Abstract
Multiple myeloma remains an incurable disease. One of the major problems is that myeloma cells develop drug resistance on interaction with bone marrow stromal cells. In this study, we examined the effects of macrophages (Mφs), a type of stromal cells, on myeloma cell survival and response to chemotherapy. We showed that Mφ, in particular tumor-associated Mφ, is a protector of myeloma cells. The protective effect was dependent on direct contact between Mφs and myeloma cells. Mφs protected both myeloma cell lines and primary myeloma cells from spontaneous and chemotherapy drug-induced apoptosis by attenuating the activation and cleavage of caspase-dependent apoptotic signaling. These findings are clinically relevant because we found that CD68+ Mφs heavily infiltrate the bone marrow of patients with myeloma but not the bone marrow of control patients. Thus, our results indicate that Mφs may contribute to myeloma cell survival and resistance to chemotherapeutic treatment in vivo.
Introduction
Multiple myeloma (MM) is a malignant B-cell tumor characterized by proliferation of monoclonal plasma cells in the bone marrow.1 Although chemotherapy is now the most effective treatment for MM, myeloma cells often fail to respond to the drugs. Studies have shown that the response of myeloma cells to cytotoxic chemotherapeutics can be attenuated by the presence of bone marrow stromal cells.2,3 However, the mechanisms of myeloma cell proliferation and failure to respond to chemotherapeutic drugs are not fully defined. To better understand the role of different stromal cell components in the bone marrow microenvironment, we examined the effects of macrophages (Mφs) on myeloma cell survival and response to chemotherapy in this study.
Methods
Myeloma cells, antibody, and reagents
Primary myeloma cells were isolated from bone marrow aspirates of myeloma patients. Interleukin-6 (IL-6) and macrophage colony-stimulating factor (M-CSF) antibodies were purchased from R&D Systems. Melphalan, dexamethasone, cytochalasin D, and fluorescein isothiocyanate (FITC)–labeled dextran were purchased from Sigma-Aldrich. The study was approved by the Institutional Review Board at the University of Texas M. D. Anderson Cancer Center.
Generation of macrophages
Mononuclear cells from the blood of healthy donors were incubated in 12-well plates for 2 hours at 37°C to remove nonadherent cells. The adherent monocytes were incubated for 7 days in medium with M-CSF4 to become normal Mφs (nMφs). nMφs were cultured for an additional 72 hours with tumor-culture conditioning medium (TCCM) of myeloma cells5 to generate tumor-associated Mφs (tMφs). Mφs were also generated from blood monocytes of patients with MM in a 7-day culture with M-CSF and used in the experiments.
Apoptosis assay
Annexin V staining was used to detect apoptosis in myeloma cells as described previously.6 To exclude apoptotic macrophages, cultured cells were stained with phycoerythrin-conjugated anti-CD138 antibody and FITC-conjugated annexin V, and apoptotic myeloma cells were identified as CD138+annexin V+ cells.
Immunohistochemistry analysis
Sections of bone marrow biopsies from MM and control patients were examined by immunohistochemistry staining as described previously.7
Results and discussion
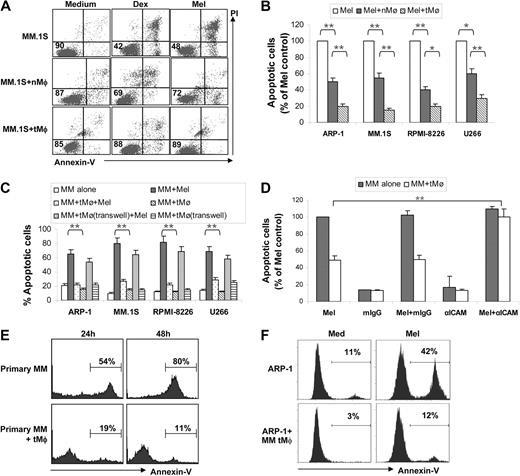
To evaluate the effect of Mφs on myeloma cells, we examined whether Mφs could protect myeloma cells from chemotherapy drug-induced apoptosis. As shown in Figure 1A, coculture of myeloma cells with Mφs protected myeloma cells from dexamethasone- and melphalan-induced apoptosis (P < .01 by Student t test). However, tMφs were more effective than nMφs at protecting myeloma cells from melphalan-induced apoptosis in 4 myeloma cell lines examined (Figure 1B; P < .05 and P < .01). Next we examined whether Mφ-mediated protection requires cell-cell contact. In the experiments, tMφs were cocultured with myeloma cells either in direct contact or separated by Transwell insets. Although direct coculture of tMφs with myeloma cells conferred protection against melphalan-induced apoptosis (Figure 1C; P < .01), coculture of tMφs with myeloma cells in Transwell inserts nearly abolished the ability of tMφs to protect myeloma cells. Likewise, the addition of culture supernatants of tMφs to myeloma cells slightly but insignificantly protected the cells from chemotherapy-induced apoptosis (data not shown). To examine the importance of cell-cell contact, blocking antibody specific for adhesion molecule ICAM-1 was used. Addition of anti–ICAM-1 antibody significantly compromised tMφ-mediated protection (Figure 1D). These results indicate that cell-cell contact plays a major role in Mφ-mediated protection of myeloma cell apoptosis. Furthermore, we examined whether Mφs can also support primary myeloma cell survival. Primary myeloma cells freshly isolated from patients with MM undergo apoptosis ex vivo in medium unless they are cocultured with stromal cells.8 As shown in Figure 1E depicting the representative data obtained from experiments with samples from 1 of 4 patients examined, approximately 50% and 80% of primary myeloma cells were apoptotic 24 hours and 48 hours, respectively, after isolation, whereas fewer than 20% of the cells were apoptotic when cocultured with tMφ. We also found that coculture with myeloma patient–derived Mφs significantly protected myeloma cell apoptosis induced by melphalan (Figure 1F). These results indicate that Mφ, especially tMφ, may be a protector of myeloma cell apoptosis. Studies have shown that tMφs are driven by tumor-derived cytokines to acquire a polarized type 2 phenotype, which differ in terms of receptor expression, effector function, and cytokine and chemokine production.9
Macrophages protect myeloma cells from apoptosis. (A) Dot plots showing apoptotic myeloma (MM.1S) cells in cultures or cocultures with nMφs or TCCM-treated Mφs (tMφ) in the presence or absence of dexamethasone (Dex) or melphalan (Mel). Numbers inside dot plots indicate the percentages of live cells. (B) Apoptotic myeloma cells (percentage of melphalan control, which is 100% of apoptotic cells) in cocultures with either nMφs or tMφs in the presence of melphalan. Four commonly used myeloma cell lines, ARP-1, MM.1S, RPMI-8226, and U266, were tested. (C) Percentages of apoptotic myeloma cells in cocultures without or with tMφs either in direct contact (MM + tMφ) or separated by Transwell inserts (MM + tMφ, Transwell) in the presence of melphalan. Culture of myeloma cells in medium and coculture of myeloma cells with tMφs served as controls. (D) Effects of anti–ICAM-1 antibody on blocking tMφ-mediated myeloma apoptosis protection. Shown is the percentage of apoptotic myeloma ARP-1 cells pretreated with 10 μg/mL anti–ICAM-1 antibody or mouse IgG, in (co)cultures with tMφs in the presence of melphalan. Culture of myeloma cells in medium and coculture of myeloma cells with tMφs served as controls. Similar results were obtained with other myeloma cell lines. (E) Percentages of spontaneous apoptotic primary myeloma cells in culture medium only (primary MM) or in cocultures with tMφs (primary MM + tMφ) at 24 hours and 48 hours after isolation of the myeloma cells. Representative results from experiments with primary myeloma cells from one patient of 4 examined are shown. (F) Percentage of apoptotic myeloma ARP-1 cells in coculture with Mφs generated from MM patients in the presence of melphalan. Representative results from experiments with Mφs from 1 patient of 3 examined using this and other cell lines are shown. *P < .05; **P < .01.
Macrophages protect myeloma cells from apoptosis. (A) Dot plots showing apoptotic myeloma (MM.1S) cells in cultures or cocultures with nMφs or TCCM-treated Mφs (tMφ) in the presence or absence of dexamethasone (Dex) or melphalan (Mel). Numbers inside dot plots indicate the percentages of live cells. (B) Apoptotic myeloma cells (percentage of melphalan control, which is 100% of apoptotic cells) in cocultures with either nMφs or tMφs in the presence of melphalan. Four commonly used myeloma cell lines, ARP-1, MM.1S, RPMI-8226, and U266, were tested. (C) Percentages of apoptotic myeloma cells in cocultures without or with tMφs either in direct contact (MM + tMφ) or separated by Transwell inserts (MM + tMφ, Transwell) in the presence of melphalan. Culture of myeloma cells in medium and coculture of myeloma cells with tMφs served as controls. (D) Effects of anti–ICAM-1 antibody on blocking tMφ-mediated myeloma apoptosis protection. Shown is the percentage of apoptotic myeloma ARP-1 cells pretreated with 10 μg/mL anti–ICAM-1 antibody or mouse IgG, in (co)cultures with tMφs in the presence of melphalan. Culture of myeloma cells in medium and coculture of myeloma cells with tMφs served as controls. Similar results were obtained with other myeloma cell lines. (E) Percentages of spontaneous apoptotic primary myeloma cells in culture medium only (primary MM) or in cocultures with tMφs (primary MM + tMφ) at 24 hours and 48 hours after isolation of the myeloma cells. Representative results from experiments with primary myeloma cells from one patient of 4 examined are shown. (F) Percentage of apoptotic myeloma ARP-1 cells in coculture with Mφs generated from MM patients in the presence of melphalan. Representative results from experiments with Mφs from 1 patient of 3 examined using this and other cell lines are shown. *P < .05; **P < .01.
To elucidate the mechanism underlying Mφ-mediated protection in myeloma cells, we examined apoptotic signaling pathways in myeloma cells. Using Western blot analysis, we showed that melphalan treatment activates and induces cleavage of caspase-3 and poly(ADP-ribose) polymerization (PARP), and down-regulated Bcl-xL in myeloma cells (Figure 2A). Coculture of myeloma cells with tMφs protected myeloma cells from melphalan-induced apoptosis by inhibiting the activation and cleavage of caspase-3 and PARP, and maintaining the levels of Bcl-xL. No changes in the expression of Bcl-2, Bad, or Bax were observed in myeloma cells treated with melphalan in the presence or absence of tMφ. These results suggest that Mφs protect myeloma cells from apoptosis via inhibiting Bcl-xL–dependent caspase activation.
The mechanism of macrophage-mediated antiapoptosis in myeloma cells. (A) Western blot analysis showing the protein expression of cleaved PARP (cPARP), cleaved caspase-3 (cCas-3), Bcl-xL, Bcl-2, Bad, and Bax in ARP-1 myeloma cells cultured alone or cocultured with tMφs in the presence of melphalan (5 μM). The level of β-actin served as loading control. Results from 1 representative experiment of 3 performed with ARP-1 are shown. Similar results were obtained with other myeloma cell lines. (B) Levels of IL-6 in normal medium, TCCM, and in the supernatants of nMφs and tMφ, measured by enzyme-linked immunosorbent assay. (C) Dot plots showing apoptotic myeloma cells in culture medium (Med), in cocultures with tMφ, and in coculture with tMφs and IL-6–neutralizing antibody (αIL-6) in the presence or absence of melphalan (Mel). Numbers inside dot plots indicate the percentages of live cells. (D) Percentages of melphalan (Mel)–induced, annexin V–positive apoptotic myeloma (ARP-1) cells in culture medium only (Med) or in cocultures with untreated tMφs or with cytochalasin D (CD)–pretreated tMφs (CD-tMφ). Infiltration of Mφs in the bone marrow of myeloma patients. (E) Immunochemistry staining by CD68 antibody to identify Mφs in bone marrow biopsies from a control patient without malignancy and from a randomly selected myeloma patient. Representative results from experiments with bone marrow biopsies from 1 of 4 myeloma and 4 control patients examined are shown. (F) Percentages of infiltrated Mφs in bone marrow biopsies of MM and control patients. Data were derived from the numbers (mean ± SD) of CD68+ Mφs in a total of 1000 cells counted in bone marrow biopsies of 4 patients with MM and 4 controls. **P < .01.
The mechanism of macrophage-mediated antiapoptosis in myeloma cells. (A) Western blot analysis showing the protein expression of cleaved PARP (cPARP), cleaved caspase-3 (cCas-3), Bcl-xL, Bcl-2, Bad, and Bax in ARP-1 myeloma cells cultured alone or cocultured with tMφs in the presence of melphalan (5 μM). The level of β-actin served as loading control. Results from 1 representative experiment of 3 performed with ARP-1 are shown. Similar results were obtained with other myeloma cell lines. (B) Levels of IL-6 in normal medium, TCCM, and in the supernatants of nMφs and tMφ, measured by enzyme-linked immunosorbent assay. (C) Dot plots showing apoptotic myeloma cells in culture medium (Med), in cocultures with tMφ, and in coculture with tMφs and IL-6–neutralizing antibody (αIL-6) in the presence or absence of melphalan (Mel). Numbers inside dot plots indicate the percentages of live cells. (D) Percentages of melphalan (Mel)–induced, annexin V–positive apoptotic myeloma (ARP-1) cells in culture medium only (Med) or in cocultures with untreated tMφs or with cytochalasin D (CD)–pretreated tMφs (CD-tMφ). Infiltration of Mφs in the bone marrow of myeloma patients. (E) Immunochemistry staining by CD68 antibody to identify Mφs in bone marrow biopsies from a control patient without malignancy and from a randomly selected myeloma patient. Representative results from experiments with bone marrow biopsies from 1 of 4 myeloma and 4 control patients examined are shown. (F) Percentages of infiltrated Mφs in bone marrow biopsies of MM and control patients. Data were derived from the numbers (mean ± SD) of CD68+ Mφs in a total of 1000 cells counted in bone marrow biopsies of 4 patients with MM and 4 controls. **P < .01.
Next we examined whether IL-6, one of the most important cytokines for myeloma growth and survival,10 plays a role in Mφ-mediated protection of myeloma cell apoptosis. As shown in Figure 2B, the level of IL-6 was significantly higher in the supernatant of tMφs than that of nMφs or TCCM (P < .01). To examine the importance of IL-6 in tMφ-mediated protection, neutralizing antibody against IL-6 was used. As shown in Figure 2C, addition of anti–IL-6 specific antibody did not affect tMφ-mediated protection of myeloma cells from melphalan-induced apoptosis, indicating that IL-6 did not contribute to the protective effects of tMφ. It may be possible that the growth and antiapoptotic signaling generated by cell-cell contact are stronger than those of IL-6 signaling. Further studies are warranted to elucidate the mechanisms.
To rule out the possibility that Mφs engulfed apoptotic myeloma cells so that fewer apoptotic cells were detected in the cocultures, cytochalasin D was used to inhibit the endocytosis ability of Mφ. Myeloma cells were cultured with tMφs pretreated with or without cytochalasin D in the presence of melphalan for 24 hours, and the number of apoptotic cells was determined after the culture. As shown in Figure 2D, 46% of myeloma cells cultured with melphalan became apoptotic, whereas fewer than 10% myeloma cells cocultured with either untreated or cytochalasin D–spretreated tMφs in the presence of melphalan became apoptotic. Using FITC-conjugated dextran, we show that pretreatment of Mφs with cytochalasin D significantly inhibited the ability of Mφs to engulf FITC-conjugated dextran (data not shown). These results indicate that the reduced numbers of apoptotic myeloma cells in the cocultures were indeed the result of apoptosis protection mediated by tMφ.
To evaluate the clinical relevance of our findings, we examined whether Mφs are present in the bone marrow of myeloma patients. Mφs in the bone marrow samples were identified with antibody against CD68, a glycoprotein expressed only by human Mφ. As shown in Figure 2E by the representative staining from 1 MM and 1 control patient of 4 examined, CD68+ Mφs were scarcely found in the bone marrow biopsies of control patients; whereas in patients with MM, CD68+ Mφs were heavily infiltrated in the bone marrow. The quantitative results of infiltrating CD68+ Mφs are shown in Figure 2F. It is evident that significantly increased numbers of CD68+ Mφs were found in the bone marrow biopsies of MM patients than controls (P < .01). Taken together, these findings indicate that Mφs may be an abundant and important component of the bone marrow stromal cells and play a critical role in vivo in protecting myeloma cells from chemotherapy-induced apoptosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank our Departmental Myeloma Tissue Bank for patient samples and Ms Alison Woo for providing editorial assistance.
This work was supported by the National Cancer Institute (grants R01 CA96569, R01 CA103978, and CA138402), the Leukemia & Lymphoma Society Translational Research Grant, Multiple Myeloma Research Foundation, and Commonwealth Foundation for Cancer Research.
National Institutes of Health
Authorship
Contribution: Y.Z., Z.C., and S.W. performed the majority of experiments and contributed to the written paper; X.Z., J.Q., S.H., and H.L. performed experiments; M.W. provided patient samples and critical suggestions; J.Y. contributed to the conceptual idea and performed experiments; and Q.Y. contributed to the conceptual idea for the paper, experimental design, and writing and editing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qing Yi, Department of Lymphoma and Myeloma, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0903, Houston, TX 77030; e-mail: qyi@mdanderson.org.
References
Author notes
Y.Z., Z.C., and S.W. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal