Abstract
Thrombocytopenia is a frequent symptom and clinical challenge in patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Eltrombopag is a small molecule thrombopoietin receptor agonist that might be a new option to treat thrombocytopenia in these diseases, provided that it does not stimulate malignant hematopoiesis. In this work, we studied the effects of Eltrombopag on proliferation, apoptosis, differentiation, colony formation, and malignant self-renewal of bone marrow mononuclear cells of patients with AML and MDS. Malignant bone marrow mononuclear cells did not show increased proliferation, or increased clonogenic capacity at concentrations of Eltrombopag ranging from 0.1 to 30 μg/mL. On the contrary, we observed a moderate, statistically nonsignificant (P = .18), decrease of numbers of malignant cells (mean, 56%; SD, 28%). Eltrombopag neither led to increased 5-bromo-2-deoxyuridine incorporation, decreased apoptosis, an increase of malignant self-renewal, nor enhanced in vivo engraftment in xenotransplantations. Furthermore, we found that Eltrombopag was capable of increasing megakaryocytic differentiation and formation of normal megakaryocytic colonies in patients with AML and MDS. These results provide a preclinical rationale for further testing of Eltrombopag for treatment of thrombocytopenia in AML and MDS.
Introduction
The management of thrombocytopenia is a clinical challenge in patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Thrombocytopenia occurs as a consequence of ineffective hematopoiesis and generation of a hematopoiesis-suppressing microenvironment by the malignant cell clone in the bone marrow (BM), and consequently, leads to an increased risk of bleeding (for review, see Hellström-Lindberg and Malcovati1 and Kantarjian et al2 ). Hemorrhagic complications are frequent in patients with MDS, in particular in patients with high-risk MDS who progress to AML, and are a direct or contributing cause of death in 14% to 30% of patients with MDS.2-6 Bleeding complications can further aggravate coexisting anemia, which is another frequent symptom and hallmark of MDS and AML. In clinical management of MDS, thrombocytopenia is an important parameter contributing to treatment decisions. Thus, alleviation of thrombocytopenia would be clinically beneficial, due to a decrease of thrombocytopenia-caused complications and mortality as well as reduced necessity for treatment, including platelet transfusions, chemotherapy, immunosuppressive agents, and others.
Recently, the novel nonpeptide thrombopoietin receptor agonist Eltrombopag was approved for treatment of patients with chronic idiopathic thrombocytopenic purpura (ITP) in the United States. Eltrombopag has been shown to effectively elevate platelet numbers and reduce thrombocytopenia-related complications in patients with chronic ITP or hepatitis C.7,8 Eltrombopag might therefore also be suitable as a new option to tackle thrombocytopenia in patients with MDS or AML. To date, Eltrombopag has not been examined in the context of myeloid malignancies, and thus, at least 2 major questions need to be addressed: First, does Eltrombopag potentially stimulate malignant hematopoiesis in patients with AML or MDS? Second, is Eltrombopag effective in stimulating megakaryopoiesis in these patients, similar to its effect in chronic ITP and hepatitis C?
To address these questions, we studied the effects of Eltrombopag on BM mononuclear cells (BM-MNC) of patients with AML and MDS as well as healthy controls. Using a variety of assays assessing proliferation, apoptosis, differentiation, colony-forming capacity, and self-renewal, we found no evidence that Eltrombopag stimulates malignant blasts or enhances the self-renewal capacity of leukemia cells in these patients. Furthermore, we found that Eltrombopag is capable of increasing megakaryopoiesis in patients with AML and MDS. Our results provide a preclinical rationale for the testing of Eltrombopag for treatment of thrombocytopenia in patients with AML and MDS.
Methods
Patients' samples
Samples were obtained from patients with AML or MDS within the framework of routine diagnostic punctures after written informed consent obtained in accordance with the Declaraiton of Helsinki. Healthy control BM samples (n = 5) were obtained from a commercial source (StemCell Technologies). We included samples from a select group of patients with AML and MDS with an excess of blasts, of which sufficient numbers of BM-MNC were available. Patients were diagnosed according to the criteria of the French-American-British classification.9,10 We examined 8 patients with de novo AML (1 FAB M1, 2 FAB M2, 2 FAB M4, 3 FAB M5), 6 patients with AML secondary to MDS, and 4 patients with primary MDS (2 RAEB II, 2 RAEB I). Cytomorphology of the samples was reviewed, and blast counts ranged between 21% and 89% in the AML patients, and between 5% and 19% in the MDS patients. We also examined 4 samples from patients in remission, including 2 AML (1 FAB M1, 1 FAB M7), 1 AML secondary to MDS, and 1 MPD/MDS (CMML). Seven of 22 samples were c-Mpl+. Experiments were approved by the institutional review board of the Albert Einstein College of Medicine (CCI 2005-536 and 2008-884).
Reagents
Eltrombopag was provided by GlaxoSmithKline. The reagent was dissolved in ddH2O at various 100× stock concentrations (3 mg/mL, 1 mg/mL, 0.3 mg/mL, 0.1 mg/mL, and 0.01 mg/mL) and stored light protected at room temperature for up to 2 weeks.
Isolation of mononuclear cells and enrichment of CD34+ cells
Mononuclear cells from anticoagulated (EDTA [ethylenediaminetetraacetic acid]) BM aspirates (BM-MNC) from patients with MDS/AML and healthy volunteers were isolated by density centrifugation using Ficoll-Hypaque (GE Healthcare). Cells were collected and washed 3 times with phosphate-buffered saline. For immunomagnetic enrichment of CD34-expressing cells, CD34+ microbeads (Miltenyi Biotec) were used, as previously described.11
Cell culture and staining
Primary human hematopoietic cells from BM were cultured for 5 to 12 days in serum-free CellGro medium (Cellgenix) supplemented with 40 μg/mL human low-density lipoprotein (Sigma-Aldrich), 10 ng/mL human recombinant (hr) interleukin 3 (IL-3; PeproTech), 25 ng/mL hrIL-6 (PeproTech), 50 ng/mL hr stem cell factor (hrSCF; PeproTech), 50 ng/mL hrFlt3L (PeproTech), and 100 ng/mL hr thrombopoietin (TPO; PeproTech), where indicated. Primary cells were exposed to various concentrations of Eltrombopag (ranging from 0.1 μg/mL to 30 μg/mL) by diluting 100× stock solutions in the culture medium.
Cytospins were prepared using a StatSpin Cytofuge (IRIS International). Cytospins and blood smears were stained with Diff-Quick Wright-Giemsa Stain Kit (IMEB). Morphology was evaluated and documented using an inverted microscope with a color camera (Carl Zeiss).
In vitro colony formation assays
For characterization of granulocytic, monocytic, and erythrocytic colony-forming units (CFU), 30 000 BM-MNC per 1 mL or 1000 CD34+ cells per 1 mL of human MethoCult GF H4434 (StemCell Technologies) were plated in duplicate and cultured, according to the manufacturer's recommendations. Cultures were either untreated, or contained 100 ng/mL hrTPO alone or Eltrombopag at various concentrations. Colonies were scored using an inverted microscope after 12 days of incubation.
For characterization of megakaryocytic CFU (CFU-Mk), we used the Megacult-C CFU-Mk assay kit (StemCell Technologies). Briefly, BM-MNC (100,000 cells/mL) or CD34+ cells (10 000 cells/mL) were incubated at 5% CO2 and > 95% humidity in double-chamber slides in a 100-mm petri dish along with an open 35-mm petri dish containing 3 mL of sterile water for 12 days. For assays with BM-MNC, the culture medium contained 1.1 mg/mL collagen, 50 ng/mL hrTPO, 10 ng/mL hrIL-6, and 10 ng/mL hrIL-3. For experiments with CD34+ cells, 50 ng/mL hrSCF, 50 ng/mL hrFlt3L, and 40 μg/mL human low-density lipoproteins (Sigma-Aldrich) were added.12 Slides were fixed with methanol-acetone solution after removing the double chamber and stored at −20°C. Immunocytochemical stainings were performed with anti–human GPIIb/IIIa (CD41a) antibody, biotin-conjugated goat anti–mouse IgG, avidin-alkaline phosphatase conjugate, and alkaline phosphatase substrate. CFU-Mk were defined as clusters of more than 3 CD41a+ cells, and subdivided into groups by size: small (3-20 cells per colony; referred to as colonies arising from a mature Mk progenitor cell), medium (21-49 cells per colony; referred to as colonies arising from an immature Mk progenitor cell), large (> 50 cells per colony; colonies arising from the most immature Mk progenitor cell), and immature megakaryocyte/erythrocyte (Mk/E) progenitors (leading to the formation of mixed colonies composed of Mk and non-Mk cells).13 Colonies containing both CD41a+ and CD41a− cells were scored as mixed colonies originating from the most immature progenitors capable of differentiating into erythroid and Mk cells.
Serial replating assays
Serial replating assays were performed, as previously described, to assess long-term self-renewal in vitro.14-16 In brief, 30 000 BM-MNC or 1000 CD34+ cells per 1 mL of human MethoCult GF H4434 (StemCell Technologies) were plated in duplicate. Cultures contained 100 ng/mL hrTPO, 0 μg/mL, or 3 μg/mL Eltrombopag, respectively. Colonies were scored using an inverted microscope after 12 days of incubation, and cells were recovered from the methylcellulose medium. Cells were washed 3 times with magnetic-activated cell sorting buffer (Hanks balanced saline solution without Ca2+, Mg2+, 0.5% bovine serum albumin [BSA; wt/vol], 2 mM EDTA, pH 8.0), and viable cells were enumerated (trypan blue staining; Invitrogen). A total of 30 000 cells/mL was replated in MethoCult GF H4434 (StemCell Technologies) under identical conditions. Colonies were scored after another 12 days, and cells were again replated until colony growth was exhausted.
Flow cytometric analyses
For measuring expression of differentiation markers, cells were stained with the following antibodies: anti–human CD45 allophycocyanin (APC; eBioscience), anti–human CD34 APC (Invitrogen/Caltag Laboratories), anti–human CD33 phycoerythrin (PE; eBioscience), anti–human CD41a fluorescein isothiocyanate (eBioscience), anti–glycophorin A PE-Cy5 (Invitrogen/Caltag Laboratories), anti–human CD15 fluorescein isothiocyanate (eBioscience), or anti–human CD14 PE-Cy5 (Caltag Laboratories). Apoptosis was assessed using Annexin V staining (Annexin V Fluos; Roche Applied Bioscience). Proliferation was assessed by incorporation of 5-bromo-2-deoxyuridine (BrdU; BrdU in situ detection kit; BD Pharmingen). Immunostained cells were analyzed using an LSRII fluorescence-activated cell sorter (FACS) analyzer (BD Biosciences). To assess the phosphorylation status of signal transducer and activator of transcription (Stat)3 and Stat5, we performed flow cytometric phosphoprotein analysis, as previously described.17-19 The following phosphospecific antibodies were used (BD Biosciences): PE-Cy7 anti-Stat5 (pY694) and Pacific Blue anti-Stat3 (pY705). After 4 hours serum starvation in RPMI medium, cells were treated with 0 or 3 μg/mL Eltrombopag, or 100 ng/mL hrTPO (PeproTech) in RPMI for 60 minutes at 37°C. Cells were fixed and permeabilized, as described previously. In brief, BD Cytofix Fixation Buffer (BD Biosciences) was added 1:1 (vol/vol), and samples were incubated for 10 minutes at 37°C. Cells were permeabilized with BD Phosphoflow Perm Buffer III and then stained with anti–phospho-Stat3 (anti–p-Stat3) and anti–p-Stat5 antibodies (1/5 dilutions). Cells were analyzed with an LSRII FACS analyzer (BD Biosciences).
Xenotransplantations
All animal experiments were performed in compliance with the institutional guidelines of the Institute for Animal Studies and approved by the Animal Institute Committee of the Albert Einstein College of Medicine (protocol 20080109). BM-MNC of 3 patients with AML were analyzed for CD34 and CD38 expression, and the BM-MNC equivalent of 10 000 CD34+/CD38− cells was transplanted into nonobese diabetic–severe combined immunodeficiency–IL-2Rγ null (NOG) mice (The Jackson Laboratory) after total body irradiation of 300 cGy (Shepard cesium-137 source), by injecting 800 000 to 2 000 000 BM-MNC into the retro-orbital vein of anesthetized mice 4 hours after irradiation. Before and after transplantation, mice were kept on antibiotics, according to the manufacturer's recommendations. Engraftment of human cells was monitored after 4 weeks by staining peripheral blood samples from the mandibular vein of transplanted mice with an APC anti–human CD45 (eBioscience) antibody. Mice showing a donor cell chimerism of more than 1% in the periphery 4 weeks after transplantation were considered successfully transplanted. For each patient, the cohort of successfully transplanted mice was divided into 2 groups. One group was given Eltrombopag at a concentration of 0.3 mg/mL in the drinking water, whereas the other group was kept on regular water. Resulting serum levels of Eltrombopag were on average 2290 ng/mL plus or minus 769 ng/mL and thus, similar to effective in vitro concentrations. Mice were killed and analyzed 12 weeks after the beginning of Eltrombopag treatment.
Statistical analysis
Data are presented as the mean plus or minus standard deviation, unless otherwise noted. Differences between 2 groups of data were analyzed by the Student t test. Significance was set at P less than .05.
Results
In the present study, we examined the effect of the novel nonpeptide thrombopoietin receptor agonist Eltrombopag on BM-MNC of patients with MDS and AML (MDS/AML). Cell growth, myeloid and Mk colony formation (suspension culture, colony-forming assays), proliferation (BrdU incorporation assays), apoptosis (Annexin V assays), and differentiation (cytomorphology; FACS analysis of differentiation markers) were assessed. Malignant self-renewal was tested in vitro and in vivo by serial replating assays and xenotransplantation experiments.
Eltrombopag does not enhance malignant cell growth of MDS/AML patient-derived BM-MNC
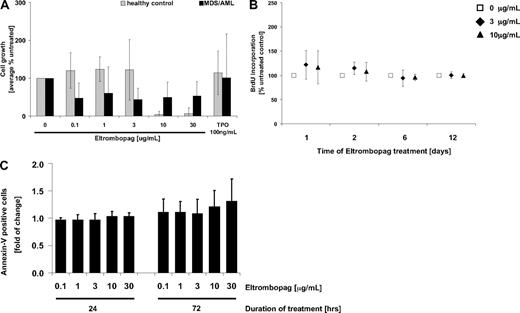
An important concern regarding the potential use of Eltrombopag in patients with AML and MDS is that it might potentially stimulate malignant hematopoiesis in these diseases. To address this question, BM cells (BM-MNC) of 10 patients (5 primary AML, 3 AML secondary to MDS, 2 primary MDS) and 5 healthy controls were exposed to different concentrations of Eltrombopag (0.1-30 μg/mL), and cell growth was monitored (Figure 1). In the examined cohort, Eltrombopag did not stimulate proliferation of MDS/AML patient-derived BM-MNC, as determined by 12 days of culture in methylcellulose (Figure 1A). On the contrary, we observed a decrease in the numbers of malignant cells in 8 of 10 patients after Eltrombopag treatment, whereas cell numbers of 2 patients were unchanged. This effect was already seen at lower concentrations of Eltrombopag (0.1-3 μg/mL). However, due to interindividual variation, this inhibition was not statistically significant (mean, 56%; SD, 28%; P = .18 at 3 μg/mL Eltrombopag). At higher concentrations (10 μg/mL, 30 μg/mL), Eltrombopag significantly inhibited cell growth of normal control BM-MNC (P < .002; Figure 1A). Moreover, we did not detect an increase of BrdU uptake of MDS/AML-derived BM-MNC after 1, 2, 6, and 12 days of suspension culture with Eltrombopag in comparison with untreated cells (Figure 1B). We also did not detect a reduction in apoptosis of the malignant cells of any of the patients tested 24 or 72 hours after Eltrombopag treatment (Figure 1C). In summary, MDS/AML patient-derived BM-MNC did not show increased proliferation or decreased apoptosis upon treatment with Eltrombopag ex vivo.
Eltrombopag does not enhance growth of malignant BM cells from patients with MDS/AML. (A) Cumulative cell numbers after 12-day culture in semisolid methylcellulose medium (n = 5 normal controls; n = 10 MDS/AML). BM-MNC were seeded in methylcellulose containing hrIL-3, hrSCF, hrIL-6, insulin, and transferrin. Cultures were supplemented with either 0, 0.1, 1, 3, 10, or 30 μg/mL Eltrombopag, or 100 ng/mL hrTPO. (B) BrdU incorporation in MDS/AML BM-MNC in the presence of either 0, 3, or 10 μg/mL Eltrombopag. BM-MNC of MDS/AML patients were kept in liquid cultures containing BSA, insulin, and transferrin, and cultures were supplemented with hrIL-3, hrIL-6, hrSCF, and hrFLT3L. Cells were incubated with the different concentrations of Eltrombopag for 1, 2, 6, or 12 days. BrdU incorporation was measured by FACS analysis. Averages and SDs (error bars) are indicated (n = 6). (C) Apoptotic activity upon exposure to increasing concentrations of Eltrombopag. BM-MNC of MDS/AML patients were kept in liquid cultures containing BSA, insulin, and transferrin, supplemented with hrIL-3, hrIL-6, hrSCF, and hrFLT3L. Cells were incubated with either 0, 0.1, 1, 3, 10, or 30 μg/mL Eltrombopag for 24 and 72 hours and analyzed for apoptotic cells (Annexin V+) by FACS. Averages and error bars (SD) are shown (n = 6).
Eltrombopag does not enhance growth of malignant BM cells from patients with MDS/AML. (A) Cumulative cell numbers after 12-day culture in semisolid methylcellulose medium (n = 5 normal controls; n = 10 MDS/AML). BM-MNC were seeded in methylcellulose containing hrIL-3, hrSCF, hrIL-6, insulin, and transferrin. Cultures were supplemented with either 0, 0.1, 1, 3, 10, or 30 μg/mL Eltrombopag, or 100 ng/mL hrTPO. (B) BrdU incorporation in MDS/AML BM-MNC in the presence of either 0, 3, or 10 μg/mL Eltrombopag. BM-MNC of MDS/AML patients were kept in liquid cultures containing BSA, insulin, and transferrin, and cultures were supplemented with hrIL-3, hrIL-6, hrSCF, and hrFLT3L. Cells were incubated with the different concentrations of Eltrombopag for 1, 2, 6, or 12 days. BrdU incorporation was measured by FACS analysis. Averages and SDs (error bars) are indicated (n = 6). (C) Apoptotic activity upon exposure to increasing concentrations of Eltrombopag. BM-MNC of MDS/AML patients were kept in liquid cultures containing BSA, insulin, and transferrin, supplemented with hrIL-3, hrIL-6, hrSCF, and hrFLT3L. Cells were incubated with either 0, 0.1, 1, 3, 10, or 30 μg/mL Eltrombopag for 24 and 72 hours and analyzed for apoptotic cells (Annexin V+) by FACS. Averages and error bars (SD) are shown (n = 6).
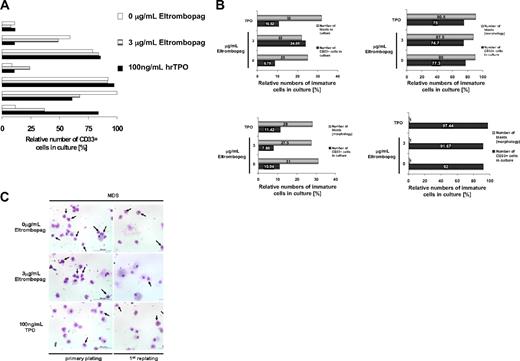
Consistent with the diagnosis of MDS/AML, initially the patients showed elevated numbers of CD34+ and/or CD33+ cells (AML, median 61%, range 21%-89%; MDS, 5%-19%). To specifically examine the malignant blast cell compartment within the BM-MNC population of MDS/AML patients upon Eltrombopag treatment, we examined the relative abundance of CD34- and CD33-expressing cells in ex vivo cultures of MDS/AML-derived BM-MNC (Figure 2). BM-MNC cultures of patients with MDS and AML were examined morphologically and for surface marker expression after 12 days of treatment with Eltrombopag in methylcellulose medium. FACS analysis did not show an increase of relative numbers of CD33+ (Figure 2A) or CD33+/CD34+ cells (data not shown) within the population of cultured cells upon treatment with 3 μg/mL Eltrombopag in comparison with untreated cells in 5 of 7 MDS/AML patients. In 2 of 7 samples, we observed a 2- to 3-fold increase of myeloid CD33 single-positive cells. However, morphologic evaluation of the Eltrombopag-treated BM-MNC revealed no increase in the percentage of blast cells in presence of Eltrombopag in these patients (Figure 2B). We also evaluated morphology after the first replating in methylcellulose. Although we did not find an increase in any of the examined patients, in one MDS patient we observed a decrease in the relative blast percentage after the first replating (34%) in presence of Eltrombopag, in comparison with cultures without treatment or in presence of hrTPO (55%; Figure 2C). Taken together, Eltrombopag did not lead to an increase of the relative abundance of malignant cells or an expansion of blast cells in any of the examined BM samples from patients with MDS/AML.
Eltrombopag does not increase relative percentages of leukemic blasts. (A) Relative numbers of CD33-expressing myeloid cells in cultures of MDS/AML patient-derived BM-MNC in the presence of Eltrombopag. BM-MNC of MDS/AML patients were seeded in liquid cultures containing BSA, insulin, and transferrin, supplemented with hrIL-3, hrIL-6, hrSCF, and hrFLT3L. Cells were incubated with either 0 or 3 μg/mL Eltrombopag or 100 ng/mL hrTPO for 12 days. CD33+ cells were assessed by FACS analysis. Results from 7 individual patients are shown. (B) Relative abundance of leukemic blasts and CD33+ cells in cultures of MDS/AML patient-derived BM-MNC in presence of Eltrombopag. BM-MNC of MDS/AML patients were grown in cytokine-supplemented medium and incubated with either 0 or 3 μg/mL Eltrombopag for 5 days. Relative blast counts were assessed from cytospin preparations, and CD33+ cells were assessed by FACS analysis. Results from 4 individual patients are shown. (C) Relative abundance of leukemic blasts after plating of MDS BM-MNC in methylcellulose. BM-MNC were cultured in cytokine-supplemented methylcellulose for 12 days and then replated, in the presence or absence of Eltrombopag or 100 ng/mL hrTPO. Cytospins were prepared and stained with Wright-Giemsa after the primary plating as well as the replating. Pictures of a representative MDS patient are shown. Blasts are indicated by arrows.
Eltrombopag does not increase relative percentages of leukemic blasts. (A) Relative numbers of CD33-expressing myeloid cells in cultures of MDS/AML patient-derived BM-MNC in the presence of Eltrombopag. BM-MNC of MDS/AML patients were seeded in liquid cultures containing BSA, insulin, and transferrin, supplemented with hrIL-3, hrIL-6, hrSCF, and hrFLT3L. Cells were incubated with either 0 or 3 μg/mL Eltrombopag or 100 ng/mL hrTPO for 12 days. CD33+ cells were assessed by FACS analysis. Results from 7 individual patients are shown. (B) Relative abundance of leukemic blasts and CD33+ cells in cultures of MDS/AML patient-derived BM-MNC in presence of Eltrombopag. BM-MNC of MDS/AML patients were grown in cytokine-supplemented medium and incubated with either 0 or 3 μg/mL Eltrombopag for 5 days. Relative blast counts were assessed from cytospin preparations, and CD33+ cells were assessed by FACS analysis. Results from 4 individual patients are shown. (C) Relative abundance of leukemic blasts after plating of MDS BM-MNC in methylcellulose. BM-MNC were cultured in cytokine-supplemented methylcellulose for 12 days and then replated, in the presence or absence of Eltrombopag or 100 ng/mL hrTPO. Cytospins were prepared and stained with Wright-Giemsa after the primary plating as well as the replating. Pictures of a representative MDS patient are shown. Blasts are indicated by arrows.
Eltrombopag does not affect malignant long-term self-renewal in ex vivo cultures of MDS/AML-derived BM-MNC
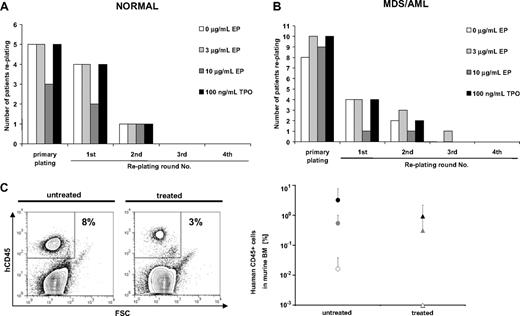
To investigate whether Eltrombopag changes long-term self-renewal of normal (n = 5) or malignant (n = 13) BM-MNC, we examined long-term in vitro colony formation in serial replating assays (Figure 3). Neither normal nor malignant BM-MNC showed an increase in plating efficiencies during the course of 4 consecutive rounds of replating over a period of 60 days. All normal BM-MNC samples showed colony formation in the primary plating regardless of treatment (Figure 3A). Four of 5 of the normal BM-MNC samples, either untreated or treated with 100 ng/mL hrTPO or 3 μg/mL Eltrombopag, showed colony formation in the first replating. Colony formation only persisted until the second round of replating in 1 of 5 samples under any of the tested conditions (Figure 3A). Treatment of normal BM-MNC with 10 μg/mL Eltrombopag reduced the number of samples replating after the primary plating and the first round of replating. In this study, only 3 of 5 samples formed colonies in the initial plating, and 2 of 5 in the first replating. Only 1 of 5 patients replated until the second replating in all conditions tested (Figure 3A).
Eltrombopag does not increase long-term self-renewal of normal or MDS/AML patient-derived BM-MNC. Serial replating assays of BM-MNC were performed in methylcellulose containing hrIL-3, hrSCF, hrIL-6, insulin, and transferrin. Cultures were supplemented with 0, 3, or 10 μg/mL Eltrombopag or 100 ng/mL hrTPO. After 12 days of culture, cells were isolated from the methylcellulose medium and replated onto the next plate. Serial replatings were carried out for 4 rounds of replating or until colony formation exhausted. (A) Serial relating of BM-MNC from healthy donors (n = 5). (B) Serial replating of BM-MNC from patients with MDS/AML (n = 13). (C) Xenotransplantation assays of BM-MNC from MDS/AML patients (n = 3). BM-MNC were transplanted into NOG mice after sublethal irradiation. Successfully engrafted mice were either treated with Eltrombopag (0.3 mg/mL) in the drinking water or left untreated. After 12 weeks of Eltrombopag treatment, all mice were killed and analyzed for human CD45-expressing cells by FACS. (Left) Representative dot plot of human CD45 analysis. (Right) Chimerism of human CD45+ cells in xenografted mice (n = 20 total) with or without Eltrombopag treatment. Averages and SDs of mouse cohorts of each of the 3 transplanted samples are shown. None of the cohorts showed higher engraftment upon treatment with Eltrombopag.
Eltrombopag does not increase long-term self-renewal of normal or MDS/AML patient-derived BM-MNC. Serial replating assays of BM-MNC were performed in methylcellulose containing hrIL-3, hrSCF, hrIL-6, insulin, and transferrin. Cultures were supplemented with 0, 3, or 10 μg/mL Eltrombopag or 100 ng/mL hrTPO. After 12 days of culture, cells were isolated from the methylcellulose medium and replated onto the next plate. Serial replatings were carried out for 4 rounds of replating or until colony formation exhausted. (A) Serial relating of BM-MNC from healthy donors (n = 5). (B) Serial replating of BM-MNC from patients with MDS/AML (n = 13). (C) Xenotransplantation assays of BM-MNC from MDS/AML patients (n = 3). BM-MNC were transplanted into NOG mice after sublethal irradiation. Successfully engrafted mice were either treated with Eltrombopag (0.3 mg/mL) in the drinking water or left untreated. After 12 weeks of Eltrombopag treatment, all mice were killed and analyzed for human CD45-expressing cells by FACS. (Left) Representative dot plot of human CD45 analysis. (Right) Chimerism of human CD45+ cells in xenografted mice (n = 20 total) with or without Eltrombopag treatment. Averages and SDs of mouse cohorts of each of the 3 transplanted samples are shown. None of the cohorts showed higher engraftment upon treatment with Eltrombopag.
We did not find an increase in long-term self-renewal of malignant MDS/AML BM-MNC in 13 samples tested (Figure 3B). Ten of 13 examined samples formed colonies on the initial plate. In the first and second replating, the number of colony-forming samples was equal in the groups treated with 3 μg/mL Eltrombopag (4 of 10) or 100 ng/mL hrTPO (4 of 10) in comparison with untreated samples (4 of 10). Only 1 of the 10 MDS/AML patients' BM-MNC samples treated with Eltrombopag showed replating until the third round. None of the samples showed replating in the fourth round of replating. Furthermore, similar to our observations in normal BM-MNC, treatment of MDS/AML BM-MNC with a higher dose of Eltrombopag (10 μg/mL) significantly reduced the number of samples replating after the primary plating (1 of 10).
Eltrombopag does not increase in vivo engraftment of MDS/AML cells in immunocompromised mice
Next, we tested the effect of Eltrombopag in vivo by using xenotransplantation assays, as previously described.20,21 We transplanted cells from 3 AML patients into sublethally irradiated nonobese diabetic–severe combined immunodeficiency–IL-2Rγ null (NOG) mice, and engraftment was checked 4 weeks after transplantation. The cohorts of successfully transplanted mice (6 mice for patient 1, 10 mice for patient 2, 4 mice for patient 3) were split into 2 groups each. One group was treated with Eltrombopag, whereas the other group was left untreated. After 12 weeks of treatment, mice were killed and analyzed (Figure 3C). Consistent with our findings ex vivo, Eltrombopag treatment did not result in higher AML cell engraftment in transplanted NOG mice in comparison with untreated mice (Figure 3C). These findings confirm the results of the in vitro assays.
In summary, our results show that in the cohort of patients used in this study, treatment with Eltrombopag does neither stimulate malignant growth, nor proliferation in vitro or in vivo. We neither detected a decrease of apoptosis nor an increase of in vitro self-renewal capabilities, nor did Eltrombopag enhance in vivo engraftment of AML cells in a xenotransplantation model.
Eltrombopag stimulates megakaryopoiesis in normal controls as well as in MDS/AML
Next, we examined the specific effect of Eltrombopag on Mk colony formation (Figure 4). Normal BM-MNC were plated in collagen- or methylcellulose-based semisolid cultures in presence of increasing concentrations of Eltrombopag. Eltrombopag effectively increased Mk colony formation of BM-MNC in a concentration-dependent manner (0.1-10 μg/mL Eltrombopag; Figure 4A). Eltrombopag treatment resulted in up to 4-fold increased Mk colony numbers in comparison with untreated cells. Stimulation with a high concentration (100 ng/mL) of hrTPO as a positive control was only slightly (20%) more effective. We next asked which progenitor stages are affected by Eltrombopag treatment. To address this question, cultures were assessed for growth of colonies arising from the following: (1) mature Mk precursors (leading to the formation of small colonies); (2) immature Mk progenitors (leading to the formation of medium and large colonies); and (3) immature Mk/E progenitors (leading to the formation of mixed colonies composed of Mk and non-Mk cells; Figure 4B). Upon treatment with 1 to 3 μg/mL Eltrombopag, colony formation of Mk progenitor and precursor cells was stimulated nearly as efficiently as those treated with 100 ng/mL hrTPO (100%). Immature Mk/E mixed colonies were formed up to 68%, immature Mk progenitor-derived colonies were formed up to 87%, and mature Mk precursor-derived colonies were formed up to 79% of the hrTPO control. Compared with untreated cells, Eltrombopag efficiently enhanced formation of immature, mixed Mk colonies up to 3-fold, formation of colonies arising from immature Mk progenitors up to 4-fold, and formation of mature Mk colonies up to 2-fold compared with untreated cells in a dose-dependent manner. Interestingly, even at very low concentrations (0.1 μg/mL) Eltrombopag increased immature Mk colony numbers by 3-fold (Figure 4B).
Eltrombopag stimulates megakaryopoietic progenitor cells in normal BM-MNC. (A) Total number of Mk colonies in the presence of increasing concentrations of Eltrombopag (n = 3). BM-MNC were seeded in semisolid collagen-based medium containing hrIL-3 and hrIL-6. Cultures were supplemented with either 0, 0.1, 1, 3, or 10 μg/mL Eltrombopag or 100 ng/mL hrTPO. Mk colonies were enumerated after 12 days of culture. (B) Differential colony count of Mk progenitor-derived colonies. Relative number of colonies from most immature bipotent Mk progenitors (□), immature Mk progenitors and precursors ( ), and mature Mk progenitor cells (■) are shown (see inset for colony morphology). Error bars indicate SD (n = 3). (C) Formation of non-Mk, myeloid colonies is not altered in the presence of Eltrombopag. BM-MNC were seeded in cytokine-supplemented methylcellulose medium. After 12 days of culture, CFU from granulocytic and monocytic progenitor cells (CFU-G, CFU-GM, CFU-M), erythrocytic progenitor cells (BFU-E, CFU-E), and granulocyte/erythrocyte/Mk/monocyte progenitor cells (CFU-EMM) were enumerated. Averages are shown. Error bars indicate SD (n = 3).
), and mature Mk progenitor cells (■) are shown (see inset for colony morphology). Error bars indicate SD (n = 3). (C) Formation of non-Mk, myeloid colonies is not altered in the presence of Eltrombopag. BM-MNC were seeded in cytokine-supplemented methylcellulose medium. After 12 days of culture, CFU from granulocytic and monocytic progenitor cells (CFU-G, CFU-GM, CFU-M), erythrocytic progenitor cells (BFU-E, CFU-E), and granulocyte/erythrocyte/Mk/monocyte progenitor cells (CFU-EMM) were enumerated. Averages are shown. Error bars indicate SD (n = 3).
Eltrombopag stimulates megakaryopoietic progenitor cells in normal BM-MNC. (A) Total number of Mk colonies in the presence of increasing concentrations of Eltrombopag (n = 3). BM-MNC were seeded in semisolid collagen-based medium containing hrIL-3 and hrIL-6. Cultures were supplemented with either 0, 0.1, 1, 3, or 10 μg/mL Eltrombopag or 100 ng/mL hrTPO. Mk colonies were enumerated after 12 days of culture. (B) Differential colony count of Mk progenitor-derived colonies. Relative number of colonies from most immature bipotent Mk progenitors (□), immature Mk progenitors and precursors ( ), and mature Mk progenitor cells (■) are shown (see inset for colony morphology). Error bars indicate SD (n = 3). (C) Formation of non-Mk, myeloid colonies is not altered in the presence of Eltrombopag. BM-MNC were seeded in cytokine-supplemented methylcellulose medium. After 12 days of culture, CFU from granulocytic and monocytic progenitor cells (CFU-G, CFU-GM, CFU-M), erythrocytic progenitor cells (BFU-E, CFU-E), and granulocyte/erythrocyte/Mk/monocyte progenitor cells (CFU-EMM) were enumerated. Averages are shown. Error bars indicate SD (n = 3).
), and mature Mk progenitor cells (■) are shown (see inset for colony morphology). Error bars indicate SD (n = 3). (C) Formation of non-Mk, myeloid colonies is not altered in the presence of Eltrombopag. BM-MNC were seeded in cytokine-supplemented methylcellulose medium. After 12 days of culture, CFU from granulocytic and monocytic progenitor cells (CFU-G, CFU-GM, CFU-M), erythrocytic progenitor cells (BFU-E, CFU-E), and granulocyte/erythrocyte/Mk/monocyte progenitor cells (CFU-EMM) were enumerated. Averages are shown. Error bars indicate SD (n = 3).
To characterize the effect of Eltrombopag on other hematopoietic lineages, including the granulocytic/monocytic and erythrocytic lineages, we assessed myeloid colony formation. Treatment with increasing concentrations of Eltrombopag (ranging from0.1 to 10 μg/mL) did not result in a significant change in differentiation or maturation characteristics of the granulocytic/monocytic or the erythrocytic lineages, as assessed by FACS analysis for expression of CD14, CD15, and glycophorin A in cells cultured for 10 days in liquid culture (data not shown). In addition, we did not find altered granulocytic/monocytic or erythrocytic colony formation potential of BM-MNC in clonogenic assays (Figure 4C). These observations indicate that Eltrombopag preferentially acts on progenitor and precursor cells harboring Mk potential.
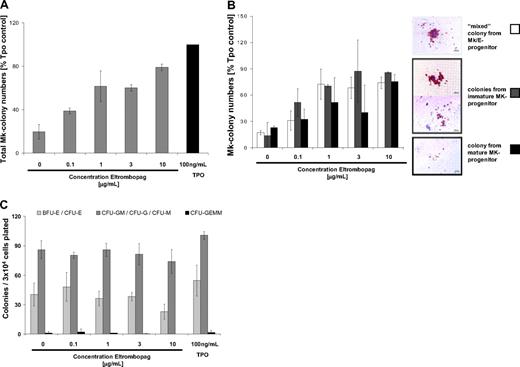
Next, we addressed the question of whether Eltrombopag is capable of stimulating megakaryopoiesis in BM-MNC of MDS/AML patients. We performed liquid differentiation cultures and colony formation assays with enriched CD34+ cells from the BM of MDS/AML patients, including MDS/AML patients in clinical remission. Treatment of CD34+ BM-MNC with either 3 μg/mL Eltrombopag alone or in combination with 20 ng/mL hrTPO increased the numbers of CD41a-expressing Mk cells by 2- to 3-fold in 3 of 5 cases (mean, 2.7-fold; SD, 0.7-fold) in a 5-day liquid differentiation culture (Figure 5A-B). In Mk colony formation assays, we observed a significant stimulation of Mk colony formation compared with untreated cells upon treatment with 3 μg/mL Eltrombopag alone or in combination with 20 ng/mL hrTPO (P = .02 and .04, respectively). Mk colony formation was increased following treatment with 3 μg/mL Eltrombopag almost as efficiently as 100 ng/mL hrTPO-treated cells in all 5 MDS/AML patient-derived CD34+ BM-MNC samples tested (mean, 90%; SD, 40%; compared with 100 ng/mL hrTPO-treated cells; Figure 5C). Using the combination treatment of 3 μg/mL Eltrombopag with 20 ng/mL hrTPO, we did not see a greater effect than with Eltrombopag alone (mean 80%, SD 40%, compared with 100 ng/mL hrTPO-treated cells; Figure 5C). Interestingly, Eltrombopag was capable of stimulating formation of Mk colonies in one patient, who did not show any colony formation in the untreated control.
Eltrombopag stimulates megakaryopoiesis in CD34+ BM-MNC cells from MDS/AML patients. (A) Number of Mk CD41a-expressing cells upon Eltrombopag treatment of CD34+ BM-MNC from MDS/AML patients (n = 5). Immunomagnetically enriched CD34+ BM-MNC were seeded in liquid cultures containing BSA, insulin, transferrin, hrIL-3, hrIL-6, hrSCF, hrFLT3L, and human low-density lipoproteins. Cells were incubated with either 0 or 3 μg/mL Eltrombopag, or 100 ng/mL hrTPO alone, or a combination of 3 μg/mL Eltrombopag and 20 ng/mL hrTPO for 5 days. CD41a+ cells were assessed by FACS analysis. The fold of change of CD41a+ cells in comparison with 100 ng/mL hrTPO-treated cultures of 5 individual patients is shown. Four of the 5 samples were treated with the combination of Eltrombopag plus TPO (one patient data not analyzed [n/a]). (B) Representative FACS histogram plot of cells upon incubation with either 0 or 3 μg/mL Eltrombopag, 100 ng/mL TPO alone, or 3 μg/mL Eltrombopag and 20 ng/mL hrTPO. (C) Eltrombopag increases the number of Mk colonies in cultures of CD34+ BM-MNC from MDS/AML patients. CD34+ BM-MNC were seeded in semisolid collagen-based medium containing hrIL-3, hrIL-6, hrSCF, hrFLT3L, and human low-density lipoproteins. Cultures were supplemented with either 0 or 3 μg/mL Eltrombopag, 100 ng/mL hrTPO alone, or 3 μg/mL Eltrombopag in combination with 20 ng/mL hrTPO. Mk colonies were enumerated after 12 days of culture. The fold of change compared with 100 ng/mL hrTPO-treated CD34+ cells is shown (n = 5). (D) Mk colony morphology of Mk colonies derived from healthy persons' and MDS/AML patients' BM-MNC. (E) Differential colony counts of Mk progenitor-derived colonies after 12 days of culture in the presence of either 0 or 3 μg/mL Eltrombopag, 100 ng/mL hrTPO alone, or 3 μg/mL Eltrombopag in combination with 20 ng/mL hrTPO. Colonies from bipotent Mk progenitors, immature Mk progenitors, and mature Mk progenitors were scored. The fold change compared with cells cultured in presence of 100 ng/mL hrTPO is shown. Results from 5 individual patients are shown.
Eltrombopag stimulates megakaryopoiesis in CD34+ BM-MNC cells from MDS/AML patients. (A) Number of Mk CD41a-expressing cells upon Eltrombopag treatment of CD34+ BM-MNC from MDS/AML patients (n = 5). Immunomagnetically enriched CD34+ BM-MNC were seeded in liquid cultures containing BSA, insulin, transferrin, hrIL-3, hrIL-6, hrSCF, hrFLT3L, and human low-density lipoproteins. Cells were incubated with either 0 or 3 μg/mL Eltrombopag, or 100 ng/mL hrTPO alone, or a combination of 3 μg/mL Eltrombopag and 20 ng/mL hrTPO for 5 days. CD41a+ cells were assessed by FACS analysis. The fold of change of CD41a+ cells in comparison with 100 ng/mL hrTPO-treated cultures of 5 individual patients is shown. Four of the 5 samples were treated with the combination of Eltrombopag plus TPO (one patient data not analyzed [n/a]). (B) Representative FACS histogram plot of cells upon incubation with either 0 or 3 μg/mL Eltrombopag, 100 ng/mL TPO alone, or 3 μg/mL Eltrombopag and 20 ng/mL hrTPO. (C) Eltrombopag increases the number of Mk colonies in cultures of CD34+ BM-MNC from MDS/AML patients. CD34+ BM-MNC were seeded in semisolid collagen-based medium containing hrIL-3, hrIL-6, hrSCF, hrFLT3L, and human low-density lipoproteins. Cultures were supplemented with either 0 or 3 μg/mL Eltrombopag, 100 ng/mL hrTPO alone, or 3 μg/mL Eltrombopag in combination with 20 ng/mL hrTPO. Mk colonies were enumerated after 12 days of culture. The fold of change compared with 100 ng/mL hrTPO-treated CD34+ cells is shown (n = 5). (D) Mk colony morphology of Mk colonies derived from healthy persons' and MDS/AML patients' BM-MNC. (E) Differential colony counts of Mk progenitor-derived colonies after 12 days of culture in the presence of either 0 or 3 μg/mL Eltrombopag, 100 ng/mL hrTPO alone, or 3 μg/mL Eltrombopag in combination with 20 ng/mL hrTPO. Colonies from bipotent Mk progenitors, immature Mk progenitors, and mature Mk progenitors were scored. The fold change compared with cells cultured in presence of 100 ng/mL hrTPO is shown. Results from 5 individual patients are shown.
The morphology and degree of maturity of Mk colonies derived from MDS/AML patients were very similar compared with colonies derived from normal controls (Figure 5D). One noticeable difference was that immature mixed colonies were on average smaller in MDS than in normal controls. To assess at which progenitor level Eltrombopag is effective, we scored different developmental subtypes of Mk colonies (Figure 5E). In 3 of 5 samples evaluated, Eltrombopag treatment was already effective in immature mixed Mk/E progenitor and immature Mk progenitor cells. In 2 samples, the increase was restricted to colonies derived from more mature Mk progenitors.
In summary, our data provide evidence that Eltrombopag is capable of increasing Mk differentiation and colony formation in BM cells from patients with MDS/AML.
Eltrombopag spares Stat3 phosphorylation
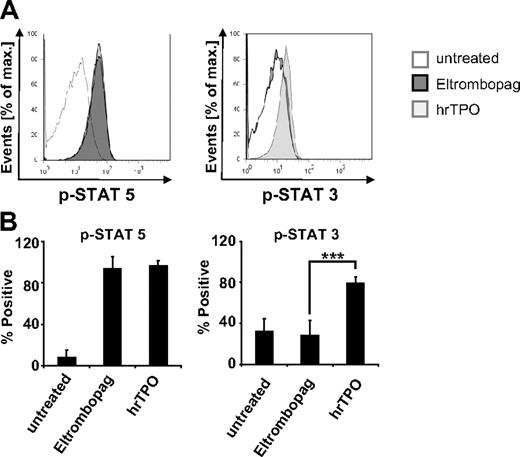
To gain insight into potential differences in activation of downstream targets of the TPO receptor by Eltrombopag, we conducted analysis of phosphorylation of Stat3 and Stat5. Flow cytometric phosphoprotein analysis of MNC derived from MDS/AML patients revealed differences in Stat3 activation after Eltrombopag treatment in comparison with hrTPO (Figure 6). Interestingly, whereas treatment with hrTPO led to a significant increase of both Stat3 and Stat5 phosphorylation, cells treated with 3 μg/mL Eltrombopag only responded with an increase in p-Stat5, but did not show an increase of Stat3 phosphorylation (Figure 6A-B).
Eltrombopag spares Stat3 phosphorylation. Flow cytometric phosphoprotein analysis of MDS/AML cells treated with either 0 or 3 μg/mL Eltrombopag or 100 ng/mL hrTPO. (A) Representative histogram plot. Cells were prestarved in serum-free RPMI medium for 4 hours, treated with Eltrombopag or hrTPO for 60 minutes, fixed, permeabilized, stained with phospho-specific antibodies against p-Stat3 and p-Stat5, and analyzed by FACS. (B) Mean values and SD of p-Stat5 and p-Stat3 analysis of 4 MDS/AML samples upon treatment with either Eltrombopag or hrTPO. ***Statistical significance (P < .001).
Eltrombopag spares Stat3 phosphorylation. Flow cytometric phosphoprotein analysis of MDS/AML cells treated with either 0 or 3 μg/mL Eltrombopag or 100 ng/mL hrTPO. (A) Representative histogram plot. Cells were prestarved in serum-free RPMI medium for 4 hours, treated with Eltrombopag or hrTPO for 60 minutes, fixed, permeabilized, stained with phospho-specific antibodies against p-Stat3 and p-Stat5, and analyzed by FACS. (B) Mean values and SD of p-Stat5 and p-Stat3 analysis of 4 MDS/AML samples upon treatment with either Eltrombopag or hrTPO. ***Statistical significance (P < .001).
Discussion
In this study, we examined the effect of the novel nonpeptide thrombopoietin receptor agonist Eltrombopag on cells from patients with AML and MDS. Eltrombopag has recently been approved for treatment of thrombocytopenia in chronic ITP in the United States. To date, there have been no published studies of Eltrombopag in hematologic malignancies. However, these patients could greatly benefit from an elevation of platelet numbers as they frequently suffer from thrombocytopenia and its clinical sequelae, including bleeding, need for platelet transfusions, as well as delay and/or dose reduction of chemotherapeutic treatment. In MDS, in particular, cytopenias represent a significant clinical problem.22,23 For treatment of anemia and neutropenia, the use of hematopoietic growth factors such as erythropoietin and/or granulocyte colony-stimulating factor has been successfully implemented into clinical practice and has improved outcome and quality of life in patients with MDS.1,24 However, both in MDS and AML, treatment of thrombocytopenia has remained an enormous challenge, with 14% to 30% of patients succumbing to thrombocytopenic bleeding.2 In this study, we examined for the first time the effects of Eltrombopag in myeloid malignancies. We found no evidence that Eltrombopag leads to increased proliferation or expansion of malignant cells from patients with MDS/AML. Furthermore, we show that Eltrombopag increases megakaryopoiesis in BM cells from patients with MDS/AML. These results provide a preclinical rationale and are encouraging with regard to future clinical trials of Eltrombopag in AML and MDS.
We carried out several independent types of assays to determine whether Eltrombopag stimulates malignant hematopoiesis and blast proliferation. Using functional assays, we neither found evidence for an increase of cell cycling, nor for the reduction of apoptotic activity of MDS/AML cells in any of the examined patients. Importantly, we did not find an increase in cell growth or the number of blasts, neither in suspension culture, in methylcellulose-based assays, nor in xenotransplantation assays. On the contrary, we observed a moderate decrease of cell growth of MDS/AML cells at lower concentrations of Eltrombopag (0.1-3 μg/mL), whereas healthy control BM cells were not affected. At higher concentrations, Eltrombopag inhibited growth of normal control BM cells. Previous studies have shown that concentrations of 0.1 to 3 μg/mL can be reached clinically by once daily administration of Eltrombopag.25,26 We observed considerable variability between individual patients, and in some specimens cell growth was not diminished at all, such that the potential inhibitory effect of Eltrombopag at 0.1 to 3 μg/mL was overall not statistically significant. In conclusion, in our cohort Eltrombopag did not seem to stimulate malignant hematopoiesis in high-risk MDS and AML in vitro or in vivo. However, due to the limited number of patient samples used in this present study, we cannot exclude the possibility that Eltrombopag might have a different effect in particular subgroups of the disease. Future studies including larger numbers of MDS/AML patients will be required to substantiate our findings and determine whether our observations are true for all subtypes of MDS/AML.
Importantly, in this setting, the effect of Eltrombopag seems to be different compared with hrTPO or other TPO mimetics, as those have been found to potentially stimulate growth of malignant cells in myeloid malignancies, including AML and MDS.27-29 A possible explanation for the different effect of Eltrombopag may lie within its different molecular mechanism of action. Whereas hrTPO and TPO mimetics bind to the extracellular domain of the TPO receptor, Eltrombopag interacts with its transmembrane domain,30 which might lead to partially different downstream effects. It has been shown that Eltrombopag activates some signaling molecules similar to hrTPO, such as Stat5, p42, and p44 MAPK, but to a lesser extent.26 Preliminary studies also suggested that Eltrombopag might spare Stat3 activation. Indeed, this view is supported by the results of our phosphoprotein analysis that showed that Eltrombopag, in contrast to hrTPO, does not lead to an increase in Stat3 phophorylation. However, it is also possible that Eltrombopag has another to date unknown mechanism of action in MDS/AML cells that accounts for the lack of stimulation of leukemia cells. Whereas further mechanistic studies will be required to elucidate this at a molecular level, the absence of leukemia cell stimulation suggests that Eltrombopag might be a candidate for testing in patients with AML and MDS.
Our data show for the first time that Eltrombopag is capable of stimulating megakaryopoiesis in BM cells of patients with AML and MDS. This suggests that Eltrombopag could be effective in elevating platelet numbers and reducing hemorrhagic events in patients with MDS and AML, similar to its effect in chronic ITP and hepatitis C.7,8,31 In contrast to previous studies on cell lines,26 we did not observe an additive effect of Eltrombopag and hrTPO on megakaryopoiesis at concentrations of 3 μg/mL and 100 ng/mL, respectively. Mk colonies obtained upon treatment with Eltrombopag of BM-MNC from MDS/AML patients in remission were cytomorphologically identical to those derived from normal controls and showed the same expression of differentiation markers. Furthermore, we found no evidence for an induction of long-term malignant self-renewal by Eltrombopag neither in serial replating assays ex vivo, nor in xenotransplantation assays in vivo.
Our study focused on patients with AML and MDS with an excess of blasts where thrombocytopenia and hemorrhagic complications occur most frequently. The results presented provide a rationale for more extensive preclinical studies incorporating a larger number of patient samples, and are encouraging with regard to clinical testing of Eltrombopag for the treatment of thrombocytopenia in MDS/AML in the future. As with erythropoietin and granulocyte colony-stimulating factor, more research will be required to elucidate the potential clinical usefulness of Eltrombopag and possible predictors of its efficacy and safety in MDS and AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Julian Jenkins, Yanli Deng, and Christian Steidl for helpful discussion and advice. We thank the Einstein Human Stem Cell FACS and Xenotransplantation Facility for expert technical assistance.
A.V. is supported by National Institutes of Health (1RO1HL082946-01 and RO1AG02913801), the Gabrielle Angel Foundation, the Hershaft Family Foundation, and a translational research award of the Leukemia & Lymphoma Society. U.S. is the recipient of a Howard Temin Award of the National Cancer Institute and a New Investigator Award of the Leukemia Research Foundation, and is the Diane and Arthur B. Belfer Faculty Scholar in Cancer Research of the Albert Einstein College of Medicine.
National Institutes of Health
Authorship
Contribution: B.W. performed most of the experiments, analyzed data, designed experiments, and wrote the manuscript; J.P.L. and M.K. performed experiments and analyzed data; A.V., I.B., and S.P. provided patient samples, analyzed data, and reviewed the manuscript; C.L.E.-M. and M.A.A. contributed to the design of experiments, analyzed data, and reviewed the manuscript; and U.S. designed the research, supervised the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: U.S. received research funding from GlaxoSmithKline. C.L.E.-M. and M.A.A. are employees and hold stocks of GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Ulrich Steidl, Albert Einstein College of Medicine, Chanin Bldg, Rm 606, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: ulrich.steidl@einstein.yu.edu.

![Figure 5. Eltrombopag stimulates megakaryopoiesis in CD34+ BM-MNC cells from MDS/AML patients. (A) Number of Mk CD41a-expressing cells upon Eltrombopag treatment of CD34+ BM-MNC from MDS/AML patients (n = 5). Immunomagnetically enriched CD34+ BM-MNC were seeded in liquid cultures containing BSA, insulin, transferrin, hrIL-3, hrIL-6, hrSCF, hrFLT3L, and human low-density lipoproteins. Cells were incubated with either 0 or 3 μg/mL Eltrombopag, or 100 ng/mL hrTPO alone, or a combination of 3 μg/mL Eltrombopag and 20 ng/mL hrTPO for 5 days. CD41a+ cells were assessed by FACS analysis. The fold of change of CD41a+ cells in comparison with 100 ng/mL hrTPO-treated cultures of 5 individual patients is shown. Four of the 5 samples were treated with the combination of Eltrombopag plus TPO (one patient data not analyzed [n/a]). (B) Representative FACS histogram plot of cells upon incubation with either 0 or 3 μg/mL Eltrombopag, 100 ng/mL TPO alone, or 3 μg/mL Eltrombopag and 20 ng/mL hrTPO. (C) Eltrombopag increases the number of Mk colonies in cultures of CD34+ BM-MNC from MDS/AML patients. CD34+ BM-MNC were seeded in semisolid collagen-based medium containing hrIL-3, hrIL-6, hrSCF, hrFLT3L, and human low-density lipoproteins. Cultures were supplemented with either 0 or 3 μg/mL Eltrombopag, 100 ng/mL hrTPO alone, or 3 μg/mL Eltrombopag in combination with 20 ng/mL hrTPO. Mk colonies were enumerated after 12 days of culture. The fold of change compared with 100 ng/mL hrTPO-treated CD34+ cells is shown (n = 5). (D) Mk colony morphology of Mk colonies derived from healthy persons' and MDS/AML patients' BM-MNC. (E) Differential colony counts of Mk progenitor-derived colonies after 12 days of culture in the presence of either 0 or 3 μg/mL Eltrombopag, 100 ng/mL hrTPO alone, or 3 μg/mL Eltrombopag in combination with 20 ng/mL hrTPO. Colonies from bipotent Mk progenitors, immature Mk progenitors, and mature Mk progenitors were scored. The fold change compared with cells cultured in presence of 100 ng/mL hrTPO is shown. Results from 5 individual patients are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/18/10.1182_blood-2009-04-219493/4/m_zh89990943620005.jpeg?Expires=1765917698&Signature=gAuAs9eFWaHonDg02O~8~xze4ehzAVYpWgyUG5FWFBA9x~wICGNeoEZzilBAVbpVhUvFAKbsTai0C9JRlzOI2Fn~qyjiR9g-M~Lbt2hS5LVb2ltctNv2cYWTi-D9mXIillRxSxHWQ54sJ8f9V40Xavg73XArkDWEGqoraR1a--cOqoJnZx3sPvKZf8rI5iQesBWXgiVBT0~c4vZ1IFKvKPGusRJtp3LzBnU1odzg~wlNmCWqzSzUJvpFh5P8gGZVPH10iSsm1qle74EIWhuCnyk1rvlEFS9hGyv~6Eeo~qvBiVWSxC-fzTyA~AOTfQAtd6Tqqugz8KMuY2d6ckfaxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)