Abstract
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disorder maintained by cancer stem cells. To target this population, we investigated the mechanism of action of BMS-214662, developed as a farnesyl transferase inhibitor (FTI) and unique in inducing apoptosis in these cells. By contrast, a related congener and equally effective FTI, BMS-225975 does not induce apoptosis, indicating a novel mechanism of action. BMS-214662 significantly and selectively induced apoptosis in primitive CD34+38− CML compared with normal cells. Apoptosis proceeded via the intrinsic pathway: Bax conformational changes, loss of mitochondrial membrane potential, generation of reactive oxygen species, release of cytochrome c, and caspase-9/3 activation were noted. Up-regulation of protein kinase Cβ (PKCβ), down-regulation of E2F1, and phosphorylation of cyclin A–associated cyclin-dependent kinase 2 preceded these changes. Cotreatment of CML CD34+ and CD34+38− cells with PKC modulators, bryostatin-1, or hispidin markedly decreased these early events and the subsequent apoptosis. None of these events was elicited by BMS-214662 in normal CD34+ cells or by BMS-225975 in CML CD34+ cells. These data suggest that BMS-214662 selectively elicits a latent apoptotic pathway in CML stem cells that is initiated by up-regulation of PKCβ and mediated by Bax activation, providing a molecular framework for development of novel therapeutics.
Introduction
Chronic myeloid leukemia (CML) is a clonal disorder of the hematopoietic stem cell caused by the formation of the BCR-ABL oncogene as a consequence of the Philadelphia chromosome (Ph) translocation.1 Current therapy for CML involves ABL tyrosine kinase inhibitors (TKIs), including imatinib (IM), dasatinib, and nilotinib.2 Although TKIs induce rapid hematologic and cytogenetic responses, they do not eliminate BCR-ABL transcripts in the majority of patients, suggesting that TKI-insensitive leukemic stem cells persist despite otherwise effective treatment.3,4 We and others have demonstrated that quiescent Philadelphia chromosome–positive stem cells exist in all CML chronic-phase patients at diagnosis and are resistant to TKIs in vitro and in vivo.4-11
BCR-ABL signaling activates the Ras signaling pathway (among others),12 contributing to proliferation and malignant transformation. The function of Ras proteins is dependent on farnesylation, a posttranslational modification responsible for anchorage to the cell membrane13 and catalyzed by the enzyme farnesyl transferase. Farnesyl transferase inhibitors (FTIs) have been designed to target activation of oncogenes, including Ras.14 We previously demonstrated that the FTI BMS-214662, but not BMS-225975 or lonafarnib, significantly reduced quiescent CML stem cells through induction of apoptosis, suggesting that BMS-214662 may have a different mechanism of action from standard FTIs.15,16 BMS-214662 synergized with TKIs and overcame the antiproliferative effect exerted by these agents on CML stem and progenitor cells, leading to their accumulation in vitro.15 These intriguing findings suggest that BMS-214662 may target CML stem cells selectively by affecting a pathway other than farnesylation (although the farnesyl transferase inhibition may be a necessary component of this effect). As it is increasingly recognized that cancer cells may be susceptible to many different forms of cell death such as autophagy and regulated necrosis17-19 and that even classic apoptosis can be mediated via disparate pathways with regulators at distinct subcellular locations,20 we have characterized the cell death caused by this agent in CML CD34+ and CD34+38− cells derived from patients and investigated the potential pathways and mechanisms involved. Where necessary, we have also investigated whether BMS-225975 caused these changes, to determine what may be due (directly or indirectly) to FTI activity and what selective changes that lead to cell death are caused by BMS-214662.
Methods
Reagents
BMS-214662, BMS-225975, and dasatinib were obtained from Bristol-Myers Squibb. Nilotinib and IM were obtained from LC Laboratories. Lonafarnib was provided by Schering-Plough. Hispidin was from Sigma-Aldrich. Bryostatin-1 was provided by the Drug Synthesis & Chemistry Branch, Division of Cancer Treatment and Diagnosis, National Cancer Institute (NCI). All reagent-grade chemicals were purchased from Sigma-Aldrich, unless otherwise stated.
Patient samples and isolation of CD34+ and CD34+38− cells
This study was approved by the Institutional Review Board at the North Glasgow University Hospitals Division of National Health Service (NHS) Greater Glasgow and Clyde, United Kingdom, and met all requirements of the Declaration of Helsinki. All the samples used were derived from peripheral blood. Fresh leukapheresis samples were obtained from patients with newly diagnosed chronic-phase CML with written informed consent in accordance with the Declaration of Helsinki. The normal CD34+ cells were from autologous donors with non–stem cell disorders (eg, lymphoma). Samples were enriched for CD34+ cells and cultured as previously described.8 For isolation of the CD34+38− population, cells were simultaneously stained with anti–human CD34-allophycocyanin and anti–human CD38–fluorescein isothiocyanate (Becton Dickinson) at room temperature (RT) for 20 minutes. Unbound antibodies were washed off with phosphate-buffered saline/2% fetal calf serum and the cells then sorted into CD34+38− and CD34+38+ subpopulations using a BD FACSAria Cell-Sorting System (Becton Dickinson). Samples used and patient characteristics are reported in supplemental Tables 1 and 2 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Intracellular flow cytometric analysis
Drug-treated and control CD34+ cells were resuspended in Fix and Perm (Merck Chemicals Ltd). The antibody was added at RT for 1 hour and the cells were analyzed by fluorescence-activated cell sorting (FACS). Antibodies were as follows: anti–annexin V, active caspase-3, Bax (clone 3; Becton Dickinson), and phospho (p)–Bcl2 (Ser70; Abcam). FMK-ZVAD (50μM; Bachem) was added to the culture 2 hours before treatment with BMS-214662. Decrease in mitochondrial membrane potential was detected using 50nM tetramethyl rhodamine (StemCell Technologies) for 20 minutes at 37°C and analysis by FACS. Cell-cycle status was analyzed by staining cellular DNA content with propidium iodide (PI, 50 μg/mL). Data analysis was performed with FlowJo software (TreeStar). Levels of intracellular reactive oxygen species (ROS) were analyzed using the redox-sensitive fluorochrome 2′,7′-dichlorofluorescein-diacetate (Sigma-Aldrich). CD34+ cells were incubated with 5μM 2′,7′-dichlorofluorescein-diacetate and 250nM BMS-214662 for the time indicated at 37°C. Next, samples were washed and analyzed by FACS.
Western blotting
CD34+ cells were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (50mM Tri-HCl, 150mM NaCl, 1% Nonidet P40, 1mM ethylenediaminetetraacetic acid), plus protease inhibitors (Roche) or in Laemmli sample buffer (Bio-Rad) and analyzed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The proteins transferred to Hybond enhanced chemiluminescence nitrocellulose membranes (Amersham) were blocked with 10% dried milk in TBST (20mM tris(hydroxymethyl)aminomethane, pH 7.6, 13.7mM NaCl, 0.1% Tween 20) for 2 hours. Incubation with the primary antibody was carried out at 4°C overnight, and with the secondary, for 1 hour at RT. Antibodies were anti–myeloid cell leukemia 1 (Mcl1), extracellular signal-related kinase, phosphoinositide-3 kinase (PI3K), nuclear factor κB (NF-κB, p65), p57, actin, Bim, Bcl2, Bclxl, cyclin E, cyclin D1, pRb, cytochrome c, caspase-3, cleaved caspase-8, caspase-9, tubulin, poly adenosine diphosphate–ribose polymerase (PARP), protein kinase Cβ (PKCβ), phospho-cyclin-dependent kinase 2 (p-Cdk2, threonine 160), p-Akt (threonine 308), Akt (New England Biolabs), and E2F1 (Santa Cruz Biotechnology).
Activated caspase detection by precipitation with biotin-X-VAD-FMK
CD34+ cells were incubated with 50μM biotin-X-VAD-FMK (Merck) for 2 hours at 37°C. Cells were then treated for 24 hours with BMS-214662. Cells were lysed in RIPA lysis buffer plus protease inhibitors and centrifuged at 15 000g for 10 minutes. Streptavidin-agarose was then added to the supernatants and agitated at 4°C for 24 hours, after which lysates were precipitated, washed with RIPA lysis buffer for 10 minutes by rocking at 4°C, and resolved by SDS-PAGE. Caspases were detected by immunoblotting.
Preparation of mitochondrial fractions
CD34+ cells were treated for 24 hours with 250nM BMS-214662. Cells were washed twice in cold phosphate-buffered saline and once in cold mitochondrial isolation buffer (MIB, 200mM mannitol, 70mM sucrose, 1mM ethyleneglycoltetraacetic acid, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.05% bovine serum albumin, pH 7.4). Pellets were then resuspended in MIB and incubated at 4°C for 5 minutes. The cells were homogenized by 60 passes in a tight-fitting dounce hand homogenizer. The homogenates were separated by centrifugation at 750g for 5 minutes, yielding a mitochondria-rich supernatant and a cell debris pellet. The mitochondria were finally pelleted by centrifugation at 10 000g for 10 minutes and resuspended in a minimal volume of MIB.
Transmission electron microscopy
CD34+ cells were harvested, fixed for 40 minutes at RT in 2.5% (vol/vol) glutaraldehyde in 0.1M cacodylate buffer, rinsed in the same buffer containing 2% sucrose, and postfixed in 1% osmium tetroxide (wt/vol) in 0.1M cacodylate buffer, pH 7.4, for 1 hour. After 3 changes in distilled water (10 minutes each), specimens were treated with 0.5% aqueous uranyl acetate, dehydrated via an alcohol series, and embedded in Araldite/EMbed 812 resin mix (Electron Microscopy Sciences). Sections (100 nm) were stained with aqueous 2% uranyl acetate and Reynold lead citrate for 10 and 5 minutes, respectively, and viewed on 300-mesh formvar-coated grids by zero-loss imaging on a LEO 912 AB energy filtering transmission electron microscope (Olympus).
Statistical analysis
Statistical analyses were performed using the Student t test. A P value of .05 or less was taken to be statistically significant. A P value of .005 or less was taken to be highly statistically significant.
Results
BMS-214662 uniquely targets primitive, quiescent CML stem cells
CD34+38− cells represent the more primitive fraction within bulk CD34+ cells and have previously been shown to be predominantly quiescent.7 Standard TKIs used in CML therapy are not able to eradicate CML, probably due to their incapacity to target this subpopulation.4,6-10,21 We separated proliferating CD34+38+ from the more primitive and quiescent CD34+38− (< 5% of total CD34+ cells) CML cells by FACS (Figure 1Ai). The 2 populations were then exposed for 48 hours to 250nM BMS-214662 or to standard TKIs (IM 5μM, dasatinib 150nM, and nilotinib 5μM) or a FTI (lonafarnib 10μM) and caspase-3 activation was measured (Figure 1Aii). By 48 hours, BMS-214662 exposure resulted in a mean of 20.3% caspase-3 activation in the CD34+38− population, compared with no-drug control (0.96%; highly significant, P < .005). For CD34+38+ cells only 16.2% showed activation of caspase-3 at this time point, however by morphology the majority of these cells were already dead, consistent with a more rapid response. The TKIs IM, dasatinib, and nilotinib showed a trend to increased apoptosis over no-drug control in both subpopulations, although not to the same extent as detected in BMS-214662–treated cells. Even if a low level of apoptosis was detected in the TKI-treated cells, most of the cells were still alive and morphologically unaffected. Although lonafarnib showed a trend toward increased apoptosis over baseline, consistent with our previous work,16 this did not achieve statistical significance in the quiescent population (for CD34+38−: lonafarnib vs no drug, P = .4). BMS-225975 is a FTI with near identical structure and in vitro FTI activity to BMS-214662, yet it differs dramatically in its ability to induce apoptosis in CML primary cells.15,22 We compared the effect of BMS-214662 and BMS-225975 in CD34+ cells derived from different CML patients (n = 3). The cells were cultured in the absence of growth factors, treated for 24 hours, and then analyzed by FACS for the apoptotic marker annexin V (Figure 1B). In BMS-214662–treated cells, 45.3% were annexin V positive after 24 hours, whereas levels of annexin V after BMS-225975 exposure were similar to the no-drug control (26.3%; BMS-214662 versus no drug and BMS-225975, P = .01). We have shown that BMS-214662 is relatively selective for CML versus normal stem cells, particularly at low concentrations (62.5nM).15 Therefore, we analyzed whether BMS-214662 exerted a different effect on the cell-cycle profile between normal and CML CD34+ cells (Figure 1C). In cell-cycle analysis using PI, the subG1 phase indicates DNA fragmentation and therefore cells undergoing apoptosis, whereas in the G1 phase the DNA is present as 2 sets of chromosomes. Whereas for normal CD34+ cells cell cycle remained almost unaffected by BMS-214662 treatment at 62.5 or 250nM (Figure 1Ci-iii), CML cells showed an initial accumulation in subG1 phase with 62.5nM (Figure 1Cv) compared with no-drug control (Figure 1Civ). At the higher concentration, the majority of CML cells were accumulated in subG1 with no cells progressing into S/G2 (Figure 1Cvi). Treatment of CML cells with BMS-225975 did not increase the subG1 phase (Figure 1Cvii). These findings confirm that BMS-214662 appears unique in targeting the subpopulation of primitive quiescent CML stem cells.
BMS-214662 treatment induces apoptosis in both CD34+38− and CD34+38+ CML cells. (A) Representative FACS dot plot showing the sort gate for CD34+38− cells (boxed region; i). CD34+38− (■) and CD34+38+ (□) cells were treated for 48 hours with no drug (1), BMS-214662 (2), IM (3), dasatinib (4), nilotinib (5), and lonafarnib (6) and activation of caspase-3 was analyzed by FACS (n = 3). The BMS-214662–treated arm showed a highly statistically significant (**) increase in caspase-3 activation in comparison with the no-drug control (P < .005; ii). (B) CD34+ CML cells (n = 3) were treated with BMS-214662 or BMS-225975 (250nM) for 24 hours and annexin V–positive cells (%) were measured by FACS as an indicator of apoptosis. *Differences that are statistically significant (P < .005). Error bars represent ± SD. (C) CD34+ normal (i-iii) and CML (iv-vi) cells (n = 3) were untreated (i,iv) or treated with 62.5nM BMS-214662 (ii,v), 250nM BMS-214662 (iii,vi), or 250nM BMS-225975 (vii) and cell-cycle analysis was performed using PI and FACS.
BMS-214662 treatment induces apoptosis in both CD34+38− and CD34+38+ CML cells. (A) Representative FACS dot plot showing the sort gate for CD34+38− cells (boxed region; i). CD34+38− (■) and CD34+38+ (□) cells were treated for 48 hours with no drug (1), BMS-214662 (2), IM (3), dasatinib (4), nilotinib (5), and lonafarnib (6) and activation of caspase-3 was analyzed by FACS (n = 3). The BMS-214662–treated arm showed a highly statistically significant (**) increase in caspase-3 activation in comparison with the no-drug control (P < .005; ii). (B) CD34+ CML cells (n = 3) were treated with BMS-214662 or BMS-225975 (250nM) for 24 hours and annexin V–positive cells (%) were measured by FACS as an indicator of apoptosis. *Differences that are statistically significant (P < .005). Error bars represent ± SD. (C) CD34+ normal (i-iii) and CML (iv-vi) cells (n = 3) were untreated (i,iv) or treated with 62.5nM BMS-214662 (ii,v), 250nM BMS-214662 (iii,vi), or 250nM BMS-225975 (vii) and cell-cycle analysis was performed using PI and FACS.
BMS-214662 induces apoptosis via the intrinsic pathway in CD34+ CML cells
We have recently shown that the effect of BMS-214662 in decreasing the number of primitive CD34+ cells derived from CML patients is likely due to apoptosis, using activation of caspase-3 as a reporter.15 Here we have further characterized this process of cell death and the potential pathway(s) involved. Exposure of CML CD34+ cells to 250nM BMS-214662 for 8 hours caused the appearance of typical structural and ultrastructural features of apoptosis, whereas no such effects were observed with BMS-225975. These included membrane shrinkage (Figure 2A), membrane blebbing, changes in the density of the matrix, reduction of nuclear size, and condensation and swelling of the mitochondria in the perinuclear region (Figure 2B). Activation of Bax was assessed by FACS using conformation-specific monoclonal antibodies that detect epitopes that are hidden in the resting state (Figure 2C). The antibodies used were clones 3 and 6A7.23,24 Similar results were obtained with both antibodies and we have reported results generated with clone 6A7. BMS-214662 did not lead to conformational changes in Bax in normal CD34+ cells, but did so in CML cells. Conformational changes of Bax were also observed in the subpopulations of CD34+38+ and CD34+38− CML cells (Figure 2C).
BMS-214662 treatment induces mitochondrial-mediated apoptosis in CD34+, CD34+38−, and CD34+38+ CML cells. (A) CD34+ CML cells were treated with 250nM BMS-214662 or BMS-225975 for up to 24 hours and observed under phase-contrast microscope. (B) Transmission electron microscopy of CD34+ CML cells treated with 250nM BMS-214662 for 12 hours. Arrows indicate mitochondria. (C) CD34+ normal and total CML CD34+, CD34+38−, and CD34+38+ cells were treated as indicated for 24 hours and conformational changes of Bax were measured (CD34+, n = 3; CD34+38− and CD34+38+, n = 2). (D) MOMP was measured in CD34+ normal and total CML CD34+, CD34+38−, and CD34+38+ cells after 24 hours of treatment with 250nM BMS-214662 (n = 3). (E) Densitometry analysis showed release of cytochrome c in CML CD34+ cells to the cytoplasm after 24-hour treatment with 250nM BMS-214662 (n = 3). Error bars represent ± SD. *P = .026. (F) CD34+ CML cells were treated as indicated and levels of ROS were measured by FACS (n = 3).
BMS-214662 treatment induces mitochondrial-mediated apoptosis in CD34+, CD34+38−, and CD34+38+ CML cells. (A) CD34+ CML cells were treated with 250nM BMS-214662 or BMS-225975 for up to 24 hours and observed under phase-contrast microscope. (B) Transmission electron microscopy of CD34+ CML cells treated with 250nM BMS-214662 for 12 hours. Arrows indicate mitochondria. (C) CD34+ normal and total CML CD34+, CD34+38−, and CD34+38+ cells were treated as indicated for 24 hours and conformational changes of Bax were measured (CD34+, n = 3; CD34+38− and CD34+38+, n = 2). (D) MOMP was measured in CD34+ normal and total CML CD34+, CD34+38−, and CD34+38+ cells after 24 hours of treatment with 250nM BMS-214662 (n = 3). (E) Densitometry analysis showed release of cytochrome c in CML CD34+ cells to the cytoplasm after 24-hour treatment with 250nM BMS-214662 (n = 3). Error bars represent ± SD. *P = .026. (F) CD34+ CML cells were treated as indicated and levels of ROS were measured by FACS (n = 3).
The activation of Bax was associated with evidence of mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome c from the mitochondria. As an indirect consequence of MOMP, we also observed a decrease in mitochondrial membrane potential, measured by staining CML CD34+ cells with the potentiometric dye tetramethyl rhodamine, which was not seen in normal cells (Figure 2D). Decrease in mitochondrial membrane potential was also detected in the subpopulations of CD34+38+ and CD34+38− CML cells (Figure 2D). Mitochondrial and cytosolic fractions of cell lysates were analyzed by immunoblotting for cytochrome c and measured by densitometry. A significant (P = .026) increase of cytochrome c in the cytoplasmic fraction was noted in treated compared with untreated cells (Figure 2E). A progressive increase in the generation of ROS was also detected in CD34+ CML cells (Figure 2F).
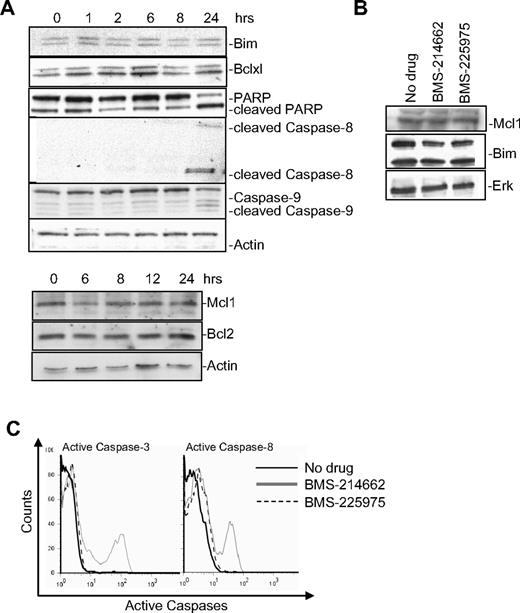
According to the displacement model, Bax activation with subsequent MOMP can result from loss of inhibition by antiapoptotic Bcl2 family members through their down-regulation or degradation.25 Therefore, we examined the effects of BMS-214662 on the expression of Mcl1, Bcl2, or Bclxl in CD34+ CML cells. No changes in the levels of these proteins were noted by immunoblotting over the period when apoptosis was induced nor in the expression of the Bax BH3-only activator Bim (Figure 3A).
Expression of Bcl2 family members in BMS-214662–induced apoptosis. (A) Time course of treatment with BMS-214662 (250nM) in CML CD34+ cells showed changes in protein levels after Western blotting analysis (n = 3). (B) Treatment with BMS-214662 or BMS-225975 (250nM) for 24 hours did not show changes in Mcl1 and Bim levels (n = 3). SDS gel 4% to 15%. (C) Activation of caspase-3 and caspase-8 in CML CD34+ cells measured after treatment with BMS-214662 and BMS-225975 (250nM; n = 3).
Expression of Bcl2 family members in BMS-214662–induced apoptosis. (A) Time course of treatment with BMS-214662 (250nM) in CML CD34+ cells showed changes in protein levels after Western blotting analysis (n = 3). (B) Treatment with BMS-214662 or BMS-225975 (250nM) for 24 hours did not show changes in Mcl1 and Bim levels (n = 3). SDS gel 4% to 15%. (C) Activation of caspase-3 and caspase-8 in CML CD34+ cells measured after treatment with BMS-214662 and BMS-225975 (250nM; n = 3).
As a consequence of caspase-3 activation by the Bax-induced cytochrome c release, PARP degradation was detected at 24 hours (Figure 3A). When CML CD34+ cells were treated for 24 hours with BMS-225975 (250nM), levels of Mcl1 and Bim did not change, as they did for BMS-214662 (Figure 3B). However, BMS-214662 caused activation of caspase-3 and caspase-8, whereas BMS-225975 did not (Figure 3C).
To rule out the possibility that caspase-3 activation caused by BMS-214662 was an upstream event in mitochondrial apoptosis, Bax conformational changes were analyzed in CD34+ CML cells exposed to 50μM pan-caspase inhibitor FMK-ZVAD 2 hours before addition of 250nM BMS-214662. Although caspase-3 activation was completely rescued by FMK-ZVAD (Figure 4Ai), Bax conformational changes were not prevented (Figure 4Aii), indicating that activation of caspase-3 was downstream in the process of mitochondrial apoptosis. It is likely that activated caspase-9 was the mediator of caspase-3 activation, even though activation of both caspases-9 and -8 was detected after 24 hours of treatment (Figure 3A). However, caspase-8 activation may reflect secondary activation by caspase-326 rather than a marker of engagement of the extrinsic pathway, as no activated caspase-8 was captured by biotin-X-VAD-FMK27 (Figure 4B), and Z-IETD-FMK, a specific caspase-8 inhibitor, did not prevent apoptosis (data not shown).
BMS-214662–induced caspase-3 activation is a downstream effect of the mitochondrial pathway. (A) Representative dot plots of the measurement of caspase-3 activation after cotreatment of CD34+ CML cells with 250nM BMS-214662 and 50μM FMK-ZVAD (i). Conformational changes of Bax (clone 3) were measured in cells treated as indicated (n = 3, ii). (B) In situ trapping of initiator caspase using biotin-X-VAD-FMK in CD34+ CML cells after 24-hour treatment with BMS-214662 (250nM) showed immunoprecipitation of caspase-3, but not caspases-8 or -9 (n = 3).
BMS-214662–induced caspase-3 activation is a downstream effect of the mitochondrial pathway. (A) Representative dot plots of the measurement of caspase-3 activation after cotreatment of CD34+ CML cells with 250nM BMS-214662 and 50μM FMK-ZVAD (i). Conformational changes of Bax (clone 3) were measured in cells treated as indicated (n = 3, ii). (B) In situ trapping of initiator caspase using biotin-X-VAD-FMK in CD34+ CML cells after 24-hour treatment with BMS-214662 (250nM) showed immunoprecipitation of caspase-3, but not caspases-8 or -9 (n = 3).
Taken together, all these data indicate that BMS-214662 treatment leads to activation of the mitochondrial pathway of apoptosis selectively in CD34+ CML cells.
BMS-214662 induces high levels of active cyclin A–associated Cdk2 and causes rapid down-regulation of E2F1
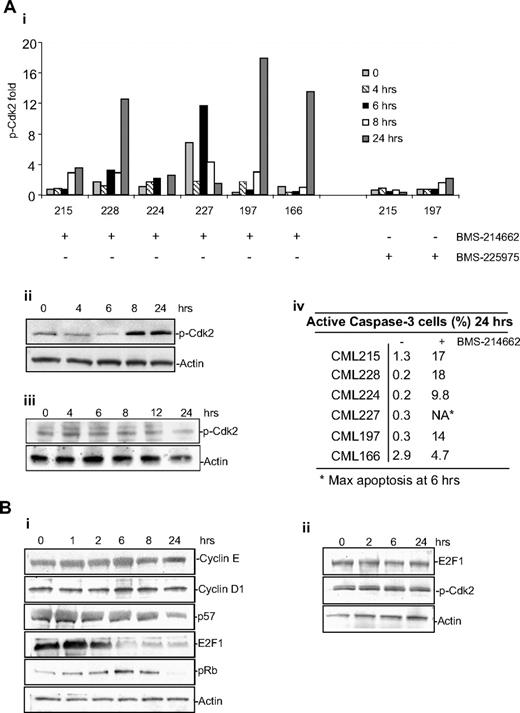
Because abnormal activation of cell-cycle proteins can result in cell death, we analyzed the activation of Cdk2, a protein that plays a critical role in cell-cycle regulation and in specific cellular contexts has been reported to mediate apoptosis, for example in etoposide-induced apoptosis28 as well as apoptosis in resting cells.29 Although each CML sample analyzed showed a different kinetic of response, BMS-214662 consistently caused a marked increase in phosphorylation of Cdk2 during the time in all samples analyzed (n = 6, Figure 5Ai; representative blot for sample CML215 shown in Figure 5Aii). The effect of BMS-214662 on the activation and levels of Cdk2 was selective, as cyclin E or cyclin D1 up-regulation was not observed (Figure 5Bi), and treatment with BMS-225975 did not cause phosphorylation of Cdk2 (Figure 5Aiii). The relative amount of p-Cdk2 was correlated with activation of caspase-3 as an index of apoptosis (Figure 5Aiv). Despite this significantly increased Cdk2 activity, CML stem cells did not enter S phase (Figure 1Cv-vi). To examine whether other cell-cycle proteins were deregulated by BMS-214662 as a potential explanation for why increased Cdk2 phosphorylation did not cause cell-cycle entry, we also noted an early decrease in E2F1 and p57 levels at 6 and 24 hours, respectively (Figure 5Bi). Furthermore, the level of pRb increased after 6 hours of treatment, but completely disappeared by 24 hours (Figure 5Bi). The alterations in E2F1 and p-Cdk2 were not seen in normal CD34+ cells after treatment with BMS-214662 over a 24-hour time course (Figure 5Bii). Thus the changes noted in CML CD34+ cells would be expected to yield discordant signals concerning cell-cycle arrest (increased pRb, decreased E2F1) or cell-cycle entry (increased phosphorylation of Cdk2). These data suggest that BMS-214662–induced apoptosis is mediated by selective deregulation of the cell-cycle proteins in a normally quiescent population—deregulation that precedes and potentially triggers the mitochondrial apoptotic pathway.
BMS-214662 activates catastrophic cell cycle. (A) BMS-214662 (250nM) induced phosphorylation of Cdk2 in CD34+ CML cells derived from patients (n = 6; i-ii), whereas BMS-225975 (250nM) did not (n = 3, iii). Percentage of active caspase-3–positive cells is reported (iv). (B) Time course of treatment with BMS-214662 (250nM) in CML CD34+ cells showed changes in protein levels after Western blotting analysis (i). Time course of treatment with BMS-214662 (250nM) in normal CD34+ cells showed no changes in protein levels after Western blotting analysis (n = 3, ii). SDS gel 4% to 15%.
BMS-214662 activates catastrophic cell cycle. (A) BMS-214662 (250nM) induced phosphorylation of Cdk2 in CD34+ CML cells derived from patients (n = 6; i-ii), whereas BMS-225975 (250nM) did not (n = 3, iii). Percentage of active caspase-3–positive cells is reported (iv). (B) Time course of treatment with BMS-214662 (250nM) in CML CD34+ cells showed changes in protein levels after Western blotting analysis (i). Time course of treatment with BMS-214662 (250nM) in normal CD34+ cells showed no changes in protein levels after Western blotting analysis (n = 3, ii). SDS gel 4% to 15%.
Cotreatment with PKC inhibitors decreases BMS-214662–induced early changes in E2F1 and p-Cdk2 and prevents apoptosis in CML CD34+ cells
Our data showed that BMS-214662 induces rapid apoptosis in noncycling cells by causing aberrant regulation of cell-cycle proteins. To determine which signal transduction pathways may cause or mediate these changes, we examined by immunoblot analysis the effect of BMS-214662 on major cellular survival pathways that are known to be affected by BCR-ABL expression and therefore deregulated in CML cells. By 24 hours, BMS-214662 exposure caused a decrease in the levels of PI3K, NF-κB, and p-Akt (Figure 6Ai).
BMS-214662 activates PKCβ. (A) BMS-214662 (250nM) exposure caused a decrease in levels of PI3K, NF-κB, p-Akt, and Akt (i). Levels of E2F1 and NF-κB were compared after treatment for 24 hours with 250nM BMS-225975 or BMS-214662 (ii; n = 3). (B) Changes in PCKβ levels were analyzed by Western blotting after treatment with 250nM BMS-214662, 100nM bryostain-1, or a combination (i) and densitometry was performed (n = 3, ii). Treatment of normal CD34+ cells with BMS-214662 did not affect levels of PKCβ (iii). Treatment of CML CD34+ cells with BMS-225975 did not increase the level of PKCβ (iv). Error bars represent ± SD.
BMS-214662 activates PKCβ. (A) BMS-214662 (250nM) exposure caused a decrease in levels of PI3K, NF-κB, p-Akt, and Akt (i). Levels of E2F1 and NF-κB were compared after treatment for 24 hours with 250nM BMS-225975 or BMS-214662 (ii; n = 3). (B) Changes in PCKβ levels were analyzed by Western blotting after treatment with 250nM BMS-214662, 100nM bryostain-1, or a combination (i) and densitometry was performed (n = 3, ii). Treatment of normal CD34+ cells with BMS-214662 did not affect levels of PKCβ (iii). Treatment of CML CD34+ cells with BMS-225975 did not increase the level of PKCβ (iv). Error bars represent ± SD.
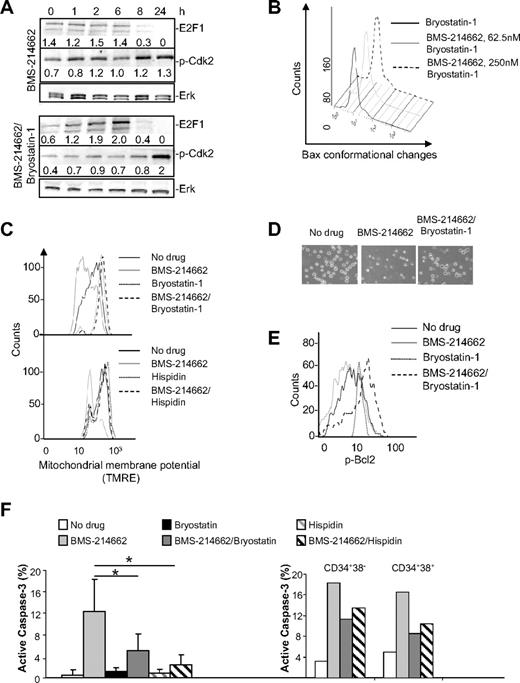
The effect of BMS-225975 on E2F1 and NF-κB protein levels was detectable, but much less than in the BMS-214662–treated cells, suggesting that the 2 drugs may affect similar pathways, but with differing potency (Figure 6Aii). However, as these changes were noted after the effect on the cell-cycle proteins, they may transduce this signal to trigger the mitochondrial apoptotic pathway, but are not involved in initiating it. By contrast, we noted early changes in PKCβ levels at 8 hours and thus at time points simultaneous with or before the deregulation of cell-cycle proteins (Figure 6Bi-ii). We therefore investigated the effects of the PKC agonist bryostatin-1, which is known to modulate apoptosis in other systems.30 Indeed, whereas bryostatin-1 alone after 8 hours decreased levels of PKCβ, cotreatment of CML CD34+ cells with BMS-214662 and bryostatin-1 prevented the up-regulation of PKCβ (Figure 6Bi-ii). Importantly, treatment of normal CD34+ cells with BMS-214662 did not affect levels of PKCβ (Figure 6Biii). Finally, treatment with BMS-225975 did not cause an increase of PKCβ in CML cells (Figure 6Biv).
Significantly, all the other biochemical changes elicited by treating cells with BMS-214662 were also mitigated or prevented by cotreatment with bryostatin-1. Specifically, the early decrease in E2F1 and increased phosphorylation of Cdk2 were modulated or delayed (Figure 7A). Densitometry was performed and fold induction relative to extracellular signal-related kinase–loading control is indicated. Bax conformational changes in CML CD34+ cells observed upon treatment with BMS-214662 (Figure 2C) were reversed by coaddition of bryostatin-1 (Figure 7B). To confirm that BMS-214662–induced apoptosis was mediated by an increase in PKCβ levels, cells were cotreated with hispidin, a specific inhibitor of the PKCβ isoform.31 As predicted, the consequences of MOMP were also not seen in CML CD34+ cells cultured in the presence of BMS-214662 and either bryostatin-1 or hispidin. In fact, cotreatment with bryostatin-1 or hispidin completely rescued the loss of mitochondrial membrane potential in CML CD34+ cells (Figure 7C). By morphology the obvious presence of dying cells in the presence of BMS-214662 was completely rescued after cotreatment with bryostatin-1 (Figure 7D).
Bryostatin-1 and hispidin rescue BMS-214662–induced apoptosis. (A) Decrease in E2F1 and increased phosphorylation of Cdk2 mediated by BMS-214662 were modulated by 100nM bryostatin-1 as shown by Western blotting analysis (n = 3). (B) Conformational changes of Bax were measured by FACS in CML cells after addition of 250nM BMS-214662, 100nM bryostatin-1, or combination for 24 hours (n = 3). (C) CD34+ CML cells were treated as in panel A (top panel) or with 250nM BMS-214662, 5μM hispidin (bottom panel), or combination for 24 hours and loss of MOMP was measured (n = 3). (D) Cells were treated as indicated and observed under phase-contrast microscope. (E) Bcl2 phosphorylation was analyzed by FACS in CD34+ CML cells after treatment with no drug or 250nM BMS-214662 or cotreatment with BMS-214662 and 100nM bryostatin-1 for 24 hours (n = 3). (F) Activation of caspase-3 was measured in CML CD34+ cells after treatment with 250nM BMS-214662, 100nM bryostatin-1, or 5μM hispidin or cotreatment (n = 5). Activation of caspase-3 was also measured in CD34+38− and CD34+38+ CML cells after treatment with 250nM BMS-214662, 100nM bryostatin-1, and 5μM hispidin (n = 2). Error bars represent ± SD. *P < .05.
Bryostatin-1 and hispidin rescue BMS-214662–induced apoptosis. (A) Decrease in E2F1 and increased phosphorylation of Cdk2 mediated by BMS-214662 were modulated by 100nM bryostatin-1 as shown by Western blotting analysis (n = 3). (B) Conformational changes of Bax were measured by FACS in CML cells after addition of 250nM BMS-214662, 100nM bryostatin-1, or combination for 24 hours (n = 3). (C) CD34+ CML cells were treated as in panel A (top panel) or with 250nM BMS-214662, 5μM hispidin (bottom panel), or combination for 24 hours and loss of MOMP was measured (n = 3). (D) Cells were treated as indicated and observed under phase-contrast microscope. (E) Bcl2 phosphorylation was analyzed by FACS in CD34+ CML cells after treatment with no drug or 250nM BMS-214662 or cotreatment with BMS-214662 and 100nM bryostatin-1 for 24 hours (n = 3). (F) Activation of caspase-3 was measured in CML CD34+ cells after treatment with 250nM BMS-214662, 100nM bryostatin-1, or 5μM hispidin or cotreatment (n = 5). Activation of caspase-3 was also measured in CD34+38− and CD34+38+ CML cells after treatment with 250nM BMS-214662, 100nM bryostatin-1, and 5μM hispidin (n = 2). Error bars represent ± SD. *P < .05.
In cytokine-dependent cell lines phosphorylation of Bcl2 on serine 70 (Ser70) leads to inhibition of apoptosis.32,33 We therefore analyzed the level of Bcl2 phosphorylation in CD34+ CML cells and observed that it was reduced by treatment with BMS-214662 for 24 hours (Figure 7E). The addition of bryostatin-1 completely rescued the decrease in Bcl2 phosphorylation observed after BMS-214662 treatment. Bryostatin-1 also caused an increase in levels of Bcl2 phosphorylation when added alone.
BMS-214662 induced 12.3% activation of caspase-3, whereas with bryostatin-1 the level of active caspase-3 was reduced to 5% in CD34+ CML cells (Figure 7F). When cells where cotreated with BMS-214662 and hispidin, the level of active caspase-3 decreased to 2.4%. To determine whether a similar rescue of apoptosis occurred in CD34+38+ and CD34+38− subpopulations, cells were sorted and treated with BMS-214662 plus or minus bryostatin-1 or hispidin. Bryostatin-1 and hispidin rescued BMS-214662–induced caspase-3 activation in both the CD34+38+ and CD34+38− subpopulations, confirming the mechanism of action for BMS-214662 in inducing apoptosis is retained for the most primitive CML cells.
Discussion
The introduction of TKI for the treatment of CML has markedly altered the natural history of this disease and has allowed most patients excellent disease control.34 However, it is evident, almost a decade after the introduction of IM, that even optimally responding patients still harbor a small number of cells that mediate relapse when the drug is discontinued.3,4 Both theoretical models and experimental data suggest that this is due to the persistence of a quiescent leukemic stem cell population that is much less or completely insensitive to these drugs.4,8,9,11,35 Therefore complete eradication of the disease will require an enhanced understanding of the nature of these cells and the development of therapeutic strategies to effectively target them. Very recently several laboratories have indicated signaling pathways that are responsible for the expansion and/or quiescence of both normal hematopoietic stem cells and leukemic stem cells, indicating potential therapeutic targets.36-42 We have previously reported another approach, which is further investigated here, whereby an agent originally designed as a FTI unexpectedly and uniquely exhibited direct cytotoxicity against CML stem cells.15 Here we report that this activity is selective for CML compared with normal stem cells and must depend on a mechanism of action that is separate from (but may still require) inhibition of farnesyl transferase, as a very closely related congener of this drug, BMS-225975, lacked this selective cytotoxicity.
The basis of this selectivity of BMS-214662 for CML stem cells is unclear. The most obvious way that these stem cells differ from normal stem cells is the kinase activity of BCR-ABL. However, maintained activity of the ABL kinase at the time of exposure to BMS-214662 cannot be the reason for selectivity as we have previously shown that combining BMS-214662 with TKIs does not abrogate its activity.15 Therefore, the basis of the selectivity either is in another cellular feature in leukemic cells that is “stamped in” by prior tyrosine kinase activity and therefore induces a durable effect even when the catalytic activity of the enzyme is inhibited, or is due to a second required hit occurring during disease development; such an event must be well conserved across patients because of the universal activity of BMS-214662 that we have observed to date. Critically, we have not observed variation in sensitivity to BMS-214662 in relation to those samples derived from IM-responsive versus IM-nonresponsive patients.
Our studies have indicated that this activity elicits apoptosis through the intrinsic apoptotic pathway and is initiated by up-regulation of PKCβ. It is noteworthy that bryostatin-1 has complex effects on the entire PKC family (early induction and late inhibition), and has been examined extensively as an agent that modifies cell proliferation, differentiation, and apoptosis in many cells types.43-48 Furthermore, we have reported that, if bryostatin-1 is used before IM in CD34+ cells from CML patient samples, the antiproliferative effect of IM is decreased, with the consequence that more cells die.49 However, if both agents are used together, there is no additional effect, that is, the CD34+ cells were resistant to IM with or without bryostatin-1. By contrast, we report a different phenomenon here: BMS-214662 causes the early up-regulation of PKCβ and pretreatment with bryostatin-1 depletes PKCβ thus inhibiting apoptosis. The requirement for PKCβ activity in this apoptotic cascade was confirmed using a structurally unrelated, more selective and “nondegradative” inhibitor of PKCβ (hispidin) that demonstrated the same effect. Therefore the fact that the proapoptotic activity of BMS-214662 is prevented by 2 completely different inhibitors of PKCβ suggests another link in our understanding of the mechanism: the drug up-regulates PKCβ that is then able to phosphorylate a substrate that is dependent on the prior tyrosine kinase activity uniquely present in CML compared with normal stem cells. The effect of this substrate phosphorylation then leads to a cascade of events eventually eliciting mitochondrial apoptosis. One consequence that may be directly relevant to inducing death in this largely nonproliferating population of cells is the induction of discordant signals altering the phosphorylation and/or level of protein of critical components of the cell-cycle machinery. It is therefore possible that this leads to a series of events akin to what has previously been described as mitotic catastrophe that ultimately initiates the mitochondrial pathway of apoptosis.28,50-52 Alternatively, because cell-cycle regulation and apoptosis are intimately regulated, these discordant signals for divide/do not divide may actually be a consequence of rather than a cause of activation of the mitochondrial pathway of apoptosis. Whatever the mechanism, it is important to note that these cells do not need to be in cycle for the drug to induce apoptosis, a unique and clinically important feature of this agent for treating this population of cells.
Based upon our current understanding of the regulation of the mitochondrial pathway of apoptosis, the activation of Bax that we observed may be the result of 2 separate processes.19 On one hand the direct activation model would predict that BMS-214662 causes the activation of one of the BH3-only proapoptotic activators of Bax such as Bim, Bid, p53, or PUMA. Our results indicate that the levels of Bim do not change, but leave open the possibility that other posttranslational modifications may have altered Bim and/or any of the other candidate BH3-only proteins to mediate cell death. Alternatively, the displacement model would predict that Bax is activated by the removal of the activity of an antiapoptotic Bcl-2 family member, such as Bcl-2. In this context, we did observe a dynamic change in the phosphorylation status of Bcl-2 that is caused by BMS-214662 and inhibited by the PKCβ inhibitors. The exact mechanism whereby Bax is activated in this context is clinically relevant as newer agents that selectively target one or other of these Bcl-2 family member interactions are currently under development and may synergize with BMS-214662 to further enhance its cytotoxic effect in these quiescent CML stem cells.53
The cancer stem cell hypothesis, widely but not universally adopted by researchers in cancer biology, poses a challenge for the appropriate design and interpretation of clinical trials of agents that target these cells, as is well illustrated by the history of BMS-214662.54 The drug has shown promising albeit somewhat modest activity in a variety of solid tumors and acute myeloid leukemia when given to patients.55,56 With respect to the acute myeloid leukemia study, the degree of activity noted may have been due to the less specific and important farnesyl transferase inhibition as other FTIs in this patient population also lead to similarly modest activity.57 It will therefore be an ongoing challenge to design clinical trials with the appropriate dose and schedule and the relevant readout to test drugs such as BMS-214662 that by themselves may do little to decrease the bulk of a non–stem cell population that is clinically evident and has traditionally been used as an assessment of tumor activity to carry drugs forward for further clinical studies. In CML, perhaps the most appropriate setting would be after TKI treatment, to significantly reduce overall tumor burden, when minimal residual disease is easily detectable and trackable. In this setting both agents would be used in combination in an attempt to eradicate the minimal residual disease. Our study demonstrating selectivity for CML stem cells and elucidating the pathway involved in this cytotoxicity provides important clues for selecting other effective drug combinations using BMS-214662 for further investigation and in identifying stem cells in other cancers that may be susceptible to this approach.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Eyal Gottlieb and Dr Zachary Shug (The Beatson Institute for Cancer Research) for providing help in the mitochondrial separation and for other useful suggestions; Dr Laurence Tetley for the transmission electron microscopy (University of Glasgow); Dr Roberto Weinmann, Dr Francis Lee (Bristol-Myers Squibb), and Dr G. Vignir Helgason (Paul O'Gorman Leukemia Research Center) for suggestions and discussion. We acknowledge Dr Richard Clark, Dr Guy Lucas, and Dr Pat Shepherd for providing most of the samples used in this study. We thank all hematologists and nonmedical staff from the United Kingdom who have contributed to our biobank.
F.P. received funding from Bristol-Myers Squibb, the Rockefeller Foundation, and Glasgow Royal Infirmary Endowments.
Authorship
Contribution: F.P. designed the research and performed all the experiments, analyzed the data, and cowrote the manuscript; M.C. edited and proofed the manuscript and provided suggestions in experimental design; J.M. provided suggestions in experimental design; H.G.J. performed FACS sorting; B.L. provided suggestions in experimental design and cowrote the manuscript; T.L.H. supervised the project and cowrote the manuscript; and all authors commented on the manuscript.
Conflict-of-interest disclosure: T.L.H. and B.L. each receives per year approximately $10 000 combined from consultancy, speaking fees, honoraria, and service on advisory boards from BMS and Novartis. T.L.H. and B.L. also received research funding from BMS. M.C. has undertaken consultancy work for BMS and is on its Advisory Board and has received honoraria from both BMS and Novartis within the last 2 years. The remaining authors declare no competing financial interests.
Correspondence: Tessa Holyoake, Paul O'Gorman Leukaemia Research Centre, Gartnavel General Hospital, 1053 Great Western Rd, Glasgow, G12 0YN, United Kingdom; e-mail: tlh1g@clinmed.gla.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal