functions. It is also possible that the enhanced calcium mobilization observed in platelets lacking WASp (Figure 6), ie, WASp KO and WIP KO platelets, compensates for the decreased PLC-γ2 phosphorylation in WIP KOlow platelets. The data also suggest that WASp negatively modulates calcium homeostasis in mouse platelets, as described for human platelets.51
In conclusion, WIP and WASp constitutively associate into a 1:1 complex in resting and in activated platelets. This association is absolutely required for WASp stability. WIP KO mice have a moderate thrombocytopenia resulting in part from enhanced platelet removal, similar to WASp KO mice. However, WIP KO, but not WASp KO, mice evolve platelet-associated IgAs that impair platelet responses initiated by the collagen receptor GPVI but normalize their survival. This study provides evidence for a role of the WIP-WASp complex in platelet signaling, function, and survival, and may contribute to a better understanding of the WAS-associated thrombocytopenia.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Narayanaswamy Ramesh, Inés Antón, María Dolores Gallego, Miguel de la Fuente, Séverine Le Bras, and Ignacio Molina for sharing reagents, Delphine Simon and Sarah Weber for technical help, and Dr François Maignen and Viktoria Rumjantseva for statistical expertise.
This work was supported by National Institutes of Health grants AI-035714 (R.S.G.), HL-056252, HL-056949 (J.H.H.), and HL-059561 (R.S.G., J.H.H., and H.F.).
National Institutes of Health
Authorship
Contribution: H.F. designed and performed research, analyzed data, and drafted the manuscript; M.P.M. performed research; K.M.H. and M.J.M. designed and performed research and analyzed data; R.S.G. and J.H.H. designed research and analyzed data; and all authors contributed to the writing of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hervé Falet, Division of Translational Medicine, Brigham and Women's Hospital, One Blackfan Cir, Karp 6, Boston, MA 02115; e-mail: hfalet@rics.bwh.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal