Abstract
CCAAT/enhancer-binding protein β (C/EBPβ), also known as nuclear factor–interleukin-6 (NF-IL6), is a transcription factor that plays an important role in the regulation of growth and differentiation of myeloid and lymphoid cells. Mice deficient in C/EBPβ show impaired generation of B lymphocytes. We show that C/EBPβ regulates transcription factors critical for proliferation and survival in multiple myeloma. Multiple myeloma cell lines and primary multiple myeloma cells strongly expressed C/EBPβ, whereas normal B cells and plasma cells had little or no detectable levels of C/EBPβ. Silencing of C/EBPβ led to down-regulation of transcription factors such as IRF4, XBP1, and BLIMP1 accompanied by a strong inhibition of proliferation. Further, silencing of C/EBPβ led to a complete down-regulation of antiapoptotic B-cell lymphoma 2 (BCL2) expression. In chromatin immunoprecipitation assays, C/EBPβ directly bound to the promoter region of IRF4, BLIMP1, and BCL2. Our data indicate that C/EBPβ is involved in the regulatory network of transcription factors that are critical for plasma cell differentiation and survival. Targeting C/EBPβ may provide a novel therapeutic strategy in the treatment of multiple myeloma.
Introduction
Multiple myeloma (MM) is a clonal B-cell neoplasia. The disorder is characterized by the accumulation of neoplastic plasma cells in the bone marrow and remains an incurable hematologic malignancy. Dysregulation of genes responsible for apoptosis and survival in plasma cells contributes to the pathogenesis of MM. New areas of research focus on targeting various dysregulated proteins involved in the tumorigenesis of MM.1 In this study, we investigated the role of the transcription factor (TF) CCAAT/enhancer-binding protein β (C/EBPβ) in MM pathology.
The C/EBPs belong to a larger family of leucine zipper TFs, termed bZip proteins, which have a basic DNA-binding domain linked to a leucine zipper dimerization motif.2 C/EBPβ, also called nuclear factor–interleukin-6 (NF-IL6), regulates a variety of genes involved in diverse functions such as acute phase response,3 immune function,3-5 inflammation,6 and cellular differentiation processes including adipogenesis,7 solid organ development, cell survival,8 tumor invasiveness,9,10 and hematopoiesis.11 Increased levels of C/EBPβ have been found in different types of tumors such as breast, renal, and colorectal cancer.12-14 Deletion of the C/EBPβ gene in mice results in impaired generation of B lymphocytes,15 and it has been shown that C/EBPβ contributes to the induction of the antiapoptotic protein, B-cell lymphoma 2 (BCL2), in t(14;18) lymphoma cells.8 Earlier studies identified C/EBPβ as a regulator of IL-6 and demonstrated that C/EBPβ itself is also induced by IL-6,16 the most important survival factor for MM cells.
Here we identify C/EBPβ as a critical TF in MM, regulating growth, proliferation, and antiapoptotic responses by regulating the expression of other key TFs.
Methods
Chemicals and antibodies
Cell culture media, sera, and penicillin-streptomycin were purchased from Gibco BRL. Polyvinyl difluoride membranes were purchased from Bio-Rad Laboratories and antibodies, from the following vendors: anti-C/EBPβ (C-19; epitope mapping at C-terminus of C/EBPβ and recommended for the detection of C/EBPβ at 45 kDa) and Author:anti-XBP1 (C-20; epitope mapping at C-terminus of XBP1 of human origin) from Santa Cruz Biotechnology Inc; anti-IRF4 (recommended for the detection of endogenous levels of IRF4 protein and does not cross-react with the other family members) and anti-BCL2 (recommended for the detection of endogenous levels of BCL2 protein and does not cross-react with the other BCL2 family members) from Cell Signaling. Thalidomide and pomalidomide were obtained from Celgene. Dimethyl sulfoxide (DMSO), dexamethasone, and melphalan were purchased from Sigma-Aldrich. PD 98059 and LY 294002 were purchased from Cell Signaling Technology. Antibody for the surface expression marker CD19 and CD38 was obtained from BD Biosciences.
Cell culture and cell selection
The human anaplastic large-cell lymphoma cell line SU-DHL-1, the acute monocytic leukemia cell line THP-1, and MM cell lines MM.1S, RPMI-8266, H929, OPM2, INA-6, and U266 were cultured in RPMI-1640 medium with l-glutamine, 1× penicillin/streptomycin, and 10% fetal bovine serum (FBS) at 37°C and 5% CO2.
Concentrations for the in vivo cell culture experiment were as follows: pomalidomide 100 μM, thalidomide 100 μM, dexamethasone 5 μM, PD 98 059 50 μM, LY 294002 50 μM, and melphalan 5 μM.
Bone marrow mononuclear cells were obtained using Ficoll (Invitrogen Corporation) according to the manufacturer's instructions, and CD138+ cells were selected using CD138+ antibody-specific microbeads followed by magnetic separation column according to the manufacturer's protocol (Miltenyi Biotec). The negative cell population was considered as CD138−.
For the generation of normal plasma cells (CD38+) from the B cells, human peripheral blood buffy coats were obtained from the Pittsburgh Blood Bank. Peripheral blood mononuclear cells were separated by density gradient centrifugation using lymphocyte separation medium. CD19+ B cells were purified using a negative isolation kit according to the manufacturer's instructions (Miltenyi Biotec). Purified B cells were cultured at 1 × 106 cells/mL in 24-well cultured plate. The cells were incubated with a combination of human IL-21 (100 ng/mL; PeproTech), 1 μg/mL anti–human CD40 (eBiosciences), and 5 μg/mL anti–immunoglobulin M (IgM; Pierce Biotechnology Inc) according to the protocol described by Ettinger et al.17 The B cells were analyzed by flow cytometry before induction and on day 6 of differentiation for the expression of CD19 as well as CD38. All blood samples were obtained as approved by the University of Pittsburgh Institutional Review Board. Informed consent was provided according to the Declaration of Helsinki.
RT-PCR analysis
For the determination of mRNA levels of C/EBPβ, total RNA was isolated using the Mini RNA isolation II kit (Zymo Research) according to the manufacturer's instructions. Total RNA was converted into cDNA using the Superscript III RT (Invitrogen Corporation). Quantitative reverse-transcription–polymerase chain reaction (RT-PCR) was performed on ABI Prism 7700 Sequence Detection System (Applied Biosystems). RT-PCR was carried out with the SYBR Green PCR master mix (Bio-Rad) using 1 μL cDNA in a 20-μL final reaction mixture (15 minutes at 95°C; 40 cycles of 15 seconds at 95°, 60 seconds at 60°C, and 10 minutes at 79°C). The average threshold cycle (Ct) for each gene was determined from triplicate reactions, and data were analyzed by taking the difference between mean thresholds of RT-PCR cycle values for target and control gene (ΔCt). Target gene expression was normalized to β-actin using the ΔCt value. This was then calibrated to the control sample in each experiment to give the ΔΔCt value, where the control had a ΔΔCt value of 0. The fold target gene expression, compared with the calibrator value, is given by the formula 2−ΔΔCT. The following primer sets were used (Real Time Primers): C/EBPβ: 5′-AACTCTCTGCTTCTCCCTCTG-3′, 5′-AAGCCCGTAGGAACATCTTT-3′; IRF4: 5′-TTAATTCTCCAAGCGGATGC-3′, 5′-AAGGAATGAGGAAGCCGTTC-3′; BCL2: 5′-AGGAAGTGAACATTTCGGTGAC-3′, 5′-GCT CAG TTC CAG GAC CAG GC-3; BLIMP1: 5′-TGA GAG TGC ACA GTG GAG AA-3′, 5′-ATT GCT GGT GCT GCT AAA TC-3′; β-actin: 5′-GGACTTCGAGCAAGAGATGG-3′, 5′ AGCACTGTGTTGGCGTACAG-3′.
Western blot analysis
Briefly, the protein was extracted from the cells using 1× radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology Inc) with the cocktail of protease inhibitor, sodium benzoate, and phenylmethylsulphonyl fluoride. Cell lysates were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinyl difluoride membranes (Bio-Rad Laboratories). The blots were incubated with respective antibodies for the protein expression and immune complexes were detected using enhanced chemiluminescence (Amersham).
Immunohistochemistry
Bone marrow biopsies from 72 patients with MM infiltrates were selected from the Lymphoma Reference Center at the Institute of Pathology, Charité, University Medicine. Nonneoplastic plasma cells were analyzed in specimens from palatine tonsils removed from 10 patients because of enlargement due to hyperplasia of lymphoid tissue and florid inflammation. For demonstration of C/EBPβ expression in normal and neoplastic plasma cells, we used the anti-C/EBPβ (clone H7) monoclonal antibody from Santa Cruz Biotechnology Inc and the anti-CD138 antibody from Dako. CD138-C/EBPβ double labeling was necessary to distinguish CD138+-C/EBPβ+ cells from C/EBPβ+-myeloid cells. For this purpose, the dewaxed sections were subjected to an antigen retrieval protocol consisting of a brief, high-temperature heating in citrate buffer (10 mmol, pH 6.0) for 2 minutes in a high-pressure cooker followed by an incubation with the anti-CD138 and visualization of bound antibody using the streptavidin-biotin-peroxidase method and diaminobenzidine as chromogen. Subsequently, the sections were incubated with the anti-C/EBPβ antibody stained by alkaline phosphatase anti–alkaline phosphatase method and FastRed as chromogen (all reagents were obtained from Dako). The result was a brown membrane staining for CD138 and a red nucleus for C/EBPβ. Negative control slides were processed with the control antibody IgG in place of primary antibody followed by incubation with the secondary antibody.
Plasmids for transfection studies
Expression vectors for the full-length wild-type C/EBPβ (pcNF-IL6) (WT-C/EBPβ) and for a truncated dominant-negative C/EBPβ (DN-C/EBPβ), with a deletion of the internal SpII-SpII fragment (pcmNF-IL6 [ΔSpl]), were generated by inserting the respective coding regions into pcDNA3.1 (Invitrogen Corporation) and provided by Dr Philip E. Auron (Duquesne University; Figure 2A).18
Transfection
MM cells (2 × 107) were transfected by electroporation with 30 μg of the empty vector pcDNA3.1 (EV), WT-C/EBPβ, or the DN-C/EBPβ plasmids. Electroporation was performed in serum- and antibiotic-free media with the Gene Pulser II apparatus (Bio-Rad) using 0.4-cm gene pulser cuvettes (Bio-Rad) with settings of 250 V and 950 μF. Twenty-four hours after transfection, transfected cells were selected for resistance to G418 by 10 days of continuous G418 (500 μg/mL) treatment. Protein of the transfected cells was extracted using 1× radioimmunoprecipitation assay buffer with a cocktail of protease inhibitors, sodium benzoate, and phenylmethylsulphonyl fluoride (Santa Cruz Biotechnology Inc). Protein extracts were analyzed for the expression of C/EBPβ by Western blot analyses.
Cell proliferation assays
MM.1S (3 × 105/well), RPMI-8266 cells (3 × 104/well), or H929 cells (3 × 104/well) were incubated in 96-well plates in the presence of RPMI-1640 medium containing 10% fetal calf serum at 37°C/5% CO2 for 48 hours. DNA synthesis was measured by 3H-thymidine incorporation (NEN Products). Cells were pulsed with 3H-thymidine (1 Ci/well [0.037 MBq]) for the last 8 hours of culture, harvested onto glass-fiber filter mats (Wallac) using an automatic cell harvester (Tomtec Harvester 96, Mach III; Tomtec Inc), and counted using a Wallac TriLux Beta plate scintillation counter (PerkinElmer). All experiments were performed in triplicate.
Apoptosis assays
Apoptosis was analyzed by annexin V–fluorescein isothiocyanate staining using the Alexa Fluor 488 annexin V kit (Invitrogen Corporation). Briefly, 0.5 × 106/mL cells were harvested, washed once with cold phosphate-buffered saline, and then resuspended in 1× annexin-binding buffer. Cell survival was determined by annexin V–fluorescein isothiocyanate/propidium iodide (PI) double staining. Samples were analyzed on FACSCalibur using the software program CellQuest (both from Becton Dickinson) .
Chromatin immunoprecipitation assay and RT-PCR
H929 human MM cells were cultured as described in “Cell culture and cell collection.” Cells were treated with formaldehyde, nuclei were isolated, and chromatin was sheared by sonication as per the protocol previously described by Shell et al.19 Sheared chromatin was immunoprecipitated by rabbit antibodies: anti-RNA polymerase II (N20; Santa Cruz Biotechnology Inc), anti-C/EBPβ (Abcam Inc), or normal IgG (Sigma-Aldrich) using the magnetic bead chromatin immunoprecipitation (ChIP)–IT express kit from Active Motif. DNA was isolated from the immunoprecipitated chromatin on protein G–linked magnetic beads using directions supplied by the manufacturer of the ChIP-IT kit, except that the de–cross-linking step was conducted at 65°C overnight, the proteinase K digestion was increased to 2 μL of 10 mg/mL proteinase K at 37°C for 2 hours, and the DNA was isolated on QIAquick PCR purification kit (QIAGEN). The DNA was analyzed by quantitative RT-PCR using an Applied Biosystems instrument with SYBR GREEN PCR master mix (Applied Biosystems). Probes for RT-PCR were purchased from Integrated DNA Technologies. Error bars on the graph (Figure 6) represent standard deviation from at least 4 determinations. The oligos used as RT-PCR primers were as follows: C/EBP, AGAAGTCGGTGGACAAGAACAGCA, ATTGTCACTGGTCAGCTCCAGCA; CCR5, (intron) TCCTGCCTCATAAGGTTGCCCTAA, AGGGCACATACTGGATGCCAATCA; IRF4 a, TCACCACTGCCAGCTGCTA, AAACTCCGGATGGCCTCAT; IRF4 b, AGGGAGCTGGGCCATTTCCTATTT, TGTAACGGAAGACGGAGGAATGGT; BLIMP a, GGACAGAGGCTGAGTTTGAAGA, CGCCATCAGCACCAGAATC, BLIMP b, AGAGCCCAAGTAAGCGTTGAGGTT, AGAGCTTCCTCTCTTCGCATGTGT; BCL2 exon 1, TGTGTACAGGGAAACGCACCTGAT, CCCTTGGCATGAGATGCAGGAAAT; XBP1, TCTATCTCGACTTTCGGCTCCACT, TCCAAACCGAGAGCTTTCCAGACT; DHFR, ACCTGGTCGGCTGCACCT, TTGCCCTGCCATGTCTCG.
The midpoint Ct values in quantitative RT-PCR were converted to ΔCt relative to the total DNA standard and converted to a percentage by taking the power of 2 to the negative ΔCt as previously described.19 The data were normalized to the value obtained with RNA polymerase II set as 100%, resulting in the percentages shown in Figure 6B.
Results
C/EBPβ is highly expressed in MM cells
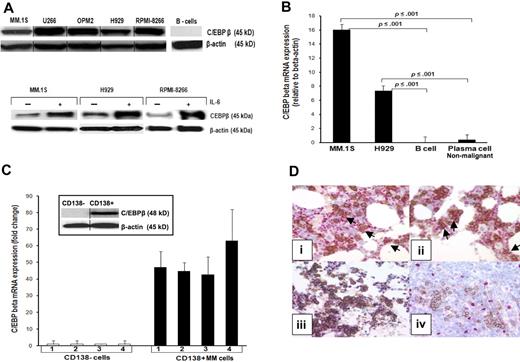
In our previous work, we found in gene array studies that C/EBPβ RNA is highly expressed in the MM cell line MM.1S.20 Therefore, we first analyzed C/EBPβ protein expression in MM cell lines, primary MM cells, and MM tissue. Our data showed that C/EBPβ LAP isoform at 45 kDa (Figure 1A top panel) and mRNA (Figure 1B) are highly expressed in all tested MM cell lines (MM.1S, U266, OPM2, H929, and RPMI-8266). Expression of C/EBPβ was also induced by IL-6 treatment in serum-starved MM cells, suggesting that C/EBPβ is up-regulated in response to cytokines critical for MM cell growth such as IL-6 (Figure 1A bottom panel). We found a heterogeneous expression pattern of the inhibitory form (LIP) in untransfected MM cells. Certain myeloma cells such as INA-6 show no expression of LIP and other cell lines such as MM.1S expressed LIP only after stimulation with IL-1β (supplemental Figure 1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Immunohistochemical analysis of MM cell lines showed the following: U226: 90% C/EBPβ+ cells; OPM2: 80% to 90% C/EBPβ+ cells; MM.S1: 60% to 70% C/EBPβ+ cells; NCL-H929: 100% C/EBPβ+ cells; RPMI: 80% C/EBPβ+ cells. In contrast to MM cells, we could not detect C/EBPβ transcripts in normal B cells (CD19+) and only very low levels of C/EBPβ mRNA in in vitro–generated plasma cells by quantitative real-time PCR (RT-PCR) (Figure 1B).
C/EBPβ is highly expressed in MM cells. (A top panel) Expression of C/EBPβ in MM cell lines was detected by Western blotting as described in “Western blot analysis.” β-Actin was used as a loading control for all samples. (A bottom panel) Induction of expression of C/EBPβ by IL-6. Cells were cultured in 1% FBS with or without IL-6 for 48 hours, and analyzed for C/EBPβ protein expression. (B) C/EBPβ mRNA expression in MM.1S and H929 MM cell lines, B cells, and nonmalignant plasma cells were analyzed by quantitative RT-PCR. Data were analyzed according to the ΔCT method. The results are expressed as C/EBPβ mRNA expression relative to β-actin. B cells were cultured in a cocktail of CD40 and human IgM for 6 days for the differentiation into plasma cells. Expression of CD19 of B cells as well as CD38 for plasma cells was confirmed by flow cytometry. (C) Mononuclear cells of bone marrow samples of 4 MM patients were collected by Ficoll. CD138+ and CD138− cells from the same patient were separated using magnetic-activated cell sorting beads selection. The total RNA was extracted, subjected to cDNA synthesis, and used for quantitative RT-PCR. Results are depicted as C/EBPβ mRNA fold expression in CD138+ cells compared with CD138− cells. The level of mRNA was normalized to β-actin expression. Expression of C/EBPβ and IRF4 was detected by Western blotting with anti-C/EBPβ and anti-IRF4 in CD138+ cells and CD138− cells from the same patient. β-Actin was used as a loading control. Error bars indicate SD of the mean. (D) Immunohistochemical double staining was performed on paraffin-embedded MM bone marrow trephines to detect C/EBPβ expression in CD138+ cells. Images were obtained using an Olympus 1 X70 microscope equipped with a 20×/0.40 numeric aperture objective lens (Olympus) and were acquired through Magnafire Version 4.1 software (Optronics). Positive staining shows a red nucleus for C/EBPβ and brown membrane for CD138. Positive double staining is indicated in panels Di and ii by arrows, negative double staining is shown in panel Diii. Nonneoplastic plasma cells in palatine tonsils did not show a C/EBPβ expression (Div). Vertical lines have been inserted to indicate a repositioned gel lane.
C/EBPβ is highly expressed in MM cells. (A top panel) Expression of C/EBPβ in MM cell lines was detected by Western blotting as described in “Western blot analysis.” β-Actin was used as a loading control for all samples. (A bottom panel) Induction of expression of C/EBPβ by IL-6. Cells were cultured in 1% FBS with or without IL-6 for 48 hours, and analyzed for C/EBPβ protein expression. (B) C/EBPβ mRNA expression in MM.1S and H929 MM cell lines, B cells, and nonmalignant plasma cells were analyzed by quantitative RT-PCR. Data were analyzed according to the ΔCT method. The results are expressed as C/EBPβ mRNA expression relative to β-actin. B cells were cultured in a cocktail of CD40 and human IgM for 6 days for the differentiation into plasma cells. Expression of CD19 of B cells as well as CD38 for plasma cells was confirmed by flow cytometry. (C) Mononuclear cells of bone marrow samples of 4 MM patients were collected by Ficoll. CD138+ and CD138− cells from the same patient were separated using magnetic-activated cell sorting beads selection. The total RNA was extracted, subjected to cDNA synthesis, and used for quantitative RT-PCR. Results are depicted as C/EBPβ mRNA fold expression in CD138+ cells compared with CD138− cells. The level of mRNA was normalized to β-actin expression. Expression of C/EBPβ and IRF4 was detected by Western blotting with anti-C/EBPβ and anti-IRF4 in CD138+ cells and CD138− cells from the same patient. β-Actin was used as a loading control. Error bars indicate SD of the mean. (D) Immunohistochemical double staining was performed on paraffin-embedded MM bone marrow trephines to detect C/EBPβ expression in CD138+ cells. Images were obtained using an Olympus 1 X70 microscope equipped with a 20×/0.40 numeric aperture objective lens (Olympus) and were acquired through Magnafire Version 4.1 software (Optronics). Positive staining shows a red nucleus for C/EBPβ and brown membrane for CD138. Positive double staining is indicated in panels Di and ii by arrows, negative double staining is shown in panel Diii. Nonneoplastic plasma cells in palatine tonsils did not show a C/EBPβ expression (Div). Vertical lines have been inserted to indicate a repositioned gel lane.
Analysis of CD138+ MM cells from bone marrow of MM patients showed high levels of C/EBPβ mRNA in all samples measured by quantitative RT-PCR, whereas CD138− cells from these same patients had barely detectable levels of C/EBPβ mRNA (Figure 1C). In accordance with these results, we detected a strong expression of C/EBPβ in primary MM samples by Western blot (Figure 1C). Additional immunohistochemical staining of 72 MM tissue samples showed variable results. To distinguish C/EBPβ-positive myeloid cells from C/EBPβ-positive plasma cells in the bone marrow, we performed CD138/C/EBPβ double labeling. A significant up-regulation of C/EBPβ was detected in 29% of MM cases by immunohistochemistry. In 7 cases, C/EBPβ expression was detected in more than 50% of the neoplastic cells, in an additional 9 cases C/EBPβ was present in 10% to 50% of the neoplastic cells, whereas 5 cases exhibited few C/EBPβ+ tumor cells (< 10% of the neoplastic population) (Figure 1Di-iv). In contrast, analysis of nonneoplastic plasma cells of palatine tonsils was completely negative in all analyzed samples for C/EBPβ-positive plasma cells (Figure 1Div).
C/EBPβ regulates expression of IRF4
The fact that C/EBPβ is not expressed in normal tissues, but is rapidly and drastically induced by cytokines such as IL-1, tumor necrosis factor, and IL-6 that support MM cell growth,21 raises the question of the role of C/EBPβ in MM and its effects on other TFs involved in plasma cell development.
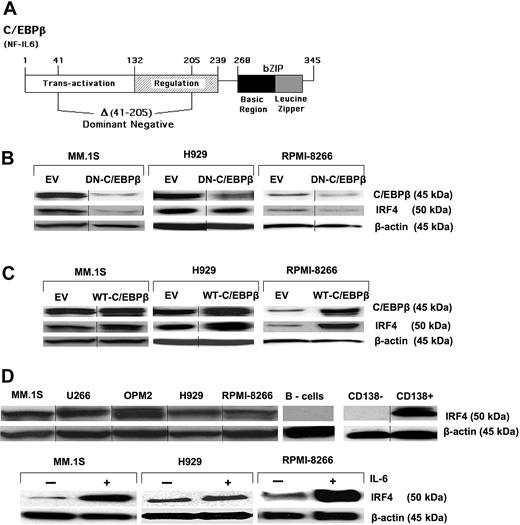
To explore the interactions between C/EBPβ and other TFs critical for MM survival and proliferation, the MM cell lines MM.1S, RPMI-8266, and H929 were transfected with expression vectors containing cDNA encoding WT-C/EBPβ, or DN-C/EBPβ (ΔSpl) lacking the internal SplI- SplI fragment encoding amino acids 41 to 205 (Figure 2A), or EV. The DN-C/EBPβ lacks the transactivation and regulatory domains, leaving intact the DNA-binding and dimerization domains, making it capable of binding to DNA but with no transcriptional activation capacity. The plasmids contain the neomycin-resistance gene, and transfected clones were selected with G418 (500 μg/mL) for 10 days. After selection, protein expression levels of C/EBPβ were determined by Western blot. Compared with the EV, transfection of MM cell lines with the DN-C/EBPβ resulted in significant inhibition of endogenous C/EBPβ (Figure 2B top panel). Inhibition of endogenous C/EBPβ expression by transfection of the DN-C/EBPβ suggests that there is autoregulation of C/EBPβ in MM cells. Autoregulation has been described as a common mechanism for the transcriptional control of all C/EBP family members, and it has been shown that C/EBPβ can stimulate its own transcription.22,23 On the other hand, transfection with WT-C/EBPβ increased the amounts of C/EBPβ protein expression in all tested MM cell lines (Figure 1 and Figure 2C top panel).
Regulation of IRF4 by C/EBPβ. (A) The human WT-C/EBPβ (also known as NF-IL6) cDNA is depicted as well as the region (an Spl1 fragment encoding amino acids 41-205) deleted to generate the DN-C/EBβ cDNA. These cDNAs inserted into empty vector (EV) pCDNA3.1 were used for the transfection studies. Transfection of the WT-C/EBPβ cDNA in MM cells results in overexpression. MM cell lines, MM.1S, H929, and RPMI-8266, were transfected with the EV, (B) DN-C/EBPβ, or (C) WT-C/EBPβ followed by G418 (500 μg/mL) selection for 10 days. Transfected MM cells were selected, and expression of C/EBPβ and IRF4 was detected by Western blotting with anti-C/EBPβ and anti-IRF4 antibody. β-Actin was used as a loading control. (D top panel) Expression of IRF4 in MM cell lines and primary MM cells was detected by Western blotting as described in “Western blot analysis.” (D bottom panel) Cells were cultured in 1% FBS with or without IL-6 for 48 hours, and analyzed for induction of IRF4 protein expression. β-Actin was used as a loading control for all samples. Vertical lines have been inserted to indicate a repositioned gel lane.
Regulation of IRF4 by C/EBPβ. (A) The human WT-C/EBPβ (also known as NF-IL6) cDNA is depicted as well as the region (an Spl1 fragment encoding amino acids 41-205) deleted to generate the DN-C/EBβ cDNA. These cDNAs inserted into empty vector (EV) pCDNA3.1 were used for the transfection studies. Transfection of the WT-C/EBPβ cDNA in MM cells results in overexpression. MM cell lines, MM.1S, H929, and RPMI-8266, were transfected with the EV, (B) DN-C/EBPβ, or (C) WT-C/EBPβ followed by G418 (500 μg/mL) selection for 10 days. Transfected MM cells were selected, and expression of C/EBPβ and IRF4 was detected by Western blotting with anti-C/EBPβ and anti-IRF4 antibody. β-Actin was used as a loading control. (D top panel) Expression of IRF4 in MM cell lines and primary MM cells was detected by Western blotting as described in “Western blot analysis.” (D bottom panel) Cells were cultured in 1% FBS with or without IL-6 for 48 hours, and analyzed for induction of IRF4 protein expression. β-Actin was used as a loading control for all samples. Vertical lines have been inserted to indicate a repositioned gel lane.
It has been reported that IRF4 is a proto-oncogene in MM, playing a critical role in survival and proliferation of MM cells.24 We found that all tested MM cell lines expressing high levels of C/EBPβ concomitantly express high levels of IRF4 protein (Figure 2D top panel). In addition, induction of C/EBPβ by IL-6 was also associated with increased expression of IRF4 (Figure 2D bottom panel), raising the possibility that C/EBPβ regulates IRF4 protein levels. Intriguingly, overexpression of WT-C/EBPβ also increased expression of IRF4 protein (Figure 2C), whereas expression of the DN-C/EBPβ resulted in down-regulation of IRF4 expression (Figure 2B), suggesting that IRF4 is under control of C/EBPβ.
C/EBPβ affects TFs critical for B-/plasma cell development such as XBP1 and BLIMP1
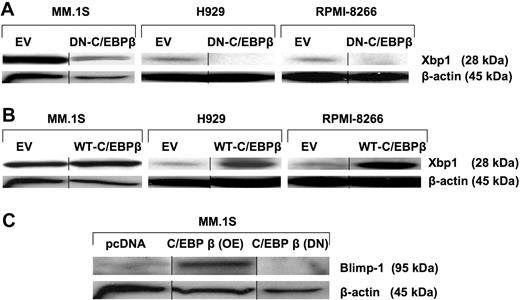
IRF4 is critical for the generation of plasma cells by regulating various TFs such as XBP1 and BLIMP1, which are essential for the development and maturation of the B/plasma cells. IRF4 acts upstream of XBP1, an activator required for plasma cell formation.25 We investigated how regulation of C/EBPβ/IRF4 impacts XBP1 and BLIMP1 protein levels. Down-regulation of C/EBPβ by DN-C/EBPβ induced an almost complete abrogation of XBP1 expression in all tested MM cell lines (Figure 3A). In contrast, overexpression of C/EBPβ induced the expression of XBP1 (Figure 3B). BLIMP1 has been reported to be a master regulator of plasma cell differentiation by repressing TFs that are important for the expression of B cell–associated genes, such as PAX5, or for cell-cycle progression, such as c-myc.26 Overexpression of C/EBPβ up-regulated the expression of BLIMP1 (Figure 3C), suggesting that the expression of XBP1 and BLIMP1 is regulated by C/EBPβ in MM cells.
C/EBPβ regulates IRF4-dependent transcription factors such as XBP1 and BLIMP1. MM cells were transfected with EV, (A) DN-C/EBPβ, or (B) WT-C/EBPβ. Transfected cells were selected for 10 days by G418 (500 μg/mL). The selected cells were analyzed for expression of XBP1 by Western blotting with the anti-XBP1 antibody. (C) EV- and WT-C/EBPβ–transfected and selected cells were analyzed for the protein expression of BLIMP1. β-Actin was used as a loading control. Vertical lines have been inserted to indicate a repositioned gel lane.
C/EBPβ regulates IRF4-dependent transcription factors such as XBP1 and BLIMP1. MM cells were transfected with EV, (A) DN-C/EBPβ, or (B) WT-C/EBPβ. Transfected cells were selected for 10 days by G418 (500 μg/mL). The selected cells were analyzed for expression of XBP1 by Western blotting with the anti-XBP1 antibody. (C) EV- and WT-C/EBPβ–transfected and selected cells were analyzed for the protein expression of BLIMP1. β-Actin was used as a loading control. Vertical lines have been inserted to indicate a repositioned gel lane.
C/EBPβ induces MM proliferation
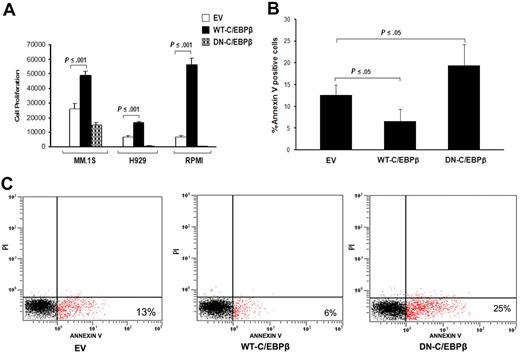
It has been shown that C/EBPβ plays an important role in the regulation of cell differentiation and proliferation.15,27 We therefore analyzed the effect of C/EBPβ on proliferation and apoptosis induction in MM cells. Overexpression of WT-C/EBPβ significantly (P ≤ .001) induced proliferation in all tested MM cell lines (MM.1S, H929, and RPMI-8266) compared with cells transfected with the control vector (Figure 4A). In contrast, transfection with DN-C/EBPβ resulted in significant inhibition of proliferation measured by thymidine uptake of MM cells (Figure 4A).
C/EBPβ induces MM cell proliferation and is involved in apoptosis. Proliferation and apoptosis of transfected MM cells with EV, WT-C/EBPβ, or DN-C/EBPβ were analyzed. (A) DNA synthesis was measured by 3H-thymidine incorporation and results are presented as mean of quadruplicates. (B) The graph represents the mean (n = 4) of the percentage of transfected MM cells that have undergone apoptosis measured by annexin V staining. Error bars indicate SD of the mean. (C) Percentage of apoptotic cells of transfected cells was analyzed by annexin V binding/PI staining using flow cytometry. Panel C figure shows a representative experiment of panel B.
C/EBPβ induces MM cell proliferation and is involved in apoptosis. Proliferation and apoptosis of transfected MM cells with EV, WT-C/EBPβ, or DN-C/EBPβ were analyzed. (A) DNA synthesis was measured by 3H-thymidine incorporation and results are presented as mean of quadruplicates. (B) The graph represents the mean (n = 4) of the percentage of transfected MM cells that have undergone apoptosis measured by annexin V staining. Error bars indicate SD of the mean. (C) Percentage of apoptotic cells of transfected cells was analyzed by annexin V binding/PI staining using flow cytometry. Panel C figure shows a representative experiment of panel B.
We next analyzed whether C/EBPβ is implicated in the induction of MM cell apoptosis. MM cells (H929), transfected with the EV, WT-C/EBPβ, or DN-C/EBPβ, were analyzed by flow cytometry using annexin V staining to measure apoptosis (annexin V+ and PI−). We detected a significant induction of apoptosis by DN-C/EBPβ (mean± SD, 20% ± 5%) relative to EV (13% ± 2%) and decreased apoptosis in the presence of overexpressed full-length C/EBPβ (7% ± 3%) (n = 4 experiments; Figure 4B). Figure 4C shows a representative experiment of 4 experiments. Our data suggest that inhibition of C/EBPβ in MM cells decreases proliferation and to a lesser extent induces apoptosis. In contrast, overexpression of WT-C/EBPβ in MM cells enhances proliferation and in part protects the cells from cell death by apoptosis, suggesting that C/EBPβ is involved in proliferation and survival.
C/EBPβ regulates BCL2
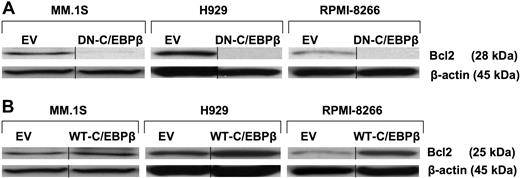
BCL2 has previously been reported to have antiapoptotic effects in MM.28 In Figure 5A, we show that inhibition of C/EBPβ by DN-C/EBPβ resulted in complete down-regulation of BCL2 protein expression (Figure 5A). On the other hand, overexpression of WT-C/EBPβ induced the expression of BCL2 in all tested MM cell lines (Figure 5B). These data suggest that BCL2 protein levels are regulated in MM cells at least in part by C/EBPβ and thereby might play a potential role in the survival of the MM cells.
BCL2 is regulated by C/EBPβ. MM cells were transfected with EV, (A) DN-C/EBPβ, or (B) WT-C/EBPβ. Transfected cells were selected for 10 days with G418 (500 μg/mL). Lysates of transfected cells were subjected to Western blotting using anti-BCL2 antibody. β-Actin was used as a loading control. Vertical lines have been inserted to indicate a repositioned gel lane.
BCL2 is regulated by C/EBPβ. MM cells were transfected with EV, (A) DN-C/EBPβ, or (B) WT-C/EBPβ. Transfected cells were selected for 10 days with G418 (500 μg/mL). Lysates of transfected cells were subjected to Western blotting using anti-BCL2 antibody. β-Actin was used as a loading control. Vertical lines have been inserted to indicate a repositioned gel lane.
C/EBPβ binds to the regulatory gene regions of IRF4, BLIMP1, and BCL2
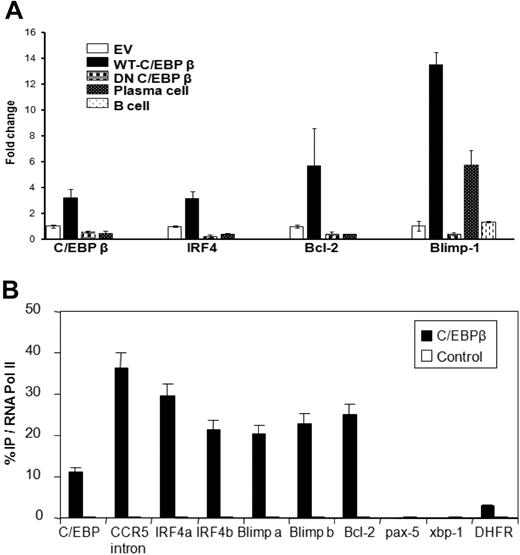
To determine whether inhibition or overexpression of C/EBPβ affects the RNA levels of the TFs critical for MM cells, we analyzed the mRNA expression of C/EBPβ, IRF4, BCL2, and BLIMP1 in MM cell lines and found that overexpression of WT-C/EBPβ significantly induces the gene expression of IRF4, BCL2, and BLIMP1. In contrast, transfection with DN-C/EBPβ resulted in down-regulation of the mRNAs of these TFs (Figure 6A, supplemental Figure 2). To further determine whether C/EBPβ protein interacts with the regulatory regions of MM-relevant TF genes, we conducted chromatin immunoprecipitations with anti-C/EBPβ antibody in MM cells. The DNA from the immunoprecipitated material was analyzed by RT-PCR using primers that span potential regulatory sequences that contain a CAAT site and could therefore serve as a binding site for C/EBPβ. In parallel, an antibody to RNA polymerase II was used in a ChIP reaction as a positive control, whereas an irrelevant antibody was used as a negative control. The amount of DNA of particular genes precipitated by anti-C/EBPβ antibody relative to that precipitated by anti-RNA polymerase II antibody is plotted (Figure 6B). As a positive control, we took advantage of the observation that the C/EBPβ gene itself has a C/EBPβ-binding site in an intron.22 Here, we observed binding of C/EBPβ at about 12% the level of RNA polymerase II. C/EBPβ has been reported to bind to an intronic region of the CCR5 gene in T lymphocytes.29 Using primers to that region, we observed binding of C/EBPβ at about 37% that of RNA polymerase II. Two sets of primers separated by 500 nt for the IRF4 5′ end were used, near closely spaced CAAT sites in the upstream region of the gene; these primers showed 30% and 20% C/EBPβ binding relative to RNA polymerase II. Primers 200-nt upstream of the PRDM-1 gene (encoding BLIMP1), where several CAAT sites are found, were used and also showed C/EBPβ binding at about 20% relative to RNA polymerase II. Primers in exon 1 of the BCL2 gene were used and also showed C/EBPβ binding to BCL2 at about 25% relative to RNA polymerase II. Meanwhile, XBP1 and dihydrofolate reductase showed little or no binding of C/EBPβ at the upstream sites examined. We conclude that C/EBPβ directly binds to potential regulatory DNA sequences within the IRF4, BLIMP1, and BCL2 genes.
C/EBPβ binds to the promoter regions of IRF4, of BLIMP1, and in exon 1 of BCL2. (A) mRNA expression of C/EBPβ, IRF4, BCL2, and BLIMP1 in H929 MM cells transfected with EV, WT-C/EBPβ, or DN-C/EBPβ were analyzed by quantitative RT-PCR. Data were analyzed according to the ΔCT method. The results are expressed as mRNA fold change compared with control pcDNA group. (B) Chromatin immunoprecipitation with anti-C/EBPβ antibody and RT-PCR on the resulting genomic DNA was conducted on H929 MM cells. PCR probes to the various promoters are indicated and described further in “RT-PCR analysis.” The a and b probes for IRF4, BLIMP1, and BCL2 represent 2 areas of CAAT sequence in the promoter regions. The C/EBP and CCR5 probes were to intron regions previously shown to bind the protein. DNA that was immunoprecipitated by RNA polymerase II was used as the standard (set to 100%) and the amount of DNA immunoprecipitated by C/EBPβ antibody was normalized to it. Control represents the amount of DNA immunoprecipitated by normal rabbit IgG (a negative control). Error bars represent the SD of the mean from at least 4 determinations.
C/EBPβ binds to the promoter regions of IRF4, of BLIMP1, and in exon 1 of BCL2. (A) mRNA expression of C/EBPβ, IRF4, BCL2, and BLIMP1 in H929 MM cells transfected with EV, WT-C/EBPβ, or DN-C/EBPβ were analyzed by quantitative RT-PCR. Data were analyzed according to the ΔCT method. The results are expressed as mRNA fold change compared with control pcDNA group. (B) Chromatin immunoprecipitation with anti-C/EBPβ antibody and RT-PCR on the resulting genomic DNA was conducted on H929 MM cells. PCR probes to the various promoters are indicated and described further in “RT-PCR analysis.” The a and b probes for IRF4, BLIMP1, and BCL2 represent 2 areas of CAAT sequence in the promoter regions. The C/EBP and CCR5 probes were to intron regions previously shown to bind the protein. DNA that was immunoprecipitated by RNA polymerase II was used as the standard (set to 100%) and the amount of DNA immunoprecipitated by C/EBPβ antibody was normalized to it. Control represents the amount of DNA immunoprecipitated by normal rabbit IgG (a negative control). Error bars represent the SD of the mean from at least 4 determinations.
Down-regulation of C/EBPβ by anti-MM agents
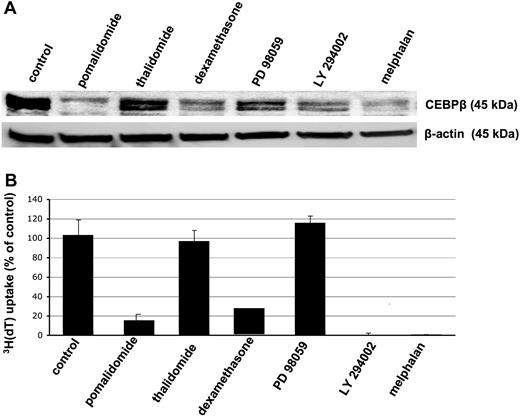
Because our studies indicated that C/EBPβ is a TF regulating growth and survival of MM cells, we next analyzed whether anti-MM agents known for their antiproliferative and apoptotic effect on MM cells inhibit expression of C/EBPβ. We cultured MM.1S MM cells with thalidomide, the immunomodulatory drug compound pomalidomide, dexamethasone, the mitogen-activated protein kinase inhibitor PD 98059, the phosphoinositide-3 kinase inhibitor LY 294002, and melphalan as described in “Cell culture and cell collection.” DMSO was used as control. Western blot analysis revealed that treatment with agents that significantly inhibited DNA synthesis (pomalidomide, dexamethasone, LY 294002, and melphalan) resulted in decreased C/EBPβ protein expression (Figure 7A-B). Of note, thalidomide, which has less strong inhibitory effects on proliferation of MM cells, induced only a weak down-regulation of C/EBPβ (Figure 7B). Taken together, this suggests that C/EBPβ is an important regulator of proliferation, survival, and apoptosis of MM cells.
Down-regulation of C/EBPβ by various anti-MM agents is associated with inhibition of proliferation. MM.1S MM cells were incubated with various anti-MM agents such as pomalidomide, thalidomide, dexamethasone, PD 98059, LY 294002, and melphalan for 48 hours. DMSO 0.1% was used as control treatment. (A) Whole-cell extracts were analyzed for C/EBPβ expression by Western blotting using anti-C/EBPβ antibody. β-Actin was used as a loading control. (B) DNA synthesis was measured by 3H-thymidine incorporation and results are presented as the mean from triplicates. Error bars indicate SD.
Down-regulation of C/EBPβ by various anti-MM agents is associated with inhibition of proliferation. MM.1S MM cells were incubated with various anti-MM agents such as pomalidomide, thalidomide, dexamethasone, PD 98059, LY 294002, and melphalan for 48 hours. DMSO 0.1% was used as control treatment. (A) Whole-cell extracts were analyzed for C/EBPβ expression by Western blotting using anti-C/EBPβ antibody. β-Actin was used as a loading control. (B) DNA synthesis was measured by 3H-thymidine incorporation and results are presented as the mean from triplicates. Error bars indicate SD.
Discussion
In this study, we demonstrate a critical role for C/EBPβ in MM. It has been shown before that C/EBPβ is important for proliferation and differentiation in a variety of cellular systems, and its aberrant expression has been implicated in the development of various epithelial tumors.10,13,30,31 In addition, C/EBPβ plays an important role in lymphopoiesis. Expression and DNA-binding activity of C/EBPβ increase during B-cell differentiation.32 Furthermore, C/EBPβ-deficient mice demonstrate an impaired generation of bone marrow B cells and, after 25 to 30 weeks of age, a peripheral lymphoproliferative disease, presumably due to deregulated cytokine expression and a dysregulation of the Th2 response.15 The fact that enforced expression of C/EBPβ in immature or mature B cells can induce transdifferentiation into macrophages33 indicates that precise control of C/EBPβ expression levels in B lymphocytes is critical to avoid interference with normal lymphoid differentiation. Recently, it has been demonstrated that C/EBPβ is overexpressed in anaplastic lymphoma kinase–positive (ALK+) anaplastic large cell lymphoma.34 This is in accordance with our results showing high expression of C/EBPβ in the ALK+ anaplastic large cell lymphoma cell line SU-DHL-1 (supplemental Figure 1). Expression of C/EBPβ is nucleophosmin-ALK dependent in ALK+ anaplastic large cell lymphoma and plays a critical role in the pathogenesis and unique phenotype of this lymphoma.35
Because C/EBPβ has been shown to be involved in the regulation of B-cell proliferation and survival, and no information is available on the role of C/EBPβ in MM, we investigated the role of C/EBPβ in relation to MM tumor biology. Herein, we describe the expression of C/EBPβ in MM cells. We found no expression of C/EBPβ in all nonmalignant plasma cell samples, including nonneoplastic plasma cells from palatine tonsil samples and in vitro–generated plasma cells. In contrast, up to 30% of primary MM bone marrow samples expressed C/EBPβ as determined by immunohistochemistry. In addition, C/EBPβ was detected in all MM cell lines and in all primary MM patient samples tested by RT-PCR or Western blot. The discrepancies in the frequency of C/EBPβ detection in malignant plasma cells might reflect different sensitivity of the methods with higher sensitivity of RT-PCR and Western blot in comparison with immunohistochemistry. In addition, the difference of C/EBPβ expression might reflect the higher proliferative capacity of MM cell lines in contrast to primary myeloma samples because we found an inverse correlation of C/EBPβ expression with doubling times (DTs) of MM cell lines, with the slowest DT5 and the lowest rate of C/EBPβ-positive cells by immunohistochemistry for MM.1S cells.
By interacting with other TFs, C/EBPβ stimulates its own transcription,2,34,36,37 and autoregulation was reported as one of the main mechanisms for regulation of C/EBPβ activity.22 In this study, we show that DN-C/EBPβ significantly inhibited endogenous C/EBPβ, which resulted in a potential block of C/EBPβ regulation of other TF expression in MM cell lines. Our studies of C/EBPβ detected the activating isoform (LAP) in MM cells. Other cell lines such as SU-DHL-1, a large anaplastic lymphoma cell line, expressed high levels of LIP. We have found a heterogeneous expression pattern of the inhibitory isoform (LIP) in untransfected MM cells. Certain MM cells such as INA-6 show no expression of LIP and other cell lines such as MM.1S expressed LIP only after stimulation with IL-1β (supplemental Figure 1). Unfortunately, due to the limited number of MM cells after transfection and selection, we were not able to obtain sufficient whole-cell lysate to allow for unambiguous detection of LIP by Western blot analysis.
MM is a malignancy of terminally differentiated B cells and the maturation into nondividing, Ig-secreting plasma cells is controlled by a network of TFs.38 Initially, IRF4 was identified as an oncogene associated with the chromosomal translocation t(6;14)(p25;q32) in MM,39 but it also is a well-defined factor for normal plasma cell differentiation.40,41 Recently, IRF4 has been reported as a critical factor controlling MM survival24 and as a prognostic marker in patients with MM associated with poor survival.42 We found that IRF4 shows a similar expression pattern as C/EBPβ in B cells, plasma cells, and MM cells. It is highly expressed in all MM cell lines expressing C/EBPβ. In contrast, normal plasma and B cells showed very low or no detectable levels of IRF4 expression. Recently, IRF4 has been described as “master regulator of an aberrant and malignancy-specific gene expression programme” of MM.24(p230) In our studies, induction of C/EBPβ increased IRF4, whereas inhibition decreased IRF4 expression, suggesting that C/EBPβ can act as an upstream regulator of IRF4. Hence, the fact that C/EBPβ is up-regulated in MM cells and regulates the expression of MM-critical IRF4 makes it a main regulator in MM. In addition, the induction of IRF4 indicates an important role of C/EBPβ in MM. Decreased XBP1 expression was found in DN-C/EBPβ–transfected MM cells, suggesting that C/EBPβ also controls XBP1, which in turn regulates the growth of MM cells.25,41,43
We further observed that overexpression of C/EBPβ induces proliferation and in part protects against spontaneous apoptosis of MM cells. Interestingly, only drugs that significantly decreased expression of C/EBPβ inhibited proliferation of MM cells, suggesting that C/EBPβ is critical for proliferation. Immunohistochemical analysis of MM cell lines indicated an inverse correlation of C/EBPβ expression with doubling times (DTs) of MM cell lines described in the literature, with the longest DT of 72 hours44 and the lowest rate of C/EBPβ-positive cells (60%-70%) by immunohistochemistry for MM.1S cells. In contrast cell lines with a shorter DT of 48 hours45-48 displayed a higher positivity of C/EBPβ, with NCI-H929: 100%; U226: 90%; OPM2: 80% to 90%; RPMI: 80% C/EBPβ+ cells.
To our knowledge, we are the first to report that C/EBPβ is involved in regulation of proliferation and survival in MM. Our findings on the role of C/EBPβ in MM cells are in agreement with a previous report describing its role in the proliferation and maintenance of lymphoma cells by regulating the promoter activity of BCL2.8 In our study, we observed that overexpression of C/EBPβ significantly induced the expression of antiapoptotic protein BCL2, suggesting that C/EBPβ positively regulates BCL2 in MM cells. Piva et al showed that ALK-induced C/EBPβ and BCL2 are involved in the proliferation and antiapoptotic actions in anaplastic lymphomas.36 In contrast, the expression of DN-C/EBPβ significantly decreased BCL2 expression accompanied by increased sensitivity of MM cells to apoptosis.
Analysis of mRNA expression of IRF4, BCL2, and BLIMP1 in MM cells with overexpressed or silenced C/EBPβ as well as in B cells and plasma cells confirmed the expression pattern that we observed. That led us to investigate the protein-promoter interaction of C/EBPβ by ChIP, revealing that C/EBPβ protein is capable of binding to the promoter regions of IRF4 and BLIMP1, and to a site in exon 1 of BCL2, potentially resulting in increases in their transcription (Figure 6B). The observation that C/EBPβ also binds to the CCR5 gene is consistent with its up-regulation in MM49 ; C/EBPβ binding to this site in CCR5 was previously seen in T lymphocytes.
The mechanism that controls the activation of C/EBPβ in MM cells is presently unclear. But our data suggest that C/EBPβ regulates the expression of various TFs associated with proliferation and survival of malignant plasma cells. Our results may help to explore the critical role of C/EBPβ in MM and may offer future therapeutic targets in plasma cell tumorigenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Philip E. Auron (Duquesne University) for providing the human WT-C/EBPβ cDNA and the DN-C/EBPβ cDNA as well as the schematic of the plasmid shown in Figure 2A.
This study was supported in part by a grant from National Institutes of Health, no. CA86433 (C.M.).
National Institutes of Health
Authorship
Contribution: R.P. performed experiments and wrote the paper; M.J. performed gene array studies and helped to design the experiments; D.L.G. provided plasmids and helped to design experiments; M.G. and S. Li performed Western blot; K.J. and I.A. performed immunohistochemical analysis of primary MM samples; B.D. and G.D.R. provided advice on design of experiments; M.Y.M., L.B., L.K., and C.M. helped to design the experiments; C.M. performed ChIP assays; L.K. and L.B. provided B cells and nonmalignant plasma cells; S. Lentzsch conceived and developed the experiments, analyzed the data, and wrote the paper; and all authors read and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne Lentzsch, Division of Hematology/Oncology, University of Pittsburgh Cancer Institute, 5150 Centre Ave, #568, Pittsburgh, PA 15232; e-mail: lentzschs@upmc.edu.