Embryonic stem (ES) cells exhibit the remarkable capacity to become virtually any differentiated tissue upon appropriate manipulation in culture, a property that has been beneficial for studies of hematopoiesis. Until recently, the majority of this work used murine ES cells for basic research to elucidate fundamental properties of blood-cell development and establish methods to derive specific mature lineages. Now, the advent of human ES cells sets the stage for more applied pursuits to generate transplantable cells for treating blood disorders. Current efforts are directed toward adapting in vitro hematopoietic differentiation methods developed for murine ES cells to human lines, identifying the key interspecies differences in biologic properties of ES cells, and generating ES cell-derived hematopoietic stem cells that are competent to repopulate adult hosts. The ultimate medical goal is to create patient-specific and generic ES cell lines that can be expanded in vitro, genetically altered, and differentiated into cell types that can be used to treat hematopoietic diseases.
Introduction
Embryonic stem (ES) cells, first isolated in 1981, are primary cell lines originally derived from the inner cell mass (ICM) of preimplantation or peri-implantation mouse blastocysts.1,2 ES cells are distinguished by their capacity to self-renew in vitro and differentiate into tissues derived from all 3 embryonic germ-cell layers. Following microinjection into host blastocysts, donor ES cells contribute to all tissues of chimeric mice, including the germ line.3 This property, combined with the ability to easily manipulate the ES-cell genome via homologous recombination, provides the tools to generate new animal strains in which genes of interest are knocked out or altered. In addition, ES cells can be induced to form various differentiated tissue types in vitro. Here, we review the production of blood lineages from ES cells.
The broad developmental capacity of ES cells and the relative ease by which resident genes can be manipulated have been valuable for understanding the basic biology of tissue development and offer tremendous potential for cell-replacement therapies in degenerative disorders, cancer, and genetic diseases. The promise of clinical applications for ES cells was stimulated recently by the development of human lines. Studies of murine hematopoiesis provide a paradigm for using ES cells as a model system to examine normal developmental processes and as a source for tissue production. This work provides a launching point for efforts to differentiate human ES cells into transplantable hematopoietic lineages that can be employed therapeutically.
Culture of ES cells
The first requirement for successful propagation of ES cells is to maintain them in an undifferentiated, pluripotent state by adherence to specific culture conditions. Subsequently, differentiation into desired lineages can be initiated in a controlled and synchronous fashion. ES cells are held in an undifferentiated state by numerous cell intrinsic and environmental signaling pathways.4,5
In practice, murine ES cells are maintained in vitro by cocultivation on a stromal layer, typically murine embryonic feeder (MEF) cells, and addition of leukemia inhibitory factor (LIF), a cytokine that activates signal transduction through a cognate receptor complex.6 Recent studies demonstrate that a combination of LIF and bone morphogenic protein 4 (BMP4) can bypass serum and feeder requirements to allow self-renewal of murine ES cells in defined medium.7,8
Human ES cells can be maintained on stromal cells in the presence of basic fibroblast growth factor (b-FGF).9 In contrast to its effects on murine ES cells, LIF does not promote self-renewal of human ES cells.10,11 Human ES cells are reported to have a particular propensity for spontaneous differentiation in culture, perhaps due to species-specific requirements for self-renewal that are incompletely understood.12-14 In this regard, further studies to optimize the propagation of human ES cells and eliminate their requirements for animal feeder cells are important for future clinical applications. For example, human ES cells can be maintained on human stroma,15,16 and numerous signaling pathways and transcription factors that participate in ES-cell self-renewal have been exploited for developing feeder-free culture systems.17-20 This includes the use of b-FGF17,21 and Wnt signaling agonists.22,23 The nuclear proteins Sox2, Oct3/4, and Nanog are critical for both human and murine ES-cell maintenance, and manipulation of their expression can bypass some cytokine and stromal requirements for self-renewal.5,24-26 It appears that these transcription factors are central components of a concerted autoregulatory network that promotes ES-cell self-renewal and pluripotency by activating and repressing numerous target genes, predominantly those encoding other nuclear proteins.27
The expansion of ES cells and maintenance of their undifferentiated state in culture are monitored by visual inspection, testing for developmental potential under various differentiation conditions and gene expression analysis. In reference to the latter point, an enormous amount of data on gene-expression profiling of human and mouse ES cell lines have been generated.21,28-36 Distilling this information to produce a defined molecular signature for ES cells and to identify all of the genes important for maintaining their self-renewal and pluripotency has not yet been achieved.
Creating blood lineages from ES cells
Major technologic advances in deriving mature tissues from ES cells were established through studies of the hematopoietic system using murine cells (for practical reviews see Kitajima et al,37 Kennedy and Keller,38 and Fraser et al39 ). The first step in differentiating ES cells in vitro is to remove them from the feeder cells and cytokines that maintain their pluripotency. Subsequently, if serum is present, a substantial fraction of ES cells differentiate into mesoderm, the germ-cell layer from which blood is derived. Under serum-free conditions, murine ES cells can be induced to form mesoderm and hematopoietic progenitors by added cytokines, most importantly, BMP4 and vascular endothelial growth factor (VEGF).40,41 BMP4 also promotes mesoderm formation from human ES cells, although these experiments were performed with serum present.42 Mesoderm formation is regulated by numerous positive and negative factors.43,44 An ultimate goal is to recapitulate these influences in defined culture systems for human and murine ES cells.
Two different experimental systems are used for the majority of experiments to generate blood precursors from ES cells: formation of embryoid bodies (EBs)45,46 and induction of hematopoietic differentiation on stromal cells.47 Upon removal from stroma and withdrawal of LIF, suspension cultures of ES cells form EBs, aggregates of differentiated cells including mesodermal derivatives capable of hematopoietic differentiation. In 1985, Doetschman et al46 noted that EBs in liquid culture form cystic structures that contain blood islands analogous to those found in the embryonic yolk sac. Subsequent studies established that erythroid and myeloid lineages develop within EBs.48,49 The emergence of hematopoietic progenitors in this culture system can be tracked by the appearance of lineage-specific markers through gene expression analysis and immunohistochemistry.45,50-52 In addition, disruption of EBs by trypsin or collagenase produces single-cell suspensions from which hematopoietic progenitors can be analyzed and enumerated by flow cytometry or methylcellulose culture assays. In this fashion, the effects of specific manipulations such as drugs, cytokines, or gene targeting can be analyzed. Cells within developing EBs synthesize various cytokines and, consequently, the initial stages of hematopoiesis occur when serum is the only source of added growth factors.38,45 However, addition of specific cytokine combinations optimizes the development of specific mature lineages (see “In vitro production of specific hematopoietic lineages from ES cells”).
Differentiation of ES cells on stromal lines provides a second means for inducing hematopoiesis. The most commonly used system is OP9, a line established from calvariae of newborn op/op mice, which lack functional macrophage colony-stimulating factor (M-CSF).47,53 OP9 cells enhance hematopoietic development by providing a supportive microenvironment for differentiation. The absence of M-CSF inhibits the survival of monocyte-macrophage cells, which tend to outgrow other lineages in culture systems using wild-type stroma. ES cells plated onto OP9 stroma form mesodermal colonies after approximately 5 days, and cells within these colonies differentiate into multipotential hematopoietic progenitors after an additional 3 days. Similar to what is observed in EBs, ES cell-derived erythroid, myeloid, and B-lineage cells are generated on OP9 stroma without exogenous growth factors, although cytokine supplementation influences lineage output. The EB and OP9 systems exhibit similar kinetics and efficiencies for many aspects of hematopoietic development, although each approach may offer unique advantages.54
Hematopoietic progenitor and stem cells develop optimally in vivo through cellular interactions and paracrine effects provided by stromal niches.55,56 Recapitulating these microenvironments in vitro could enhance cell production. Toward this goal, 3-dimensional culture systems incorporating extracellular matrix proteins have been used to provide a scaffold for EB development.57-59 It is likely that refining this approach to more closely mimic the in vivo niches that support cell specification and organogenesis will further optimize tissue production from ES cells. For example, mKirre is a type Ia membrane protein that is expressed by OP9 cells and contributes to their ability to support hematopoiesis.60 Incorporation of recombinant mKirre into artificial matrix systems might, therefore, enhance hematopoietic culture of ES cells under more defined conditions.
Examining early hematopoietic ontogeny through studies of ES cells
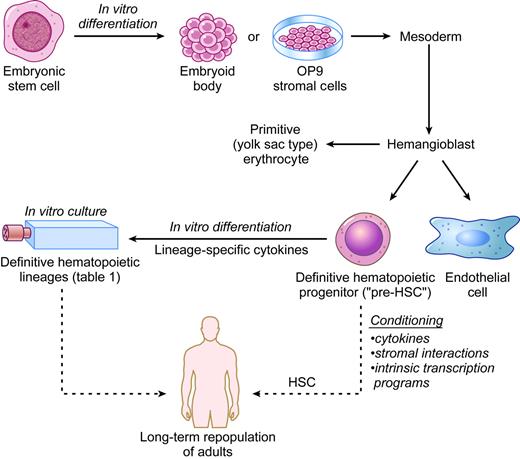
During embryogenesis, the hematopoietic system is established spatiotemporally through waves of distinct progenitors arising in different tissues.61-64 The first recognizable blood cells in the embryo are yolk sac-derived primitive erythrocytes that differ from adult-type definitive erythrocytes in many respects, including gene expression, morphology, and cytokine requirements. Hematopoietic stem cells (HSCs) capable of reconstituting adult hosts are found initially in the murine yolk sac and in an intraembryonic region termed the aorta-gonad-mesonephros (AGM).65,66 Around midgestation, the major site of HSC activity and production of mature blood cells shifts to the fetal liver where definitive erythrocytes and other adult-type lineages are produced.67 Numerous studies in avian and murine species suggest that the earliest embryonic hematopoietic progenitors and endothelial cells derive from a common progenitor termed the hemangioblast.68-70
Remarkably, hematopoiesis in cultured ES cells largely recapitulates embryonic events. For example, distinctly timed waves of primitive and definitive erythropoiesis develop in vitro from ES cells in a fashion that parallels the onset of these developmental programs in early embryos.45 Moreover, Keller and colleagues (Kennedy et al71 and Choi et al72 ) discovered a transient progenitor in early EBs, termed blast-colony-forming cell (BL-CFC), which gives rise to primitive erythrocytes, definitive lineages, and endothelial cells. Nishikawa et al73 extended these studies, using cell-surface markers to identify a developmental hierarchy in ES-cell differentiation in which hematopoietic cells arise from a precursor expressing the endothelial markers Flk-1 and VE-cadherin. They also identified single progenitor cells capable of hematopoietic and endothelial differentiation. Together, these studies helped to establish lineage relationships between the first murine hematopoietic precursors and verify the existence of the hemangioblast (Figure 1). These and subsequent studies are beginning to define the characteristics of hemangioblasts, including cytokine and transcription factor requirements. For example, BL-CFCs express the cytokine receptor Flk-1 and are dependent on its ligand, VEGF. Gene-targeting studies have identified numerous transcription factors and signaling molecules important for the generation or maintenance of murine ES cell-derived hemangioblasts.74-79
Recently, Huber et al80 identified hemangioblasts from the primitive streak of the early mouse embryo. Importantly, these progenitors have similar properties to ES cell-derived BL-CFCs but are an extremely rare and transient population in vivo. Most embryos examined contained fewer than 10 hemangioblasts that could be identified by current culture conditions. This study further illustrates the parallels between developmental hematopoiesis in early embryos and ES-cell differentiation cultures, validating the biologic relevance of the latter. Using ES cells, hemangioblasts can now be generated and purified on a scale much greater than what could be achieved from embryos. This, in turn, allows for further studies into the basic biology and therapeutic potential of this progenitor.
The holy grail: hematopoietic stem cells from ES cells
By definition, transplantation of a single HSC into a suitable adult host can repopulate the entire hematopoietic system for an extended period of time. While cultured ES cells give rise to multipotent hematopoietic progenitors with extensive proliferative capacity, they repopulate animals only weakly and inconsistently in most reported studies.81,82 Burt et al83 recently reported more robust in vivo reconstitution from purified EB-derived CD45+ c-Kit+ progenitors. It is now important to further characterize these progenitors with HSC activity, refine the conditions for their derivation, and reproduce these encouraging findings in other laboratories.
Most ES cell-derived blood progenitors studied may exhibit relatively limited HSC activity because they approximate an immature embryonic lineage that cannot effectively survive and proliferate in adult hematopoietic niches.84,85 Accordingly, “pre-HSCs” are found in the yolk sac and the para-aortic splanchnopleura region of early embryos.86-90 Presumably, during fetal maturation, pre-HSCs are somehow made competent to repopulate adult hosts, either through interactions with specific embryonic/fetal microenvironments, execution of latent cell-autonomous genetic programs, or both (Figure 1).
Recent work suggests that pre-HSCs from ES cells and embryos can be coaxed to become adult repopulating cells through genetic manipulation.91,92 The homeobox (Hox) family of transcription factors participates in normal hematopoiesis and leukemogenesis.93-98 Work by Humphries' group (Antonchuk et al,99,100 Sauvageau et al,101 Buske et al,102 and Helgason et al103 ) showed that ectopic expression of HoxB4 can enhance the self-renewal of hematopoietic progenitors and HSCs from mice and ES cells. Daley and colleagues (Kyba et al104 ) extended these findings by showing that enforced expression of HoxB4 in immature murine yolk sac- or ES cell-derived hematopoietic progenitors rendered them competent for long-term multilineage engraftment of irradiated adult hosts. In these experiments, a doxycycline-inducible HoxB4 transgene was introduced into ES cells and expressed during a narrow window of hematopoietic differentiation. Hence, ectopic HoxB4 can facilitate the maturation of pre-HSCs but is not required subsequently. This result leads to several interesting questions and areas for further study. First, for unknown reasons, lymphoid repopulation appeared to be less robust than myeloid repopulation in the HoxB4-transduced cells, a finding warranting further examination.104,105 Second, 2 million cells were used to reconstitute mice in these studies. It is now important to fractionate this mixture, identify the repopulating cells, and demonstrate that a single cell is capable of generating all hematopoietic lineages. Third, basic mechanistic studies are essential to understand how HoxB4 might educate pre-HSCs. For example, structure-function studies identified a HoxB4 mutant with more potent regenerating activity.106 In addition, it is important to define the HoxB4 transcriptional program associated with pre-HSC maturation.107 It is possible that this line of research will identify important cell signaling interactions that can be modulated by drugs.
In vitro hematopoiesis from ES cells. The solid arrows indicate established methods to produce specific hematopoietic lineages. The dashed arrows indicate potential future therapeutic applications to be derived from human ES cells. HSC indicates hematopoietic stem cell.
In vitro hematopoiesis from ES cells. The solid arrows indicate established methods to produce specific hematopoietic lineages. The dashed arrows indicate potential future therapeutic applications to be derived from human ES cells. HSC indicates hematopoietic stem cell.
Additional strategies to generate HSCs from ES cells will be facilitated by ongoing and future studies to better understand how pre-HSCs are conditioned in the embryo. For example, stromal interactions almost certainly contribute to HSC development and maturation as hematopoietic populations shift during embryogenesis.108 In support of this, multilineage progenitors and HSCs develop autonomously in ex vivo organ cultures of the para-aortic splanchnopleura and AGM regions.87,89,90,109 Additionally recent work by several groups suggests that HSC activity is induced and supported by stromal lines from various embryonic sources.110-112 It will be interesting to determine whether such stromal lines can support the maturation of HSCs from ES-cell differentiation cultures. In this case, the next step will be to identify the specific molecules that influence HSC development by either direct stromal interactions or paracrine effects.113 Ultimately, the goal is to reconstruct these inductive events using recombinant molecules.
In vitro production of specific hematopoietic lineages from ES cells
Early studies showed that mixtures of different hematopoietic-cell types develop in ES-cell differentiation cultures.45-48,50,53,114 Subsequently, methods were refined to generate relatively pure populations of specific lineages. Important variables that affect lineage output include duration and mode of differentiation and supplemental cytokines or chemicals. Conditions for generating various hematopoietic cell types have been established (Table 1). These methods are useful for studying the development and physiology of specific mature blood lineages generated from both wild-type and gene-targeted ES cells. Analysis of hematopoiesis from genetically altered ES cells is particularly useful when the mutation produces a complex phenotype with early embryonic lethality. This application has been used extensively. The methods summarized in Table 1 were established using murine ES cells, but these are now being adapted for use with human ES cells.
Generation of specific hematopoietic lineages from murine ES cells
Hematopoietic lineage . | Method of culture . | Cytokines . | References . | Other . |
|---|---|---|---|---|
| Megakaryocytes and platelets | OP9, EBs | Major: Tpo | 38, 115-119 | Megakaryocytes show agonist-dependent fibrinogen binding. Platelets exhibit agonist-dependent fibrinogen binding, aggregation, and spreading. |
| Other: IL-6, IL-11 | ||||
| Erythroid | OP9, EBs | Major: Epo, KL | 38, 45, 47, 114, 120 | EryPs are KL independent. EryDs require Epo/KL. Iron-saturated transferrin added to improve hemoglobin synthesis during terminal maturation. Dexamethasone can be used for erythroid progenitor expansion. |
| Other: Insulin, IGF-1, IL-3 | ||||
| Mast cells | EBs | IL-3, KL | 121, 122 | EB-derived mast cells proliferate extensively in culture and reconstitute mast cell-deficient KitW/KitW-v mice after adoptive transfer. |
| Macrophages | OP9, EBs | IL-1, IL-3, M-CSF, GM-CSF | 47, 123-125 | Macrophage overgrowth tends to inhibit the production of other lineages in ES-cell differentiation cultures. |
| T lymphocytes | OP9, OP9-DL1 | Flt-3 ligand, IL-7 | 126, 127 | Full T-lymphocyte development requires maturation in thymic stroma (FTOCs or RTOCs). T-lymphocyte production is augmented by cultivation on OP9 cells ectopically expressing the notch ligand DL-1. |
| B lymphocytes | OP9 | Flt-3 ligand, IL-7 | 47, 128, 129 | Stimulation with LPS augments production of IgM+-secreting B lymphocytes. |
| Eosinophils | OP9 | IL-5, IL-3, GM-CSF, eotaxin | 130 | Express characteristic granules and cell-surface markers, produce oxidative response to Leishmania. |
| Neutrophils | EBs, OP9 | Progenitor expansion: OSM, b-FGF, IL-6, IL-11, LIF, KL | 131, 132 | Functional neutrophils produced. This includes characteristic markers and morphology, superoxide production, and chemotactic responses. |
| Neutrophil differentiation: G-CSF, GM-CSF, IL-6 | ||||
| DCs | EBs, OP9 | Progenitor expansion: GM-CSF, IL-3 | 133-135 | DC production facilitated by plating EBs on tissue culture dishes. Exhibit characteristic surface phenotype, process protein antigens, and stimulate T lymphocytes in the MLR. |
| Differentiation/maturation: IL-4, TNF-α, LPS, anti-CD40 | ||||
| NK cells | OP9 | Flt-3 ligand, IL-15, IL-6, IL-7, KL | 129, 136 | Express characteristic NK proteins; kill selected tumor lines and MHC 1-deficient lymphoblasts. |
| Osteoclasts | OP9, ST2 | M-CSF, RANKL | 137-141 | Recognized by TRAP expression and bone resorbtion activity. Production augmented by vitamins C and D and by dexamethasone. |
Hematopoietic lineage . | Method of culture . | Cytokines . | References . | Other . |
|---|---|---|---|---|
| Megakaryocytes and platelets | OP9, EBs | Major: Tpo | 38, 115-119 | Megakaryocytes show agonist-dependent fibrinogen binding. Platelets exhibit agonist-dependent fibrinogen binding, aggregation, and spreading. |
| Other: IL-6, IL-11 | ||||
| Erythroid | OP9, EBs | Major: Epo, KL | 38, 45, 47, 114, 120 | EryPs are KL independent. EryDs require Epo/KL. Iron-saturated transferrin added to improve hemoglobin synthesis during terminal maturation. Dexamethasone can be used for erythroid progenitor expansion. |
| Other: Insulin, IGF-1, IL-3 | ||||
| Mast cells | EBs | IL-3, KL | 121, 122 | EB-derived mast cells proliferate extensively in culture and reconstitute mast cell-deficient KitW/KitW-v mice after adoptive transfer. |
| Macrophages | OP9, EBs | IL-1, IL-3, M-CSF, GM-CSF | 47, 123-125 | Macrophage overgrowth tends to inhibit the production of other lineages in ES-cell differentiation cultures. |
| T lymphocytes | OP9, OP9-DL1 | Flt-3 ligand, IL-7 | 126, 127 | Full T-lymphocyte development requires maturation in thymic stroma (FTOCs or RTOCs). T-lymphocyte production is augmented by cultivation on OP9 cells ectopically expressing the notch ligand DL-1. |
| B lymphocytes | OP9 | Flt-3 ligand, IL-7 | 47, 128, 129 | Stimulation with LPS augments production of IgM+-secreting B lymphocytes. |
| Eosinophils | OP9 | IL-5, IL-3, GM-CSF, eotaxin | 130 | Express characteristic granules and cell-surface markers, produce oxidative response to Leishmania. |
| Neutrophils | EBs, OP9 | Progenitor expansion: OSM, b-FGF, IL-6, IL-11, LIF, KL | 131, 132 | Functional neutrophils produced. This includes characteristic markers and morphology, superoxide production, and chemotactic responses. |
| Neutrophil differentiation: G-CSF, GM-CSF, IL-6 | ||||
| DCs | EBs, OP9 | Progenitor expansion: GM-CSF, IL-3 | 133-135 | DC production facilitated by plating EBs on tissue culture dishes. Exhibit characteristic surface phenotype, process protein antigens, and stimulate T lymphocytes in the MLR. |
| Differentiation/maturation: IL-4, TNF-α, LPS, anti-CD40 | ||||
| NK cells | OP9 | Flt-3 ligand, IL-15, IL-6, IL-7, KL | 129, 136 | Express characteristic NK proteins; kill selected tumor lines and MHC 1-deficient lymphoblasts. |
| Osteoclasts | OP9, ST2 | M-CSF, RANKL | 137-141 | Recognized by TRAP expression and bone resorbtion activity. Production augmented by vitamins C and D and by dexamethasone. |
DCs indicates dendritic cells; NK, natural killer; OP9, M-CSF-/- stromal line; EB, embryoid bodies; Tpo, thrombopoietin; IL-6, interleukin 6; Epo, erythropoietin; KL, c-kit ligand; IGF-1, insulin-like growth factor 1; EryP, primitive erythroid progenitor; EryD, definitive erythroid progenitor; M-CSF, macrophage colony-stimulating factor; GM-CSF granulocyte/macrophage colony-stimulating factor; FTOCs, fetal thymic organ cultures; RTOCs, reaggregate thymic organ cultures; DL-1, Delta-like-1; LPS, lipopolysaccharide; OSM, oncostatin M; b-FGF, basic fibroblast growth factor; LIF, leukemia inhibitory factor; G-CSF, granulocyte colony-stimulating factor; TNF-α, tumor necrosis factor α; MLR, mixed lymphocyte reaction; ST2, bone marrow-derived stromal line; and TRAP, tartrate-resistant acid phosphatase.
Erythroid cells
When 7- to 8-day-old EBs are disaggregated and plated into methylcellulose cultures with erythropoietin (Epo) and Kit ligand (KL), the majority of resultant colonies are erythroid.45 We used this strategy to show that ES cells with disrupted Gata1, which encodes an essential hematopoietic transcription factor, produce normal-appearing proerythroblasts that subsequently undergo maturation arrest and apoptosis.142,143 In these experiments, use of ES cells allowed us to easily generate a synchronous cohort of erythroid precursors to pinpoint the developmental stage at which GATA-1 function becomes essential. We used the same system to generate an immortalized GATA-1- proerythroblast line that undergoes GATA-1-dependent terminal erythroid maturation.144 This line, termed G1E, for GATA-1-Erythroid, has been a useful tool for various applications including erythroid gene discovery and examining mechanistic aspects of GATA-1 function in erythroid development.145-153 Zheng et al154 generated ES cells in which the endogenous Gata1 gene was ablated and a tetracycline-regulated Gata1 cDNA expression cassette was inserted, permitting conditional rescue of the gene defect. Using this system the authors identified unique functions for GATA-1 at various stages of erythroid development. Together, these studies illustrate how gene functions can be studied through genetic manipulation and in vitro differentiation of ES cells.
Methods to derive definitive erythrocytes from ES cells were advanced by Beug's group (Carotta et al120 ), who developed culture conditions in which EB-derived immature erythroid progenitors can be expanded in a relatively pure state roughly 107-fold over the course of several months. This approach relies on prior findings that dexamethasone promotes erythroid progenitor self-renewal.155-157 When dexamethasone is removed and the cytokine mixture is adjusted, terminal maturation ensues. Carotta et al120 used this approach to demonstrate an unrecognized function for the cytokine receptor Flk-1 in erythroid development. This role was not appreciated from in vivo studies, as Flk1-/- embryos die early with complex, non-cell-autonomous defects in hematopoiesis.158
Megakaryocytes
Culturing ES cells on OP9 stroma with thrombopoietin (Tpo) generates populations enriched for megakaryocytes with characteristic functional features including proplatelet production and fibrinogen binding after exposure to platelet agonists.115-119 Eto et al118 used this system to show that CalDAG-GEF1, a guanine nucleotide exchange factor that is deficient in megakaryocytes lacking the transcription factor NFE2, promotes integrin-mediated megakaryocyte signaling. Fujimoto et al115 reported similar methods to derive functional megakaryocytes and platelets from ES cells. Our laboratory adapted these culture methods to show that Gata1 gene-disrupted ES cells produce a unique self-renewing bipotential progenitor with the capacity to undergo GATA-1-dependent megakaryocyte and erythroid maturation.159 A defined role at this stage of hematopoietic development is relevant to recent findings that mutations in the GATA1 gene contribute to Down syndrome-associated acute megakaryoblastic leukemia (AMKL), which exhibits both erythroid and megakaryocytic features, suggesting that malignant transformation may occur within a megakaryocyte-erythroid progenitor (MEP).160
Granulocytes
Lieber et al131,132 generated greater than 75% pure neutrophil cultures from ES cells. This approach involves inducing the maturation of EB-derived hematopoietic progenitors on OP9 cells with added cytokines. The resultant neutrophils exhibit signature markers, chemotactic response, and superoxide production. The authors used this system to demonstrate migration defects in neutrophils lacking mitogen-activated protein (MAP)/extracellular signal-related kinase (MEKK1) through targeted mutagenesis.
Mast cells
Culturing EB-derived hematopoietic progenitors in suspension with the cytokines IL-3 and KL generates pure populations of mast cells that can be expanded significantly in vitro.45,48,121,161 Upon transplantation into mast cell-deficient mice (KitW/KitW-v), ES cell-derived mast cells survive, proliferate, mature, and mediate IgE-dependent immune responses. This provides a powerful strategy to analyze the effects of genetic and chemical alterations on mast-cell development and function in vitro and in vivo. For example, IgE-induced cross-linking of its high-affinity receptor triggers mast-cell activation with release of inflammatory mediators, contributing to the pathophysiology of allergic response. Several groups are dissecting this pathway by creating ES cells with mutations in candidate genes suspected to participate in IgE receptor signaling and using these lines to generate mast cells for functional studies.122,161
Eosinophils
Hamaguchi-Tsuru et al130 generated eosinophils (∼50% enriched) by differentiating ES cells on OP9 stroma with IL-5 and either IL-3 or granulocyte-macrophage colony-stimulating factor (GM-CSF). The ability to generate eosinophil-enriched populations and study their development and function at defined stages has implications for human disorders such as allergies and asthma. For example, this approach may be a useful basis for screens to identify new anti-inflammatory agents that inhibit eosinophil development or function.
T and B lymphocytes
Nakano et al47 cultured ES cells on OP9 stroma with IL-7 to generate early B cells. Most of these were IgM- and completed diversity-joining (DJ) gene rearrangement, but a small proportion differentiated into IgM+ cells that expressed the complete μ chain mRNA.47 B-cell maturation from ES cells was developed further by stimulating differentiation in the presence of IL-7 and Flt3 ligand.128,129 This provides an efficient method to produce relatively large amounts of mature Ig-secreting B cells, which, in principle, could be adapted to engineer and produce specific murine or human antibodies.
It has been particularly challenging to differentiate ES cells into T lymphocytes, probably because their development requires complex thymic stromal interactions that are not easily recapitulated in vitro. Zuniga-Pflucker's group (de Pooter et al126 ) differentiated ES cells on OP9 stroma and then reseeded Flk1+CD45- cells into fetal thymic organ cultures (FTOCs). Although the fraction of T cells was low, normal CD4 and CD8 thymic subsets were generated.126 Recognizing that Notch signaling is uniquely important for the production of T cells, the same group engineered OP9 cells to express the delta-like 1 (DL-1) Notch ligand.127 Differentiation of ES cells on this modified stroma produced more extensive and complete T-cell maturation including γδ and αβ T-cell receptor (TCR)-bearing cells, as well as mature CD8+ cells that express the TCR and produce interferon γ (IFN-γ) in response to stimulation. However, further maturation in FTOCs is required to render these ES cell-derived late-stage T-cell progenitors competent to functionally reconstitute nonobese diabetic-severe combined immunodeficiency (NOD/SCID) mice. This system represents an important technical advance in the manipulation of ES cells and should be useful for examining the cell-autonomous and environmental factors that promote T-lymphocyte differentiation and maturation. The finding that ES cell-derived T-lymphocyte progenitors can become self-tolerant and functional after transplantation into mice has potential clinical utility if these experimental methods can be adapted to humans.
Macrophages
Macrophages can be produced from ES cells using either EB or OP9 culture systems. ES cell-derived macrophages express Mac1 and lysozyme RNA and stain with F4/80, a macrophage-specific antibody.47,48 In addition, they exhibit numerous properties of arterial lesion macrophages and, therefore, offer research opportunities in the field of atherosclerosis.123,124 In vitro differentiation of ES cells has been used to study the effects of specific gene knockouts on macrophage function. For example, Guillemot et al125 used this approach to demonstrate that GDID4, a guanine diphosphate dissociation inhibitor that regulates the cycling of Rho family GTP-binding proteins, is required for normal superoxide production by macrophages.
Dendritic cells
Dendritic cells (DCs) are potent antigen-presenting cells that can promote either immune response or self-tolerance. ES cells offer a potential source of normal and genetically manipulated DCs that could be used for a variety of clinical applications.133-135 ES cell-derived DCs expand in the presence of IL-3 and GM-CSF and are induced to mature by tumor necrosis factor α (TNF-α) and IL-4 plus lipopolysaccharide or anti-CD40. Similar to their bone marrow-derived counterparts, ES cell-derived DCs express characteristic markers, process foreign antigens, and stimulate primary T-cell responses in the mixed leukocyte reaction. In one interesting application, Senju et al133 randomly introduced a reporter gene flanked by loxP recombination sequences into ES cells and identified a clone in which reporter expression was particularly high in DC progeny generated by in vitro differentiation. Then, they introduced ovalbumin cDNA into this active locus by Cre-mediated recombination to generate a DC line that efficiently presented antigens to prime ovalbumin-specific cytotoxic T lymphocytes in vivo. This method has potential clinical applications if it can be widely applied to other antigens.
NK lymphocytes
Natural killer (NK) cells, an important arm of the innate immune system, destroy cells that fail to present the correct major histocompatibility complex (MHC) class I receptor, including some virally infected and malignant clones. Lian et al136 induced ES cells to differentiate into functional NK cells that killed certain tumor lines and MHC class I-deficient lymphoblasts. These findings provide a mechanism to further examine NK-cell biology and possibly to manufacture these cells for antiviral and anticancer therapies.
Osteoclasts
Osteoclasts, which are derived from HSCs, are involved in bone resorption and remodeling. Therefore, understanding the biology of this lineage has important implications for various bone disorders. Methods to derive osteoclasts from ES cells in vitro have been developed and refined, mainly by Hayashi's group (Yamane et al,137,138 Tsuneto et al,139 Okuyama et al,140 and Hemmi et al141 ). Cytokines that promote osteoclast differentiation and maturation from ES cells include M-CSF and receptor activator of nuclear factor κB ligand (RANKL). Osteoclast production is also augmented by ascorbic acid, dexamethasone, and 1α, 25-dihydroxyvitamin D3. Analysis of gene-targeted ES cells using these culture methods identified several signaling pathways that participate in osteoclastogenesis. For example, ES cells lacking transcription factor NFATc1 are deficient in RANKL-induced osteoclastogenesis, suggesting that NFATc1 is downstream of RANKL signaling.162 In a different study, Tsuneto et al163 showed that enforced expression of the myeloid transcription factor PU.1 stimulates osteoclast production in ES cells lacking stem-cell leukemia (SCL/Tal-1), a nuclear protein essential for early stages of hematopoiesis. This indicates that PU.1 is not only important for osteoclast development, but it may also bypass some early hematopoietic requirements for SCL.
Recent progress with human ES cells
In 1998, Thompson et al9 derived human ES cells from embryos. More recently, murine ES cells were developed by somatic-cell nuclear transfer (SCNT), a method in which the nucleus of an adult somatic cell is injected into a normal enucleated oocyte to generate cloned blastocysts.164 SCNT was also reportedly used to generate human ES cell and this approach should eventually open up new opportunities to generate generic and patient-specific lines.165,166
Blood from human ES cells
Methods previously established for examining hematopoiesis in murine ES cells are applicable to human studies.167,168 Human ES cells differentiate to hematopoietic lineages via EBs or culture on stromal-cell lines.169 It appears that human ES-cell differentiation cultures accurately mirror early human embryonic hematopoiesis, similar to what is observed in murine systems. For example, distinct sequential waves of primitive and definitive erythropoiesis occur in human EBs. Moreover, cells within human EBs give rise to colonies that contain mesodermal, hematopoietic, and endothelial cells arranged into structures reminiscent of yolk sac blood islands. The nature of the progenitor that gives rise to these colonies remains to be determined.170 It is possible that hemangioblasts originate from within these colonies. Along these lines, Wang et al171 showed that CD45-, PECAM-1+, Flk-1+, VE-cadherin+ cells within human EBs give rise to hematopoietic and endothelial lineages. This hints at the existence of human hemangioblasts. However, in clonality studies, the viability and capacity for hematoendothelial differentiation of these cells were low. Hence, it will be important to further fractionate these cells and optimize conditions for their culture and differentiation.
While extensive studies of murine ES cells provide a broad basis for analyzing hematopoiesis in human lines, it is important to point out that there are significant interspecies differences that could influence culture protocols.12,172 There are also differences in colony morphology, cloning efficiency, surface markers, and patterns of gene expression. In contrast to murine ES cells, serum-free methods to generate mesoderm and hematopoietic lineages from human ES cells are not yet reported. It is likely that protocols to specifically optimize hematopoiesis from human ES cells will be further developed and refined over the next few years.
Generating human HSCs
As in murine systems, generating HSCs capable of long-term multi-lineage engraftment is a vexing problem. It has been possible to produce human ES cell-derived progenitors with some features of adult-type HSCs, including CD34 expression, multi-lineage hematopoietic potential in clonal assays, the ability to efflux dyes such as Hoechst 3334 and Rhodamine 123, and high aldehyde dehydrogenase (ALDH) activity.167 However, these cells exhibit limited repopulating activity after transplantation into immunodeficient mice. Wang et al173 demonstrated that a population of human ES cell-derived progenitors incapable of repopulating the marrow after intravenous injection exhibits limited regional repopulation after intrafemoral injections. At minimum, this suggests defects in migratory capacity, homing, or niche interactions, similar to what is observed in pre-HSCs of the early mouse embryo.174 Consistent with this interpretation, multipotential progenitors derived from human ES cells exhibit a distinct gene expression signature that resembles primitive embryonic progenitors.173,175
In contrast to murine ES cells, ectopic expression of HoxB4 does not promote the expansion of bona fide HSCs from human ES cells.173 This finding could be due to technical issues or could reflect intrinsic differences in the biologies of human and mouse HSC development. In case of the latter, it is possible that manipulated expression of different proteins in human ES-cell cultures will stimulate HSC production. Candidate molecules include Hox paralogues and numerous other nuclear regulators.176-178 In considering this experimental approach for human HSC production, it will be important to use strategies that avoid stable genetic alterations by transgenes or viral vectors, which pose a risk for malignant transformation. One innovative approach fused the cell-penetrating peptide TAT to HoxB4 protein.179 This fusion protein was taken up into murine bone marrow-derived HSCs and stimulated their expansion ex vivo. This method, which allows cells to be manipulated by transcription factors without altering the genome, could be applied to ES cells.
The future
The ability to create hematopoietic cells from human ES cells opens up profound therapeutic possibilities (Figure 1). Currently, bone marrow transplantation (BMT) can cure many hematopoietic diseases, but HSC availability is often limiting and many patients lack matched tissue donors. Human ES cells offer a potentially renewable source of tissue-compatible HSCs for BMT. In addition, compared with somatic HSCs used currently for BMT, human ES cells are potentially more amenable to genetic manipulation for rescuing specific defects or for altering antigenicity to augment transplantation. Eventually, it should be possible to use SCNT to generate individualized ES-cell lines from patients with various inherited or acquired disorders; treat the defect by genetic manipulation using homologous recombination, lentiviral, or siRNA approaches; and then generate transplantable HSCs by in vitro differentiation. However, numerous unsolved questions related to the biology of ES cells derived from adult somatic tissues must be further investigated. These include the nature of epigenetic influences such as X-chromosome inactivation and genomic imprinting, telomere status, and genomic stability.
It is also possible that replacement therapies using mature hematopoietic lineages derived from human ES cells could be of clinical utility. For example, in vitro-manipulated human DCs are being explored as a mechanism to tolerize against autoimmune disease or to stimulate antitumor immune responses. However, the number of DCs that can be isolated from patients using current technologies is limiting.180 Using human ES cells as a source for generating DCs could circumvent this problem. Additionally, ES cells appear to be a particularly robust source for expanding erythroid progenitors compared with fetal liver and adult tissues.120 By merging several established technologies, it may be possible to produce large quantities of mature red blood cells from human ES cells.181 In principle, this represents a limitless source for erythrocyte transfusions and for laboratory reagents used in blood bank testing. This may be especially useful for rare red blood-cell types with limited donor availability. In addition, it may be possible through genetic manipulation to design improved universal donor erythrocytes with a limited antigenic repertoire. Similarly, functional granulocytes and megakaryocytes or their committed progenitors could be used to treat neutropenia and thrombocytopenia, respectively. One potentially interesting application for the latter would be to manipulate hemostasis by engineering human ES cells to produce modified platelets that deliver ectopically expressed procoagulant or anticoagulant factors directly to the site of blood clots.182,183
Several additional problems must be further addressed to facilitate the transit of ES cells from bench to bedside. Like all organs, hematopoietic tissues develop in the context of a complex and poorly understood 3-dimensional microenvironment composed of paracrine factors, stromal contacts, and physical forces that vary during development. Defining these microenvironments and recapitulating them in vitro may provide a key to more efficiently recapitulating hematopoiesis, particularly HSC generation. In addition, continued refinement of serum-free and stroma-free culture systems will minimize pathogen contamination and provide tighter control of cellular differentiation.
It is also critical to develop new approaches for testing ES cell-based therapies. For example, transplantation into NOD/SCID immunodeficient mice may not be the most sensitive or effective method to evaluate ES cell-derived human hematopoietic cells. To gain more information on the safety and utility of ES cell-based therapies, it is important to use model systems that more closely approximate humans. Accordingly, several groups are examining hematopoietic development from nonhuman primate ES cells.184-188 In general, these lines exhibit hematopoiesis similar to what is observed from human ES cells. While these studies are relatively preliminary, continued investigation of nonhuman primate ES cells will provide improved preclinical models for ES cell-derived hematopoietic transplantation therapies.
In addition to therapeutic applications, new technologies for making blood from human ES cells will shed light on hematopoietic ontogeny, which is difficult to study in the human embryo for ethical and practical reasons. Due to inherent developmental differences, not all conclusions drawn from murine ES cells will be applicable to the human embryo. Comparative studies to examine the onset of hematopoiesis in human and murine ES-cell differentiation cultures should elucidate fundamental similarities and differences between these species.
Note added in proof: The generation of human ES cells by somatic-cell nuclear transfer described in references 165 and 166 has recently come under question, and it seems likely that one or both papers will be retracted. Nonetheless, we remain hopeful that this technology is feasible and can eventually be utilized to develop patient-specific ES cells for therapeutic purposes.
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2005-09-3621.
Supported by National Institutes of Health (NIH) grants R01 DK064037 (M.J.W.) and T32-HL007971-04 (D.L.S.).
We thank Gerd Blobel, Gordon Keller, Marcela Maus, and Toru Nakano for comments and suggestions on the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal