Abstract
Studies of zebrafish hematopoiesis have been largely performed using mutagenesis approaches and retrospective analyses based upon gene expression patterns in whole embryos. We previously developed transplantation assays to test the repopulation potentials of candidate hematopoietic progenitor cells. We have been impaired, however, in determining cellular differentiation potentials by a lack of short-term functional assays. To enable more precise analyses of hematopoietic progenitor cells, we have created zebrafish kidney stromal (ZKS) cell lines. Culture of adult whole kidney marrow with ZKS cells results in the maintenance and expansion of hematopoietic precursor cells. Hematopoietic growth is dependent upon ZKS cells, and we show that ZKS cells express many growth factors and ligands previously demonstrated to be important in maintaining mammalian hematopoietic cells. In the absence of exogenous growth factors, ZKS cells maintain early hematopoietic precursors and support differentiation of lymphoid and myeloid cells. With the addition of zebrafish erythropoietin, ZKS cells also support the differentiation of erythroid precursors. These conditions have enabled the ability to ascertain more precisely the points at which hematopoietic mutants are defective. The development of robust in vitro assays now provide the means to track defined, functional outcomes for prospectively isolated blood cell subsets in the zebrafish.
Introduction
Hematopoiesis has served as the paradigmatic tissue stem cell system since the pioneering studies of Till and McCulloch suggested the presence of rare, multipotent hematopoietic stem cells (HSCs) in murine bone marrow.1,2 The advent of bone marrow transplantation (BMT) showed that these HSCs could sustain donor-derived hematopoiesis for life in both mice and humans.1,3-5 The development of in vitro culture technologies subsequently suggested that HSCs commit to downstream myeloerythroid fates through a stepwise progression of lineage-restricted progenitor cells.6-9 The lineage relationships suggested by the studies of Metcalf and colleagues have recently been solidified through the prospective isolation of lineage-restricted progenitors at each of the major branch points of the hematopoietic hierarchy
The precision afforded by in vitro culture systems has led to the isolation of the common lymphoid progenitor (CLP)10 and common myeloid progenitor (CMP)11 that, at the single cell level generate B lymphocytes and T lymphocytes and the major myeloerythroid fates, respectively. Similarly, oligopotent progenitors, including the megakaryocyte/erythrocyte-restricted progenitor (MEP)11 and granulocyte/monocyte-restricted progenitor (GMP),11 and monopotent precursors, such as the megakaryocyte-restricted precursor (MkP)12 and eosinophil-restricted precursor (EoP),13 have been demonstrated to arise downstream of these multipotent progenitors by use of sensitive culture techniques. The long-term culture initiating cell (LTCIC) assay has served as a surrogate assay for human HSCs in the absence of autologous transplantation.14 A variety of transformation culture assays have also been used to better understand the multiple steps to cancer.15-17
To enable similar approaches in the zebrafish, we have developed an in vitro culture system. The zebrafish has proven an excellent system in which to dissect the genetic bases of blood cell formation through forward mutagenesis approaches (reviewed in de Jong and Zon18 ). Experimental methodologies to functionally test resulting mutants, however, have been lacking. We have recently described the prospective isolation of the first hematopoietic stem and progenitor cells to arise in the zebrafish embryo.19,20 We have also developed a variety of hematopoietic cell transplantation assays to provide the most stringent test of HSC function, long-term reconstitution.21,22 To complement these advances, we have developed the means to maintain and grow hematopoietic cells in vitro through the creation of zebrafish kidney stromal (ZKS) cell lines.
We show that growth and survival of zebrafish hematopoietic cells is dependent upon coculture with ZKS cells, and that addition of recently identified zebrafish growth factors, such as zebrafish erythropoietin (zEpo), enables multilineage differentiation from whole kidney marrow (WKM) cells. In addition, we have analyzed genetic mutants with erythroid defects, demonstrating that a mutation in iron transport can be rescued via in vitro delivery of membrane-permeable iron. Together, these studies should enable a better understanding of the biology of zebrafish hematopoietic stem and progenitor cells, and of the points along the hematopoietic hierarchy at which blood mutants are defective.
Methods
Generation of ZKS cells
Kidneys were isolated from AB* WT fish, bleached for 5 minutes in 0.000525% sodium hypochlorite (Fisher Scientific), then rinsed in sterile phosphate-buffered saline (PBS; Mediatech). Tissue was mechanically dissociated by trituration then filtered through a 40-μm filter (BD Biosciences). Flow-through was discarded, and the remaining kidney tissue was cultured in 12.5-cm2 vented flasks (Corning Incorporated Life Sciences) at 32°C and 5% CO2. ZKS cells were maintained in culture media consisting of 10% embryonic stem (ES) cell qualified fetal bovine serum (FBS; ATCC), 55% L-15, 32.5% Dulbecco modified Eagle medium (DMEM), and 12.5% Ham F-12 (Mediatech). Media was supplemented with 150 mg/L sodium bicarbonate, 2% penicillin/streptomycin (10 U/mL stock), 1.5% N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 1% l-glutamine, and 0.1 mg/mL gentamycin (all from Mediatech). Cells were grown until 60%-80% confluence, then trypsinized (0.25%; Invitrogen) for 5 minutes and split 1:10 to passage. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Diego (UCSD).
Morphologic characterization of ZKS cells
ZKS cells were grown on glass coverslips in a 24-well culture plate (BD Biosciences). After removal from culture, slides were fixed and stained with May-Grünwald Giemsa (Sigma-Aldrich) and visualized with an Olympus BX51 microscope (Olympus). To visualize cells using transmission electron microscopy, ZKS cells grown in 35-mm plastic culture dishes were fixed in 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 7.3), buffer washed, and fixed in 1% osmium tetroxide in 0.1 M Na cacodylate buffer. They were subsequently treated with 0.5% tannic acid, followed by 1% sodium sulfate in cacodylate buffer, and then dehydrated in graded ethanol series. The cells were cleared in 2-hydroxypropyl methacrylate (HPMA; Ladd Research) and embedded in LX112 resin. After overnight polymerization at 60°C, small pieces of resin were attached to blank blocks using SuperGlue. Thin sections (70 nm) were cut on a Reichert Ultracut E (Leica) using a diamond knife (Diatome; Electron Microscopy Sciences), mounted on parlodion-coated, copper slot grids, and stained in uranyl acetate and lead citrate. Sections were examined on a Philips CM100 transmission electron microscope (TEM; FEI Company) and data-documented on a Megaview III charge-coupled device (CCD) camera (Soft Imaging System Corp).
Zebrafish stocks and embryos
Isolation of WKM
Zebrafish kidney was isolated under sterile conditions and mechanically dissociated by trituration. Tissue was passaged through a 40-μm filter, rinsed in sterile PBS, then centrifuged for 10 minutes at 300g. Supernatant was aspirated, and cells were resuspended in culture media. Cell counts were performed with the use of a bright line hemacytometer (Hausser Scientific), using trypan blue (Invitrogen) to assess viability.
Fluorescence-activated cell sorting
βactin:GFP WKM was isolated, resuspended in PBS with 0.9 × FBS, and sorted on a FACSAria flow cytometer (BD Biosciences).
Cytology
Hematopoietic cells were concentrated by cytocentrifugation at 250g for 5 minutes onto glass slides using a Shandon Cytospin 4 (Thermo Fisher Scientific). Slides were fixed and stained with May-Grünwald Giemsa (Sigma-Aldrich). Briefly, the slides were air-dried and stained in May-Grünwald solution for 10 minutes. Then, the slides were placed in a 1:5 dilution of Giemsa in distilled water (dH2O) for 20 minutes, rinsed in dH2O, and allowed to dry before covering with a coverslip. For histochemical analysis of hemoglobin, o-dianisidine staining was performed. Cells were concentrated in 50 μL media, and 100 μL fresh o-dianisidine (Sigma-Aldrich) working solution (100 μL o-dianisidine solution [50 mg o-dianisidine in 35 mL 100% ethanol (EtOH)], 25 μL NaOAc, pH 4.5, 5 μL 30% H2O2) was added, and cells were incubated in the dark for 10 minutes before being cytospun. Slides were then fixed/stained with May-Grünwald solution for 10 minutes, dried, and covered with a coverslip.
Reverse transcription polymerase chain reaction analysis
For reverse transcription polymerase chain reaction (RT-PCR) analysis, RNA was isolated from hematopoietic cells using the RNeasy kit (QIAGEN), and cDNA was generated with a Superscript III RT-PCR kit (Invitrogen). Primers used for characterization of ZKS cells are listed in Table 1. Primers used in hematopoietic maintenance assays were pax5-forward: CTG ATT ACA AAC GCC AAA AC, pax5-reverse: CTA AAT TAT GCG CAG AAA CG; lck-forward: TAC GTA AAC ATG GGG AAC TG, lck-reverse: TCT TCT CCC CTT TCT CAA AC; igM-forward: AGC TTC TCT AGC TCC ACC AG, igM-reverse: GCT AAA CAC ATG AAG GTT GC. Primers to detect zebrafish ef1α,19 gata3,20 cd45,20 rag2,24 mpx,25 pu.1,25 and gata125 have previously been described.
Primer sets used for RT-PCR characterization
| Cytokine . | Accesson no. . | Definition . | Forward primer . | Reverse primer . | Product size, bp . |
|---|---|---|---|---|---|
| zkitla | AY929068 | Danio rerio kit ligand a* | GGATTCAATGCTTGACTTTG | TGTACTATGTTGCGCTGATG | 205 |
| zkitlb | AY929069 | Danio rerio kit ligand b* | CTTGATTGCCGTTCAGTTAG | TGACCGGAGATTGTAGACAC | 107 |
| zbmp 1 | DQ424857 | Danio rerio bone morphogenic protein 1* | GGATGGATATTGGAGGAAAG | CTTTGTTCGGTCTGTAATCG | 230 |
| zil-1 la | BN000717 | Danio rerio interleukin-l la* | GACAAGCTGAGCAATCAGAC | GGAGCTGAGAAAGAGTAGGC | 172 |
| zil-1lb | BN000718 | Danio rerio interleukin-11b* | TTGAACATTCGCTATCATCC | GAGTAATCGTTCCCCAATTC | 166 |
| zdla | NM 130954 | Danio rerio δA† | ACGACGATTTGAGTATGACG | GGGATTGGCACTTTATATCC | 186 |
| zdlb | NM 130958 | Danio rerio δB† | TTCCGTGTTTAATGATTTGG | CACTCCACAGAAACTCTTGC | 158 |
| zdlc | NM 130944 | Danio rerio δC† | TGGTGGACTACAATCTGAGC | ACCTCAGTAGCAAACACACG | 169 |
| zdld | NM 130955 | Danio rerio δD† | TGAAACATTACCAAGCCAAC | GCAGTGCTTCAATAATCAGC | 189 |
| zdll4 | NM 001079835 | Danio rerio δ-like 4† | CTCTTTCAGCACACCAATTC | TGAACATCCTGAGACCATTC | 189 |
| zjagla | BC162468 | Danio rerio jagged 1a† | TGATTGGTGGATACTTCTGC | AATCCATTGAGTGTGTCCTG | 238 |
| zjaglb | NM 131863 | Danio rerio jagged 1b† | CTGTGAGCCATCTTCTTCAG | AGCAAAGGAACCAGGTAGTC | 213 |
| zjag2 | BC163889 | Danio rerio jagged 2† | AATGACTGTGTGAGCAATCC | GTCATTGACCAGATCCACAC | 174 |
| zil-12a | NM 001007107 | Danio rerio interleukin 12a‡ | GTGAGTCTGCTGAAGGAGTG | AGTGACATCATTTCCTGTGC | 167 |
| zil-15–2 | AB194243 | Danio rerio interleukin 15–2‡ | CCAAGTCCACAATTACATGC | TCTTTGTAGAGCTCGCAGAC | 166 |
| zil-10 | AY887900 | Danio rerio interleukin 10‡ | ATGAATCCAACGATGACTTG | TCTTGCATTTCACCATATCC | 222 |
| zil-22 | NM 001020792 | Danio rerio interleukin 22‡ | CTACCTGCGATATGAAGTGC | GAAATATGGAAGCAGTCGTG | 173 |
| zil-26 | NM 001020799 | Danio rerio interleukin 26 | TGAAAAGATGTGGGATGAAC | ACTGATCCACAGCAAAACAC | 214 |
| zepo | NM001 115128 | Danio rerio erythropoietin transcript variant 1‡ | ACTTGTAAGGACGATTGCAG | TATCTGTAATGAGCCGATGG | 156 |
| zfgfl1 | N M200760 | Danio rerio fibroblast growth factor 1‡ | ATACTGCGCATAAAAGCAAC | AGTGGTTTTCCTCCATCTTC | 154 |
| zfgf2l | N M001045324 | Danio rerio fibroblast growth factor 21‡ | CGGTGGTGTATGTATGTTCC | GTAGCTGCACTCTGGATGAC | 203 |
| Cytokine . | Accesson no. . | Definition . | Forward primer . | Reverse primer . | Product size, bp . |
|---|---|---|---|---|---|
| zkitla | AY929068 | Danio rerio kit ligand a* | GGATTCAATGCTTGACTTTG | TGTACTATGTTGCGCTGATG | 205 |
| zkitlb | AY929069 | Danio rerio kit ligand b* | CTTGATTGCCGTTCAGTTAG | TGACCGGAGATTGTAGACAC | 107 |
| zbmp 1 | DQ424857 | Danio rerio bone morphogenic protein 1* | GGATGGATATTGGAGGAAAG | CTTTGTTCGGTCTGTAATCG | 230 |
| zil-1 la | BN000717 | Danio rerio interleukin-l la* | GACAAGCTGAGCAATCAGAC | GGAGCTGAGAAAGAGTAGGC | 172 |
| zil-1lb | BN000718 | Danio rerio interleukin-11b* | TTGAACATTCGCTATCATCC | GAGTAATCGTTCCCCAATTC | 166 |
| zdla | NM 130954 | Danio rerio δA† | ACGACGATTTGAGTATGACG | GGGATTGGCACTTTATATCC | 186 |
| zdlb | NM 130958 | Danio rerio δB† | TTCCGTGTTTAATGATTTGG | CACTCCACAGAAACTCTTGC | 158 |
| zdlc | NM 130944 | Danio rerio δC† | TGGTGGACTACAATCTGAGC | ACCTCAGTAGCAAACACACG | 169 |
| zdld | NM 130955 | Danio rerio δD† | TGAAACATTACCAAGCCAAC | GCAGTGCTTCAATAATCAGC | 189 |
| zdll4 | NM 001079835 | Danio rerio δ-like 4† | CTCTTTCAGCACACCAATTC | TGAACATCCTGAGACCATTC | 189 |
| zjagla | BC162468 | Danio rerio jagged 1a† | TGATTGGTGGATACTTCTGC | AATCCATTGAGTGTGTCCTG | 238 |
| zjaglb | NM 131863 | Danio rerio jagged 1b† | CTGTGAGCCATCTTCTTCAG | AGCAAAGGAACCAGGTAGTC | 213 |
| zjag2 | BC163889 | Danio rerio jagged 2† | AATGACTGTGTGAGCAATCC | GTCATTGACCAGATCCACAC | 174 |
| zil-12a | NM 001007107 | Danio rerio interleukin 12a‡ | GTGAGTCTGCTGAAGGAGTG | AGTGACATCATTTCCTGTGC | 167 |
| zil-15–2 | AB194243 | Danio rerio interleukin 15–2‡ | CCAAGTCCACAATTACATGC | TCTTTGTAGAGCTCGCAGAC | 166 |
| zil-10 | AY887900 | Danio rerio interleukin 10‡ | ATGAATCCAACGATGACTTG | TCTTGCATTTCACCATATCC | 222 |
| zil-22 | NM 001020792 | Danio rerio interleukin 22‡ | CTACCTGCGATATGAAGTGC | GAAATATGGAAGCAGTCGTG | 173 |
| zil-26 | NM 001020799 | Danio rerio interleukin 26 | TGAAAAGATGTGGGATGAAC | ACTGATCCACAGCAAAACAC | 214 |
| zepo | NM001 115128 | Danio rerio erythropoietin transcript variant 1‡ | ACTTGTAAGGACGATTGCAG | TATCTGTAATGAGCCGATGG | 156 |
| zfgfl1 | N M200760 | Danio rerio fibroblast growth factor 1‡ | ATACTGCGCATAAAAGCAAC | AGTGGTTTTCCTCCATCTTC | 154 |
| zfgf2l | N M001045324 | Danio rerio fibroblast growth factor 21‡ | CGGTGGTGTATGTATGTTCC | GTAGCTGCACTCTGGATGAC | 203 |
Primer sets used for RT-PCR characterization of ZKS cells. Gene names are listed at left, followed by accession number and definition listed in the database. Forward and reverse primer sequences are listed 5′ to 3′, and product size is listed at far right. Since not all products span exons, negative RT controls were performed alongside all reactions.
Transcripts involved in proliferation and differentiation of hematopoietic stem and progenitor cells.
Transcripts involved in signaling, lymphoid development, and differentiation.
Transcripts involved in lineage-specific signaling, maintenance, and differentiation.
Proliferation, maintenance, and differentiation analyses
Before culture, isolated WKM was washed twice with 0.1% bovine serum albumin (BSA) to remove FBS. Cells were suspended in 0.1% BSA, and 2 μL/mL 5 mM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) were added while mixing. Cells were incubated in the dark for 10 minutes, and culture media, with an additional 10% FBS, was used to wash cells twice. Cells were resuspended in complete ZKS media. WKM cultures were grown at 32°C and 5% CO2 on 80%-90% confluent ZKS cells and harvested by gentle pipeting to obtain single-cell suspensions without disruption of ZKS monolayer. Samples were resuspended in PBS and counted. A fraction of the hematopoietic cells were then used for RT-PCR analysis. Another fraction was cytospun and stained. Flow cytometry analysis was conducted on a LSRII (BD Bioscience), using propidium iodide (Sigma-Aldrich) to exclude dead cells. Data were analyzed using FlowJo software (TreeStar).
Expression and purification of recombinant zEpo
The region corresponding to the mature form of zepo was reamplified by PCR from zepo cDNA26 using primers zepo-1: CGG GAT CCT CCC CAT TAC GCC CCA TCT GTG, and zepo2: CGG GAT CCT CAG CTG ACA CCC TGT CGA CA. The 499-bp PCR product was digested with BamHI restriction endonuclease, isolated by gel electrophoresis, and ligated into the prokaryotic expression vector pETH2a. This generated the expression plasmid pHis-zEpo, which was used for transformation of T7 polymerase expressing BL21(DE3) cells. Induction of 19 kDa His-zEpo protein synthesis by isopropyl-β-D-thiogalactopyranoside (IPTG) and purification of recombinant protein on a Ni2+-NTA agarose column was done according to the manufacturer's procedure (Qiagen) with some modifications as published before.27
Cytokine assay
WKM cultures were grown at 32°C and 5% CO2 with purified, recombinant zEpo, 1% carp serum, or 1:1000 ferric salicylaldehyde isonicotinoyl hydrazone (Fe-SIH, catalog number I3153; Sigma-Aldrich), then harvested by gentle pipeting to obtain single-cell suspensions without disruption of ZKS monolayer. Samples were resuspended in PBS for counting. A fraction of the hematopoietic cells were cytospun and stained with o-dianisidine and May-Grünwald Giemsa. Another fraction was subjected to flow cytometry analysis.
Results
ZKS cells are derived from adult zebrafish kidney stroma
Since the kidney is the main hematopoietic site in adult zebrafish,28 we generated stromal cell lines from dissociated kidney cells. Kidneys were removed from fish, gently bleached, and physically dissociated by trituration. After trituration, the cells were passed through a 40-μM filter and rinsed thoroughly with media. The flow-through contains WKM, which is comprised mainly of hematopoietic cells.21 Non-flow-through tissue was washed from the filter and allowed to grow in gas vacuum plasma-treated tissue culture dishes until confluent. Upon reaching confluence several weeks later, these cells were trypsinized and passaged for further culture (see “Methods”).
The optimum culture conditions for ZKS cells were determined to be 32°C, in a humidified 5% CO2 environment. Cells divided slower than mammalian stromal cells (once every 36-48 hours), probably due to their growth at cooler temperatures. While ZKS cells appeared healthy at the physiologic temperature of zebrafish (28°C), they divided faster, and appeared similarly healthy at 32°C. Raising the temperature to 37°C for extended periods of time was toxic to ZKS cells. Multiple lines have been maintained under these conditions for more than 200 passages. None of the cell lines exhibited crisis at any point and were capable of repeated passage in culture. ZKS cells were density-dependent and grew optimally when maintained at 25%-50% confluence.
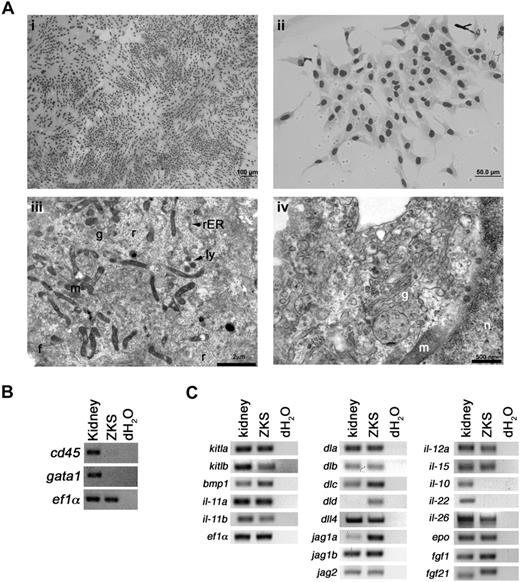
ZKS cells displayed a fibroblastic appearance when observed by May-Grünwald Giemsa staining (Figure 1Ai-ii). To confirm that these cells were stromal and not hematopoietic, ZKS cells were analyzed by TEM. ZKS cells displayed several ultrastructural characteristics of teleost kidney fibroblasts.29 They were irregularly branched, with large processes containing extensive filament bundles (not shown), and they contained a heterochromatic nucleus and a prominent endomembrane system, including well-defined Golgi apparati, rough endoplasmic reticuli, and secretory vesicles (Figure 1Aiii-iv). We never observed cellular morphologies indicative of hematopoietic cell types in ZKS cultures. ZKS cells did not show expression of the pan leukocyte marker cd45 or the red blood cell gene gata1 by RT-PCR, confirming no hematopoietic cell types were present in these cultures (Figure 1B).
ZKS cells are primary kidney stromal cells. (A) Morphologic characterization of ZKS cells. (i,ii) May-Grünwald/Giemsa staining of ZKS cells on glass coverslips reveals stromal morphology. Images in subpanel i were photographed at 100× at 100% confluence; those in subpanel ii were photographed at 400× at low confluence. Transmission electron micrographs of ZKS stromal cells show ultrastructural features of fibroblasts. (iii) Close-up of ZKS cell, 7900×. Note abundant mitochondria and free ribosomes. (iv) Close-up of a ZKS cell, 19 000×. Note abundant Golgi and rough endoplasmic reticular network. n indicates nucleus; g, Golgi; r, ribosome; rER, rough endoplasmic reticulum; ly, lysosome; f, filaments; and m, mitochondria. (B) ZKS cells do not express hematopoietic specific transcripts. Gene expression analysis of ZKS cells by RT-PCR for the pan leukocytic transcript cd45 and erythroid specific gata1. Gene names listed at left. Whole kidney used as positive controls, dH2O as negative controls. (C) Gene expression analysis of ZKS cells by RT-PCR for transcripts involved in proliferation and differentiation of progenitor cells (left column), niche signaling and lymphoid development (middle column), and lineage-specific signaling, maintenance, and differentiation (right column). Gene names listed at left. Whole kidney used as positive controls, dH2O as negative controls.
ZKS cells are primary kidney stromal cells. (A) Morphologic characterization of ZKS cells. (i,ii) May-Grünwald/Giemsa staining of ZKS cells on glass coverslips reveals stromal morphology. Images in subpanel i were photographed at 100× at 100% confluence; those in subpanel ii were photographed at 400× at low confluence. Transmission electron micrographs of ZKS stromal cells show ultrastructural features of fibroblasts. (iii) Close-up of ZKS cell, 7900×. Note abundant mitochondria and free ribosomes. (iv) Close-up of a ZKS cell, 19 000×. Note abundant Golgi and rough endoplasmic reticular network. n indicates nucleus; g, Golgi; r, ribosome; rER, rough endoplasmic reticulum; ly, lysosome; f, filaments; and m, mitochondria. (B) ZKS cells do not express hematopoietic specific transcripts. Gene expression analysis of ZKS cells by RT-PCR for the pan leukocytic transcript cd45 and erythroid specific gata1. Gene names listed at left. Whole kidney used as positive controls, dH2O as negative controls. (C) Gene expression analysis of ZKS cells by RT-PCR for transcripts involved in proliferation and differentiation of progenitor cells (left column), niche signaling and lymphoid development (middle column), and lineage-specific signaling, maintenance, and differentiation (right column). Gene names listed at left. Whole kidney used as positive controls, dH2O as negative controls.
RT-PCR expression analyses of genes previously shown to play important roles in hematopoietic maintenance, development, or proliferation demonstrated that ZKS cells expressed many transcripts known to support hematopoietic progenitor cells (Figure 1C left column). In addition, ZKS cells expressed Notch ligands, known to play a role in HSC niche signaling, lymphocyte development, and maturation (Figure 1C middle column). Finally, ZKS cells expressed many cytokines involved in lineage-specific signaling, maintenance, and differentiation (Figure 1C right column; see Table 1 for primer sequences).
ZKS cells support hematopoiesis
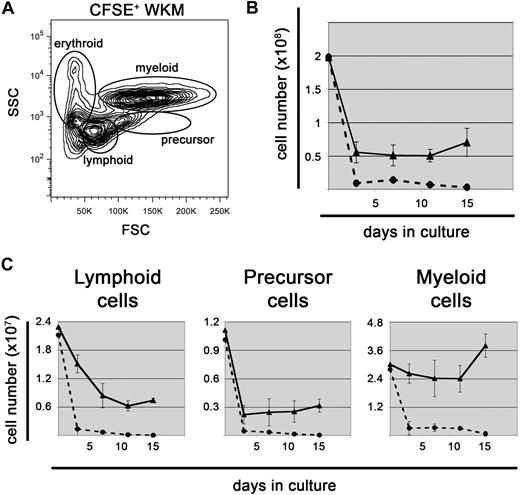
To determine whether ZKS cells would support hematopoiesis, we isolated WKM from WT adult zebrafish and plated it on confluent ZKS cells. To ensure our measurements were specific to hematopoietic cells and not confounded by stromal elements, we labeled WKM with CFSE dye before plating. CFSE diffuses through the plasma membrane, after which it becomes modified in the cytoplasm to a polar compound that serves as a fluorescent, membrane-impermeable dye that is useful for cell tracing.30 Furthermore, CFSE is inherited by daughter cells after cell division and is not transferred to adjacent cells.31-33 After hematopoietic cells were labeled with CFSE, we plated half of the cells on ZKS stroma and half in complete media without stroma. WKM after CFSE labeling retained the same light scatter characteristics as unlabeled WKM, allowing us to distinguish between different lineages of hematopoietic cells (Figure 2A). Live cell counts showed that WKM cultured with ZKS cells survived and proliferated over time, whereas cells cultured in media alone rapidly died (Figure 2B). Stromal cocultures resulted in at least a 3-fold increase in live cell number at all time points compared with cultures without stroma. Separate experiments confirmed that hematopoietic cultures survived on ZKS stroma over the course of 30 days, while cells cultured without stroma senesced by 15 days (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
ZKS cells support hematopoiesis. (A) All hematopoietic cell populations are represented in CFSE+ cultures. Representative flow cytometric light scatter profile showing the different populations of cells present in CFSE-labeled WKM at day 0, before plating on ZKS. (B) ZKS support cell survival. Total cell numbers of WKM cultured with ZKS cells (—▲—) and without (---●---) over time (n = 4). (C) Lymphoid, precursor, and myeloid cells all survive over time. Total number of lymphoid (left), precursor (center), and myeloid (right) cells present in WKM cultures with ZKS cells (—▲—) and without (---●---; n = 4). In all cases, total cell numbers are given at left, and days in culture are given at bottom. In all cases, the graph depicts the mean and SD for 4 independent replicates.
ZKS cells support hematopoiesis. (A) All hematopoietic cell populations are represented in CFSE+ cultures. Representative flow cytometric light scatter profile showing the different populations of cells present in CFSE-labeled WKM at day 0, before plating on ZKS. (B) ZKS support cell survival. Total cell numbers of WKM cultured with ZKS cells (—▲—) and without (---●---) over time (n = 4). (C) Lymphoid, precursor, and myeloid cells all survive over time. Total number of lymphoid (left), precursor (center), and myeloid (right) cells present in WKM cultures with ZKS cells (—▲—) and without (---●---; n = 4). In all cases, total cell numbers are given at left, and days in culture are given at bottom. In all cases, the graph depicts the mean and SD for 4 independent replicates.
CFSE+ cells were examined by flow cytometry, and the percentage of cells within each lineage gate outlined in Figure 2A was multiplied by the total cell counts to obtain the actual numbers of erythroid, lymphoid, precursor, and myeloid cells. Compared with cells cultured without stroma, lymphoid, precursor, and myeloid cells were maintained by ZKS cells. To confirm that these lineages survived over time in ZKS cultures, we analyzed cellular morphologies on cytocentrifuge preparations (Figure 3A). Lymphoid, precursor, and myeloid cells were observed at all time points. In contrast, erythroid cells were not observed after only 3 days after culture, suggesting that they did not survive in these culture conditions (Figure 3A). Expression of the pan hematopoietic marker cd45 confirmed that cells present on the ZKS cultures were hematopoietic (Figure 3B), in contrast to the ZKS cells themselves (Figure 1B). The expression of the myeloid-specific transcripts pu.1 and mpx and the expression of the lymphoid-specific genes pax5, igM, gata3, lck, and rag2 confirmed the presence of myeloid and lymphoid cells. In addition, the loss of the erythroid-specific transcript gata1 (Figure 3B) confirmed the observation that erythroid cells are not maintained well in ZKS cultures (Figure 3A). Differential cell counts of cytocentrifuge preparations over 11 days of culture support the flow cytometric data (Figures 3C and 2C).
ZKS cells support hematopoiesis; morphology and gene expression data. (A) Representative hematopoietic cell types recovered from in vitro culture on ZKS stroma. May-Grünwald/Giemsa stained cells were photographed at 1000×. After photographing, cells were cut and pasted from multiple fields to create composite image. (B) Gene expression analysis of cells by RT-PCR recovered from ZKS cultures for the panleukocytic transcript cd45, the erythroid-specific transcript gata1, myeloid-specific transcripts pu.1 and mpx, and the lymphoid-specific transcripts pax5, igM, gata3, lck, and rag2. Gene names listed at left, days in culture on ZKS stroma at top. Whole kidney used as positive controls, dH2O as negative controls. (C) Percentages of lineages in recovered cell populations over 11 days of culture. Percent population listed at left, days in culture on ZKS stroma at bottom. Green indicates myeloid; purple, precursor; blue, lymphoid; and red, erythroid.
ZKS cells support hematopoiesis; morphology and gene expression data. (A) Representative hematopoietic cell types recovered from in vitro culture on ZKS stroma. May-Grünwald/Giemsa stained cells were photographed at 1000×. After photographing, cells were cut and pasted from multiple fields to create composite image. (B) Gene expression analysis of cells by RT-PCR recovered from ZKS cultures for the panleukocytic transcript cd45, the erythroid-specific transcript gata1, myeloid-specific transcripts pu.1 and mpx, and the lymphoid-specific transcripts pax5, igM, gata3, lck, and rag2. Gene names listed at left, days in culture on ZKS stroma at top. Whole kidney used as positive controls, dH2O as negative controls. (C) Percentages of lineages in recovered cell populations over 11 days of culture. Percent population listed at left, days in culture on ZKS stroma at bottom. Green indicates myeloid; purple, precursor; blue, lymphoid; and red, erythroid.
ZKS cells support proliferation of hematopoietic cells
In addition to serving as an effective labeling tool for hematopoietic cells, CFSE can be used to examine cell division and proliferation. CFSE has the unique property of being split equally between daughter cells during division, thus each round of cell division reduces the initial relative fluorescence intensity by half.31-33 WKM labeled with CFSE and cultured on ZKS cells showed more cell division by day 3 than in cultures with no stroma (supplemental Figure 2). Over 11 days, CFSE-labeled cells divided only once, whereas cells cultured on ZKS proceeded through many rounds of division. Examination of CFSE+ cells further showed that the majority of cells that divided once without stroma were myeloid (supplemental Figure 2, green). By contrast, WKM cultured on ZKS showed proliferation of myeloid (supplemental Figure 2, green), lymphoid (blue), and precursor (purple) fractions, with the lymphoid and precursor fractions dividing more. These results demonstrate that ZKS cells both maintain and support proliferation of adult leukocyte subsets.
ZKS cells support differentiation of hematopoietic precursors
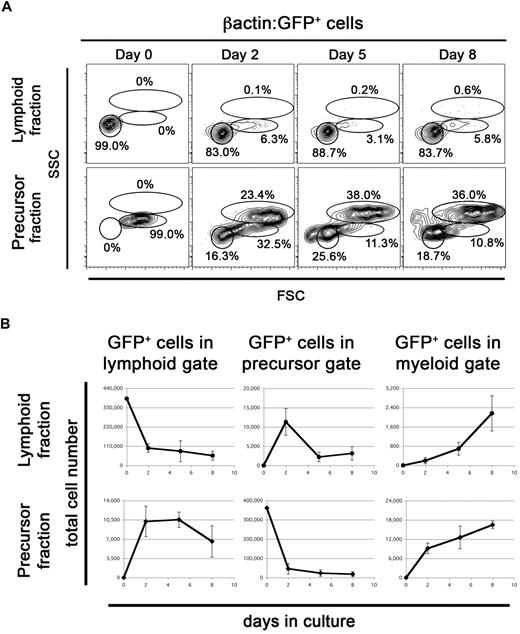
To determine whether ZKS cells support differentiation of hematopoietic precursors, we isolated and plated the lymphoid and precursor fractions from WKM of adult βactin:GFP fish21 (Figure 4A) on top of confluent ZKS stroma. The lymphoid fraction of adult zebrafish WKM contains B lymphocytes, lymphoid precursors, and rare HSCs.21 The precursor fraction of zebrafish WKM contains myeloid, lymphoid, and erythroid precursors.21,34 After 5 days of culture, the lymphoid and precursor fractions generated green fluorescent protein–positive (GFP+) colonies on the ZKS stromal surface (not shown).
ZKS cells stimulate differentiation of hematopoietic precursors. (A) Populations containing hematopoietic progenitors differentiate in culture on ZKS cells. Lymphoid fraction from βactin:GFP+ WKM, which contains rare HSCs, was sorted, plated, and analyzed after culture for the time period indicated at top (top row). βactin:GFP+ WKM precursor fraction, which contains erythroid, myeloid, and lymphoid progenitors was sorted, plated, and analyzed after culture for time period indicated at top (bottom row). Reanalysis of sorted GFP+ lymphoid and precursor fractions from βactin:GFP+ WKM (day 0) shows 99% purity. Data are representative of 3 independent experiments. Scatter gates drawn as in Figure 2. (B) Total number of GFP+ lymphoid (left), precursor (center), and myeloid (right) cells present over time from cultured βactin:GFP+ lymphoid (top, circles) and precursor (bottom, diamonds) fractions (n = 3). Each graph depicts the mean and SD for 3 independent replicates.
ZKS cells stimulate differentiation of hematopoietic precursors. (A) Populations containing hematopoietic progenitors differentiate in culture on ZKS cells. Lymphoid fraction from βactin:GFP+ WKM, which contains rare HSCs, was sorted, plated, and analyzed after culture for the time period indicated at top (top row). βactin:GFP+ WKM precursor fraction, which contains erythroid, myeloid, and lymphoid progenitors was sorted, plated, and analyzed after culture for time period indicated at top (bottom row). Reanalysis of sorted GFP+ lymphoid and precursor fractions from βactin:GFP+ WKM (day 0) shows 99% purity. Data are representative of 3 independent experiments. Scatter gates drawn as in Figure 2. (B) Total number of GFP+ lymphoid (left), precursor (center), and myeloid (right) cells present over time from cultured βactin:GFP+ lymphoid (top, circles) and precursor (bottom, diamonds) fractions (n = 3). Each graph depicts the mean and SD for 3 independent replicates.
Purified GFP+ lymphoid cells began differentiating into precursor cells within 2 days of coculture when examined by flow cytometry (Figure 4). Precursor cell production peaked on day 2 and continued over 8 days of culture (Figure 4A-B). Myeloid cell production steadily increased through day 8 (Figure 4B). These results suggested that hematopoietic precursors within the lymphoid fraction generated both myeloid and lymphoid cell types. To confirm this, we removed the cultured hematopoietic cells and analyzed their cellular morphologies on cytocentrifuge preparations (Figure 5A top). Cytospins confirmed the presence of lymphoid, precursor, and myeloid cell types at all time points. They also confirmed that while more mature cell types were not initially present in our sorted populations (day 0), they appeared over time. Differential cell counts showed similar percentages to those obtained by flow cytometry (Figure 5B left panel). Multiple experiments showed that cultures generated from sorted lymphoid cells could be maintained for 25 days in culture, and that lymphoid, precursor, and myeloid cells were present through out the time course (not shown).
ZKS cells stimulate differentiation of zebrafish hematopoietic precursors; morphologic evidence. (A) Representative hematopoietic cells recovered from purified βactin:GFP+ lymphoid (top) and precursor (bottom) fractions cultured on ZKS cells stained with May-Grünwald/Giemsa. All images were photographed at 1000×. After photographing, cells were cut and pasted from multiple fields to create composite image. (B) Percentages of lineages derived from lymphoid fraction (left) and precursor fraction (right) recovered cell populations over 8 days of culture. Percent population listed at left, days in culture on ZKS stroma at bottom. Blue indicates lymphoid; purple, precursor; and green, myeloid.
ZKS cells stimulate differentiation of zebrafish hematopoietic precursors; morphologic evidence. (A) Representative hematopoietic cells recovered from purified βactin:GFP+ lymphoid (top) and precursor (bottom) fractions cultured on ZKS cells stained with May-Grünwald/Giemsa. All images were photographed at 1000×. After photographing, cells were cut and pasted from multiple fields to create composite image. (B) Percentages of lineages derived from lymphoid fraction (left) and precursor fraction (right) recovered cell populations over 8 days of culture. Percent population listed at left, days in culture on ZKS stroma at bottom. Blue indicates lymphoid; purple, precursor; and green, myeloid.
Purified GFP+ precursor cells differentiated more rapidly to lymphoid and myeloid cells compared with the purified lymphoid fraction. Plated cells immediately differentiated into lymphoid cells after only 2 days in culture, followed by a steady increase in myeloid cells over 8 days (Figure 4A-B). Morphologic analyses confirmed that purified precursors differentiated into cells displaying lymphoid and myeloid characteristics (Figure 5A bottom panel). Differential counts confirmed the flow cytometric data presented in Figure 4A (Figure 5B right panel). Subsequent experiments showed that cultures generated from sorted precursor cells could be maintained for 25 days in culture, but cells in the precursor gate rapidly disappeared from the culture after 10 days. Myeloid cells comprised the majority of live cells at day 25 (not shown).
Parallel experiments culturing βactin:GFP+ purified myeloid cells on ZKS stroma showed no differentiation of lymphoid or precursor cell types (not shown). In addition, culturing purified lymphoid and precursor fractions without ZKS cells resulted in a 90% reduction in cell number by day 2 and complete loss of cells by day 5 (not shown).
Purified Epo increases in vitro erythroid differentiation
ZKS cells supported the maintenance, proliferation, and differentiation of myeloid, lymphoid, and immature precursor cells, but they did not similarly support the erythroid lineage.
Most zebrafish cytokines that have been identified are highly divergent from their mammalian counterparts, and few attempts to purify, produce, or test zebrafish cytokines have been made. However, identification and characterization of zEpo was recently published.26 Epo is a cytokine essential for definitive hematopoiesis during development and for maintenance of erythroid cells in the adult.35 For these reasons, we added exogenous zEpo to our in vitro cultures to determine whether it would similarly maintain, stimulate proliferation, and encourage differentiation of erythroid progenitors. Adding purified zEpo to WKM cocultures resulted in an increase in precursor cells after 5 days (Figure 6A). Progressive increases in precursor expansion correlated with increasing amounts of purified zEpo, with a maximal increase at 100 μg/mL (Figure 6A). This dose, however, led to only minimal increases in mature erythrocytes (Figure 6C), measured by o-dianisidine, a histochemical hemoglobin stain.
zEpo increases precursor cell survival and differentiation. (A) Epo stimulates precursor survival. Fold change in precursor number after 5 days in culture. Cytokine conditions listed at bottom (n = 3). (B) RT-PCR expression of the zebrafish transferrin-a (tfa) gene in zebrafish tissues. (C) Increased erythroid differentiation achieved by exogenous iron delivery. Percent o-dianisidine-positive erythroid cells after 5 days of culture with erythroid stimulating factors. Factors added to media listed at bottom (n = 3). In all cases, the graph depicts the mean and SD for 3 independent replicates.
zEpo increases precursor cell survival and differentiation. (A) Epo stimulates precursor survival. Fold change in precursor number after 5 days in culture. Cytokine conditions listed at bottom (n = 3). (B) RT-PCR expression of the zebrafish transferrin-a (tfa) gene in zebrafish tissues. (C) Increased erythroid differentiation achieved by exogenous iron delivery. Percent o-dianisidine-positive erythroid cells after 5 days of culture with erythroid stimulating factors. Factors added to media listed at bottom (n = 3). In all cases, the graph depicts the mean and SD for 3 independent replicates.
Iron transport into the mitochondria of erythroid cells is essential for hemoglobin biosynthesis36 and ultimately for the survival and maturation of erythrocytes. In vertebrates, the major pathway for iron uptake is dependent on the interaction of iron-bound transferrin (Tf) with the transferrin receptor 1 (Tfr1; reviewed in Andrews37 ). Receptor engagement stimulates clathrin-mediated endocytosis, followed by release of iron from the Tf/Tfr1 complex upon endosomal acidification.37 Iron is then released from the endosome and trafficked preferentially to mitochondria by the divalent metal transporter 1 (DMT1) for incorporation into hemoglobin.37
Lack of Tf might explain the inability of ZKS cells to support the erythroid lineage, so we assayed for its production by ZKS cells. ZKS cells did not express tfa (Figure 6B), and we reasoned that this deficiency might explain the poor survival and maturation of erythrocytes. Whereas our culture media contained bovine transferrin, it is extremely divergent from zebrafish Tf and is unlikely to cross-react with the zebrafish receptor. We therefore supplemented cultures with carp serum (carp Tf is 67% similar to zebrafish Tf, while human Tf is only 49%38 ) or an Fe-SIH reagent that is able to diffuse across membranes and bypass the Tfr and DMT1 transporters normally required for mitochondrial iron import.39 Addition of either reagent alone did not increase hemoglobinized erythroid cells compared with addition of zEpo (Figure 6C). In contrast, addition of Fe-SIH and zEpo, or carp serum and zEpo, increased erythroid differentiation approximately 4- or 6-fold, respectively, compared with zEpo alone (Figure 6C). These results demonstrate that either carp serum or Fe-SIH can rescue erythrocyte survival and maturation, likely in part due to the proper trafficking of iron to mitochondria, and suggest that the inability of ZKS cells to support erythroid cells is due to a lack of proper iron transport.
ZKS cells aid in vitro study of erythroid mutants
We next examined 2 genetic mutants identified previously in forward mutagenesis screens that possess defects in erythroid maturation. Chardonnay (cdy) fish exhibit hypochromic, microcytic anemia due to a mutation in the DMT1 transporter.40 DMT1 function is essential for iron transport out of the endosome and is important for red blood cell maturation.41 Mutants in DMT1 can survive into adulthood, but display increased erythroid precursors due to impaired survival of mature erythrocytes (not shown). WKM cultured from cdy homozygotes in the presence of zEpo and Fe-SIH showed rescue of erythrocyte differentiation when analyzed by o-dianisidine staining (Figure 7A). No measurable increase in precursor production occurred after 5 days of culture upon addition of zEpo and Fe-SIH (Figure 7B). Rather, the precursor pool appeared to be maintained over the culture period, and resulting precursors differentiated predominantly to erythrocytes (Figure 7C). As a control, we used WKM from an iron-independent erythroid mutant retsina, a mutant animal with a band3 deficiency that leads to dyserythropoiesis.42 As expected, the number of precursor (Figure 7B) or o-dianisidine positive cells (Figure 7C) was not significantly increased by the addition of membrane-permeable iron. Together, these results provide an example of how the ZKS cell culture system can be used to better understand the biology of zebrafish blood mutants.
Fe-SIH rescues erythroid differentiation in cdyte216/te216 mutant WKM cultured on ZKS cells. (A) Morphologic (May-Grünwald/Giemsa, top row) and histochemical (o-dianisidine, bottom row) staining of cdyte216/216 mutant WKM cultured on ZKS for 5 days with no factors added (left column) or 100 μg zEpo and Fe-SIH added (right column). Arrowheads denote erythroid cells. (B) Fold expansion of precursor cells from erythroid mutants over 5 days in culture on ZKS cell without (−) or with (+) recombinant Epo and Fe-SIH (n = 4). (C) Percent o-dianisidine-positive erythroid cells after 5 days of culture without (−) or with (+) recombinant Epo and Fe-SIH (n = 4; *P < .01). In all cases, the graph depicts the mean and SD for 4 independent replicates.
Fe-SIH rescues erythroid differentiation in cdyte216/te216 mutant WKM cultured on ZKS cells. (A) Morphologic (May-Grünwald/Giemsa, top row) and histochemical (o-dianisidine, bottom row) staining of cdyte216/216 mutant WKM cultured on ZKS for 5 days with no factors added (left column) or 100 μg zEpo and Fe-SIH added (right column). Arrowheads denote erythroid cells. (B) Fold expansion of precursor cells from erythroid mutants over 5 days in culture on ZKS cell without (−) or with (+) recombinant Epo and Fe-SIH (n = 4). (C) Percent o-dianisidine-positive erythroid cells after 5 days of culture without (−) or with (+) recombinant Epo and Fe-SIH (n = 4; *P < .01). In all cases, the graph depicts the mean and SD for 4 independent replicates.
Discussion
The objective of this study was to create a tissue culture system to enable the functional analysis of hematopoietic stem and progenitor cells in the zebrafish. It is well documented that the hematopoietic niche plays a crucial role in the support and maintenance of hematopoiesis, providing a favorable environment for self-renewal, amplification, and differentiation of hematopoietic stem cells.43 We therefore set out to replicate aspects of the niche by creating stromal cell lines from the major hematopoietic organ of the adult zebrafish, the kidney.
Similar strategies have been used to support murine hematopoiesis in vitro. Long-term bone marrow stromal cultures (LTBMCs), comprised of a variety of different cell types including endothelial cells, adipocytes, fibroblasts, and macrophages, were one of the first attempts to create a supportive, in vitro hematopoietic niche that would support hematopoietic progenitor cells.8,44 Plating fresh bone marrow on LTBMCs can maintain active hematopoiesis for several months.45 This culture system has proven invaluable for investigating the roles of specific growth factors46 and extracellular matrix proteins47 in the maintenance of specific hematopoietic lineages. Clonal cell lines were subsequently generated from the adherent layer of LTBMCs that could support myelopoiesis and lymphopoeisis (S17 cells) or myelopoiesis alone (S10 cells).48 Many other methods of studying hematopoiesis ex vivo have also been used. The generation of the OP9 stromal line49 from the calvaria of newborn m-csf−/− mice ushered in an effective way to generate murine megakaryocytes, erythroid cells, B lymphocytes, neutrophils, eosinophils, dendritic cells, natural killer cells, and osteoclasts in vitro (reviewed in Olsen et al50 ). Modification of OP9 cells by constitutive expression of the Notch ligand [delta], which is essential for thymocyte development,51,52 has allowed further study of T-cell development and maturation in vitro. Although T-cell development does not normally occur in the BM, prolonged contact with OP9-Δ lines or thymic tissue permits lymphoid precursors to differentiate into T lymphocytes (reviewed in Zuniga-Pflucker53 ). In addition, stromal cell lines such as MS-5 have proven to be effective at elucidating human hematopoietic development in the tissue culture dish.54
There are several benefits to using hematopoietic stromal cultures. First, semisolid culture methods, which use methylcellulose or soft agar, require addition of several cytokines to support hematopoietic stem and progenitor cells. Our attempts to stimulate zebrafish hematopoietic precursors using mouse or human growth factors were unsuccessful, likely due to poor sequence conservation between species. To date, very few zebrafish cytokines have been identified and characterized. Second, stromal cell interactions are required for many hematopoietic cell types to differentiate and develop (such as T lymphocytes). Third, hematopoietic cells can be repeatedly harvested from stromal cultures to determine the longevity and differentiation kinetics of each lineage over time. Finally, ZKS cultures establish a baseline system in which to further examine the effects of zebrafish growth factors. As we have demonstrated with the rescue of erythropoiesis by addition of zEpo, it should be possible to similarly test candidates for additional zebrafish growth factor orthologs, such as thrombopoietin, granulocyte colony-stimulating factor (g-csf), and macrophage colony-stimulating factor (m-csf). Since it is often difficult to predict discovery of these molecules by sequence homology, it should now be possible to identify these and other factors through their functions in our culture system. Progress here, and possibly use of conditioned media from ZKS cells, should enable the development of semisolid culture systems for the detection of zebrafish colony-forming cells.
Our results using WKM have shown that ZKS cells cannot only support myeloid and erythroid differentiation, but can also support lymphocyte survival and maturation. Surprisingly, we have observed the simultaneous differentiation and proliferation of cells expressing pax5, igM, gata3, rag2, and lck, likely reflecting the presence of both B lymphocytes and T lymphocytes in our cultures. This is unexpected, since mammalian cell lines, like OP9 tend to support only one of these 2 lymphoid lineages. OP9 cells support B-cell development normally, but at the apparent expense of T cells. Transduction of the Notch ligand, Δ, into OP9 cells conversely supports T-cell development at the expense of B lymphocytes.53 ZKS cells express all of the zebrafish Δ ligands, which may be responsible for differentiation of lck+ T cells over time. We are working to verify whether mature T lymphocytes and B lymphocytes are present in ZKS cultures through development of molecular probes against the T- and B-cell antigen receptors.
The remarkable conservation between mammals and zebrafish in the lineages and functions of mature hematopoietic cells make it likely that a similar cascade of lineage commitment proceeds downstream of HSCs as previously demonstrated in the mouse. Using the ZKS stromal system, we can now address the ontogeny of the major blood lineages through identification of defined progenitor subsets. Our current results suggest that a lymphoid progenitor is present within the precursor scatter fraction, a finding not previously appreciated. Since ZKS cells appear to support both T- and B-lymphoid development, it may be possible to determine whether this activity is due to the presence of a common lymphoid progenitor (CLP) equivalent in this fraction by using prospective isolation strategies based on, for example, fluorescent transgene expression. With further optimization of the ZKS system, it should be possible to perform the clonal experiments necessary to identify a zebrafish CLP and assess its full developmental potential. Similarly, identification of clonogenic myeloid-restricted progenitors should be possible using the ZKS system to determine whether zebrafish HSCs commit to the mature myeloerythroid lineages through CMP, GMP and MEP intermediates, as demonstrated in the mouse.11
Several large-scale, forward genetic screens have generated an array of zebrafish mutants, many with essential defects in hematopoiesis. Many of these mutants have not been fully characterized due to a lack of appropriate functional assays. Here we show by rescuing cdy mutant erythroid cell maturation that the ZKS system may be used to examine genetic mutants in ways not previously possible. The ability to study and rescue mutant gene function in vitro should provide additional means to elucidate normal gene function in other mutants. Further refinement of the ZKS system and development of semisolid culture techniques will provide more precision and flexibility in analysis of genetic mutants. The majority of hematopoietic mutants were identified by visible defects in erythrocyte maturation or survival. In vitro assays will serve to address whether any of these mutants possess additional, nonerythroid hematopoietic defects. This should greatly aid the identification of genetic mutants possessing defects in hematopoietic stem and progenitor cells.
In conclusion, we have generated primary stromal cell lines from the adult zebrafish kidney able to support multilineage hematopoiesis. Optimization of culture conditions, along with identification of zebrafish hematopoietic growth factors, should ultimately enable the in vitro survival, proliferation, and differentiation of all blood cell lineages in the zebrafish. Furthermore, this system should likewise enable more precise characterization of mutant phenotypes resulting from forward mutagenesis screens designed to identify erythroid, myeloid, and lymphoid mutants.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mitch Weiss, Dawne Page, and Wilson Clements for critical evaluation of the manuscript; Shrey Purohit for technical assistance; Malcolm Wood for electron microscopy; Roger Rainville, Evie Wright, and Lisa Phelps for excellent animal care; and Kerstin Richter for excellent laboratory management.
This work was supported by the Prevent Cancer Foundation (D.L.S.), the LC06077 project of Ministry of Education, Youth, and Sports (MSMT) and Fullbright Scholars award (P.B.), the March of Dimes Foundation (B.H.P.), the American Society of Hematology (D.T.), the Arnold and Mabel Beckman Foundation (D.T.), the Senyei Family Foundation (D.T.), and National Institutes of Health (NIH) grant nos. R01-DK074482 (D.T.), R01-DK070838, (B.H.P.), and P01-HL32262 (B.H.P.).
National Institutes of Health
Authorship
Contribution: D.L.S. and J.R.R. performed the research and analyzed the data; D.L.S. and D.T. designed the research and wrote the article; and P.B., B.P., and L.I.Z. contributed vital reagents.
Conflict-of-interest disclosure: D.L.S., J.R.R., P.B., B.P., and D.T. declare no competing financial interests. L.Z. has stock in and is a consultant for FATE Therapeutics and Stemgent.
Correspondence: David Traver, University of California at San Diego Division of Biological Sciences, Section of Cell and Developmental Biology, 9500 Gilman Dr, La Jolla, CA, 92093-0380; e-mail: dtraver@ucsd.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal