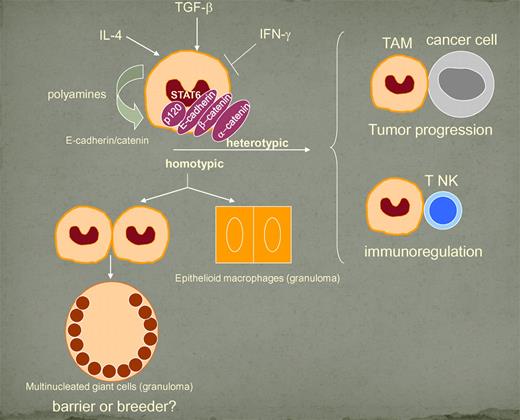
In this issue of Blood, Van den Bossche and colleagues identify E-cadherin as a new IL-4/IL-4Rα/STAT6-induced and polyamine-dependent marker of M2 polarized macrophages.1 This new pathway promotes formation of the E-cadherin/β-catenin complex, leading to homotypic and heterotypic cellular fusion (see figure) and plays an important role in guiding macrophage functions associated with Th2 immune responses.
Cell fusion leading to multinucleation is a characteristic feature of multicellular organisms organization, accompanying events such as fertilization (fusion of sperm and egg), placental morphogenesis (trophoblasts fusion with muscle development relies on the fusion of myoblasts), and osteoclast formation (macrophage fusion).2 Both homo- and heterotypic fusion of macrophages have been observed in disease, including granulomatous infections (eg, tuberculoid lesions, sarcoidosis, and schistosomiasis),2 giving rise to multinucleated giant cells (homotypic fusion of macrophages) and metastatic foci (fusion of myeloid cells and tumor cells).3 However, the key triggers of macrophage fusion, as well as the specialized functions of multinucleated giant cells, remain undetermined.
Regulation and significance of E-cadherin up-regulation. The scheme is based on the paper by Van den Bossche et al. The role of E-cadherin in formation of epithelioid cells is proposed.
Regulation and significance of E-cadherin up-regulation. The scheme is based on the paper by Van den Bossche et al. The role of E-cadherin in formation of epithelioid cells is proposed.
Macrophages are innate immune cells characterized by a high degree of functional plasticity, and, in response to environmental signals, these cells express classic or M1 and alternative or M2 polarized programs.4-6 In agreement with previous findings, showing an IL-4–dependent fusion of macrophage precursors,2,7 Van den Bossche et al indicate that macrophage fusion activity is part of a polarized M2 program.
Multinucleated cells form in granuloma macrophages and also acquire an epithelioid morphology. It is tempting to speculate that the E-cadherin up-regulation may underlie the acquisition of an epithelioid phenotype. This would mirror the E-cadherin down-regulation observed during the epithelial-mesenchymal transition (EMT) event preceding metastasis.8
The report by Van den Bossche et al sheds new light on molecular mechanisms underlying homotypic and heterotypic interaction of macrophages and formation of multinucleated cells. It also raises a number of questions. It remains unclear how the diverse morphology of multinucleated cells, as seen for instance in different forms of leprosy lesions,9 affects disease progression. The significance of multinucleated cells and granuloma itself in antimicrobial resistance is still controversial.10,11 Although a general consensus exists on the containment of pathogens as a specialized function of granulomas, epithelioid cells and multinucleated giant cells express high levels of FasL, thus creating a potential immune privileged site for mycobacteria.12
Tumor-associated macrophages (TAM) generally have an M2 phenotype13 ; it remains unclear whether the propensity to fuse with tumor cells contributes to tumor promotion in cancer-related inflammation. This potential involvement of E-cadherin expression in M2-polarized inflammation associated with cancers supports this possibility. Fusion of myeloid cells and malignant cells has been suggested to generate aggressive cancer cell clones and to be a source of myeloid traits in cancer/myeloid cell hybrids.3
Irrespective of its pathophysiologic significance, the finding of up-regulation of the E-cadherin/catenin complex in M2 macrophages may offer an invaluable tool to dissect the presence and significance of these cells in human pathology.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal