Acute myeloid leukemia (AML) has a different clinical and biologic behavior in patients at older age. To gain further insight into the molecular differences, we examined a cohort of 525 adults to compare gene expression profiles of the one-third of youngest cases (n = 175; median age 31 years) with the one-third of oldest cases (n = 175; median age 59 years). This analysis revealed that 477 probe sets were up-regulated and 492 probe sets were down-regulated with increasing age at the significance level of P < .00001. After validation with 2 independent AML cohorts, the 969 differentially regulated probe sets on aging could be pointed to 41 probe sets, including the tumor-suppressor gene CDKN2A (encoding p16INK4A). In contrast to the induced p16INK4A expression that is associated with physiologic aging, p16INK4A is down-regulated in AML samples of patients with increasing age. However, this was only noticed in the intermediate- and unfavorable-risk group and not in the favorable-risk group and the molecularly defined subset “NPM1 mutant without FLT3-ITD.” Multivariate analysis revealed p16INK4A, besides cytogenetic risk groups, as an independent prognostic parameter for overall survival in older patients. We conclude that, in addition to altered clinical and biologic characteristics, AML presenting at older age shows different gene expression profiles.
Introduction
The hematopoietic system is composed largely of cells with short life spans and therefore requires continuous replenishment, which is ensured by hematopoietic stem cells (HSCs).1 During aging, the most clinically significant changes in the hematopoietic system are the decreased competence of the adaptive immune system, the onset of anemia in older humans, and the increased incidence of myeloid diseases, including leukemia.2,,–5 These changes are underscored by microarray analyses of HSCs from aged mice, which demonstrated up-regulation of genes associated with myeloid differentiation and down-regulation of genes specifying lymphoid fate, chromatin remodeling, and gene silencing.5,6
Central in aging of cells is cumulative cellular and genomic damage caused by endogenous as well as exogenous factors. In response to this damage, tumor suppressor pathways are activated, including those mediated by the tumor suppressor proteins p16INK4a and p53, to ensure that potentially dangerous lesions do not lead to malignancy.7,8 For example, the expression of p16INK4a is virtually undetectable in tissues of young humans and rodents and increases with increasing age.9,10 In a murine study, p16INK4a expression was elevated in HSCs from old mice, whereas age-associated repopulating deficits and serial-transplantation capacities improved in aged p16INK4a-deficient mice.11 Taken together, these data would indicate that, when aging advances and damage accumulates, tumor suppressor pathways are activated with the potential to induce apoptosis or senescence to protect against genomic damage, resulting in a negative modulation of stem cell function.
Aging may not only affect normal hematopoietic development but also impact on the clinical biology of acute myeloid leukemia (AML). In particular, the incidence of AML increases with increasing age.12,13 Moreover, older AML patients have a markedly reduced long-term survival resulting from the combination of poor chemotherapeutic tolerance and inherent chemotherapy resistance compared with younger AML patients.12,,,–16 AML in older patients shows also a lower frequency of favorable core-binding chromosomal abnormalities and a higher incidence of complex aberrant karyotypes.17,18 These differences in clinical and cellular behavior of AML in older patients suggest activation of different target genes by oncogenic events in aged stem cells or progenitor cells compared with younger stem cells or progenitor cells.
We wondered whether, in addition to altered clinical and biologic characteristics, AML at older age is associated with an altered gene expression profile. Therefore, we have compared the gene expression in AML samples of a group of 175 young patients with a median age of 31 years with a group of 175 older patients with a median age of 59 years. Our results show distinct gene expression profiles in AML samples of older patients compared with AML samples of younger patients. In particular, we report here that the tumor suppressor p16INK4a is down-regulated in AML samples of patients with increasing age and is an independent prognostic factor for overall survival in older patients.
Methods
Patient samples
Gene expression profiling has been performed on 525 consecutive patients with newly diagnosed AML who have been treated according to sequential HOVON/SAKK AML-04, -04A, -29, -32, -42, and -43 protocols (available at http://www.hovon.nl).19,–21 From this cohort, we concentrated on the one-third of the youngest (n = 175) and one-third of the oldest patients (n = 175) for gene expression comparison. The diagnosis of primary AML has been confirmed by a cytologic examination of blood and bone marrow. Cytomorphology and cytogenetics have been reviewed by an independent review board. Bone marrow aspirates or peripheral blood samples were collected at the time of diagnosis. All subjects provided written informed consent in accordance with the Declaration of Helsinki. A total of 285 of 525 adult patients have been included in a previous gene expression array study.22 Patients younger than 60 years were treated more intensively and received more often an allogeneic hematopoietic cell transplantation (HCT) compared with patients older than 60 years (Table 1). An independent second series of specimens from 53 newly diagnosed patients with AML from a single center (University Medical Center Groningen) was used for validation of the data of the initial cohort. In the validation set, isolated mononuclear AML cells were sorted on MoFlo (Dako North America), based on CD34 expression (BD Biosciences). A third series of CD34+ cells derived from neonatal cord blood from healthy full-term pregnancies, potential donors for allogeneic bone marrow transplantation, and bone marrow aspirates of patients who underwent elective total hip replacement also served as normal controls. Cytogenetic risk group distinction (favorable, intermediate, unfavorable) is according to HOVON/SAKK protocols23 and is also given in Table 1. The medical ethical committee of the University Medical Center Groningen and the Erasmus Medical Center of Rotterdam provided approval for this study.
Patient characteristics
| Characteristic . | All patients . | Young . | Middle . | Old . |
|---|---|---|---|---|
| No. | 525 | 175 | 175 | 175 |
| Age at diagnosis, y | 46.6 (15.2-77.2) | 31.1* (15.2-39.4) | 46.6* (39.4-53.6) | 59.4* (53.6-77.2) |
| White blood cell count (× 109/L) | 26 (0.3-510) | 25 (1-44) | 34 (0.3-278) | 27 (1-510) |
| Bone marrow blasts (%) | 65 (1-99) | 69 (4-98) | 66 (1-99) | 62 (1-99) |
| Platelets (× 109/L) | 56 (3-998) | 44* (3-339) | 55* (5-998) | 66* (8-722) |
| Cytogenetics | ||||
| Favorable | 89 (17%) | 50* (29%) | 27* (15%) | 12* (7%) |
| t(8;21) | 34 | 19* | 13* | 2* |
| t(15;17) | 20 | 13* | 5* | 2* |
| inv16 | 35 | 18* | 9* | 8* |
| Intermediate | 331 (63%) | 93 (53%) | 119 (68%) | 119 (68%) |
| Normal karyotype | 218 | 57 | 82 | 79 |
| +8 | 25 | 12 | 7 | 6 |
| −9q | 7 | 2 | 1 | 4 |
| Other | 81 | 22 | 29 | 30 |
| Unfavorable | 85 (16%) | 26 (15%) | 22 (13%) | 37 (21%) |
| 11q23 | 11 | 4 | 5 | 2 |
| Complex | 20 | 4 | 4 | 12 |
| −5(q)/−7(q) | 42 | 15 | 6 | 21 |
| abn(3q) | 2 | 0 | 2 | 0 |
| t(6;9) | 6 | 1 | 4 | 1 |
| t(9;22) | 2 | 2 | 0 | 0 |
| Other | 2 | 0 | 1 | 1 |
| Not available | 20 (4%) | 6 (3%) | 7 (4%) | 7 (4%) |
| NPM1 mutation without FLT3-ITD | 77 (15%) | 13* | 35* | 29* |
| NPM1 mutation with FLT3-ITD | 82 (15%) | 20 | 29 | 33 |
| NPM1 wild-type without FLT3-ITD | 305 (58%) | 120 | 90 | 95 |
| NPM1 wild-type with FLT3-ITD | 61 (12%) | 22 | 21 | 18 |
| No SCT | 312 | 81* | 104* | 127* |
| Allogeneic SCT | 140† | 65* | 52*‡ | 23*§ |
| Autologous SCT | 68 | 29 | 19 | 20 |
| Not available | 5 | 0 | 0 | 5 |
| Cycles to CR, n (%) | ||||
| 1 | 297 (57) | 88 (50.3) | 109 (62.3) | 100 (57.1) |
| 2 | 111 (21) | 53* (30.3) | 33* (18.9) | 25* (14.3) |
| 3 | 8 (2) | 2 (1.1) | 4 (2.3) | 2 (1.1) |
| More than 3 | 5 (1) | 2 (1.1) | 2 (1.1) | 1 (0.6) |
| No CR | 104 (20) | 30 (17.2) | 27 (15.4) | 47 (26.9) |
| Relapse, n (%) | 202 (39) | 70 (40) | 70 (40.0) | 62 (35.0) |
| Dead/alive | 316/209 | 92/83 | 111/64 | 113/62 |
| Characteristic . | All patients . | Young . | Middle . | Old . |
|---|---|---|---|---|
| No. | 525 | 175 | 175 | 175 |
| Age at diagnosis, y | 46.6 (15.2-77.2) | 31.1* (15.2-39.4) | 46.6* (39.4-53.6) | 59.4* (53.6-77.2) |
| White blood cell count (× 109/L) | 26 (0.3-510) | 25 (1-44) | 34 (0.3-278) | 27 (1-510) |
| Bone marrow blasts (%) | 65 (1-99) | 69 (4-98) | 66 (1-99) | 62 (1-99) |
| Platelets (× 109/L) | 56 (3-998) | 44* (3-339) | 55* (5-998) | 66* (8-722) |
| Cytogenetics | ||||
| Favorable | 89 (17%) | 50* (29%) | 27* (15%) | 12* (7%) |
| t(8;21) | 34 | 19* | 13* | 2* |
| t(15;17) | 20 | 13* | 5* | 2* |
| inv16 | 35 | 18* | 9* | 8* |
| Intermediate | 331 (63%) | 93 (53%) | 119 (68%) | 119 (68%) |
| Normal karyotype | 218 | 57 | 82 | 79 |
| +8 | 25 | 12 | 7 | 6 |
| −9q | 7 | 2 | 1 | 4 |
| Other | 81 | 22 | 29 | 30 |
| Unfavorable | 85 (16%) | 26 (15%) | 22 (13%) | 37 (21%) |
| 11q23 | 11 | 4 | 5 | 2 |
| Complex | 20 | 4 | 4 | 12 |
| −5(q)/−7(q) | 42 | 15 | 6 | 21 |
| abn(3q) | 2 | 0 | 2 | 0 |
| t(6;9) | 6 | 1 | 4 | 1 |
| t(9;22) | 2 | 2 | 0 | 0 |
| Other | 2 | 0 | 1 | 1 |
| Not available | 20 (4%) | 6 (3%) | 7 (4%) | 7 (4%) |
| NPM1 mutation without FLT3-ITD | 77 (15%) | 13* | 35* | 29* |
| NPM1 mutation with FLT3-ITD | 82 (15%) | 20 | 29 | 33 |
| NPM1 wild-type without FLT3-ITD | 305 (58%) | 120 | 90 | 95 |
| NPM1 wild-type with FLT3-ITD | 61 (12%) | 22 | 21 | 18 |
| No SCT | 312 | 81* | 104* | 127* |
| Allogeneic SCT | 140† | 65* | 52*‡ | 23*§ |
| Autologous SCT | 68 | 29 | 19 | 20 |
| Not available | 5 | 0 | 0 | 5 |
| Cycles to CR, n (%) | ||||
| 1 | 297 (57) | 88 (50.3) | 109 (62.3) | 100 (57.1) |
| 2 | 111 (21) | 53* (30.3) | 33* (18.9) | 25* (14.3) |
| 3 | 8 (2) | 2 (1.1) | 4 (2.3) | 2 (1.1) |
| More than 3 | 5 (1) | 2 (1.1) | 2 (1.1) | 1 (0.6) |
| No CR | 104 (20) | 30 (17.2) | 27 (15.4) | 47 (26.9) |
| Relapse, n (%) | 202 (39) | 70 (40) | 70 (40.0) | 62 (35.0) |
| Dead/alive | 316/209 | 92/83 | 111/64 | 113/62 |
Young, middle, and old indicate the youngest, middle, and oldest third of the patients, respectively. Characteristics—age, WBC, percentage bone marrow blasts and platelets—are given as median (range).
Significant difference, younger versus middle versus older patients (P < .01).
A total of 12 of 140 patients with an allogeneic HCT after nonmyeloablative conditioning.
A total of 1 of 52 patients with an allogeneic HCT after nonmyeloablative conditioning.
A total of 11 of 23 patients with an allogeneic HCT after nonmyeloablative conditioning.
Isolation and quality control of RNA, gene profiling, and quality control
The samples for the gene expression profiling were obtained and analyzed as described previously.22 Staining, washing, and scanning procedures were carried out as described in the GeneChip Expression Analysis technical manual (Affymetrix). Detailed clinical, cytogenetic, and molecular cytogenetic information is available at the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo, accession no. GSE6891). The RMA method in R, Version 2.4.0, was used to compute probe set summaries.24
RT-PCR
In the samples for reverse-transcriptase polymerase chain reaction (RT-PCR) validation, total RNA was isolated from 0.3 to 106 cells using the RNeasy kit (QIAGEN) according to the manufacturer's recommendations. RNA was reverse transcribed, and aliquots of cDNA were real-time amplified using iQ SYBR Green supermix (Bio-Rad) on an MyIQ thermocycler (Bio-Rad) and quantified using MyIQ software (Bio-Rad). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used to calculate relative expression levels. Sequences of the used primers are: p16INK4A forward, 5′-ATG GAG CCT TCG GCT GAC TG-3′; p16INK4A reverse, 5′-GCG CTG CCC ATC ATC ATG AC-3′; HPRT forward, 5′-AGT TCT GTG GCC ATC TGC TTA G-3′; HPRT reverse, 5′-CGC CCA AAG GGA ACT GAT AGT C-3′.
Class comparison
Differentially expressed genes were identified for 175 youngest versus 175 oldest AML patients using a multivariate permutation test in Biometric Research Branch ArrayTools (BRB ArrayTools, Version 3.6). Differential expression was considered significant at P less than .001. A random variance t test was selected to permit the sharing of information among probe sets within class variation without assuming that all of the probe sets possess the same variance.
Gene ontology analysis
To investigate the biologic significance of the gene lists, we used gene ontology (GO; http://www.geneontology.org). After mapping each gene to the GO tree structure, the number of genes was determined at or below any given node in the GO hierarchy and the amount of statistical enrichment (Fisher exact test) for each GO node relative to chance observation, using a previously developed procedure (GeneTrail).25
Statistical analysis
Statistical analyses were performed with SPSS software, release 14.0. Actuarial probabilities of overall survival (OS; with death from any cause) as well as event-free survival (EFS; with failure in case of no complete remission [CR1] or relapse or death) were estimated according to the Kaplan-Meier method. For quantitative parameters, overall differences between the cohorts were evaluated using an F test (or Student t in case of 2 groups) for normally distributed variables or a Kruskal-Wallis test (or Mann-Whitney U test in case of 2 groups) for skewed distributed variables. For qualitative parameters, overall group differences will be evaluated using a χ2 test (or Fisher exact in 2 × 2 setting). Correlations between age and OS were calculated with the Spearman rank correlation coefficient (ρ). Cox regression analysis was applied to determine the association of age or p16INK4A expression and OS with adjustment for disease-related risk factors, such as cytogenetic risk profile (ie, favorable, intermediate, or unfavorable), FLT3 internal tandem duplication (FLT3-ITD), as well as nucleophosmin 1 (NPM1) mutation. The proportional hazard assumption was checked using log-log survivor functions (parallel curves). In addition, the presence of time dependence indicating violation of the proportional hazard assumption was assessed. All tests were 2-tailed, and a P value of less than .05 was considered statistically significant.
Results
Patient population
Patient characteristics of 525 AML samples of the first cohort of patients are summarized in Table 1 and supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). AML with favorable cytogenetic abnormalities was more apparent in persons of younger age, whereas AMLs with intermediate and unfavorable cytogenetic abnormalities were seen more frequently at older age (Table 1). There was no difference in age at diagnosis between patients with AML with FLT3-ITD and those without FLT3-ITD (P = .21). However, patients with AML and the favorable NPM1 mutation had a significantly older age (median age, 50 years) compared with patients with wild-type NPM1 (median age, 44 years; P < .0001). In addition, the favorable genotypic combination “NPM1 without FLT3-ITD” was more frequently observed with increasing age (Table 1).
A highly significant inverse correlation between the continuous variables age and OS was found in all tested AML patients (P < .0001, ρ = −0.229, n = 525). Univariate analysis demonstrated that age and the disease-related parameters, NPM1 mutation, FLT3-ITD, and cytogenetic risk group (ie, unfavorable, intermediate, and favorable risk), significantly affected OS (data not shown). Subsequently, all significantly different variables in the univariate analysis were included in a multivariate analysis. This analysis established the continuous variable age (in years) as an independent risk indicator for OS (hazard ratio [HR] = 1.012 a year; 95% confidence interval [CI], 1.004-1.021, P = .005). Subsequently, the effect of age on OS was studied in defined cytogenetic risk groups (favorable, intermediate, and unfavorable risk). In each of these risk classes, increasing age correlated with reduced OS (supplemental Figure 2). The intermediate cytogenetic risk groups were further subdivided with molecular markers NPM1 mutation and FLT3-ITD. In the most favorable genotype “NPM1 mutant without FLT3-ITD,” increasing age showed no impact on OS (supplemental Figure 2). However, in the genotypic subgroups “NPM1 mutant plus FLT3-ITD” and “NPM1 wild-type without FLT3-ITD,” older patients had a significantly worse OS compared with their younger counterparts.
Distinct gene expression profiles between AML samples of younger and older patients
To extend the observation that biologic and clinical parameters differ between AML samples of younger and older patients, the transcriptome of AML samples of the 175 oldest (median age, 59.4 years; range, 53.6-77.2 years) patients (oldest one-third of the entire cohort) was compared with the transcriptome of AML samples of the 175 youngest (median age, 31.1 years; range, 15.2-39.4 years) patients (youngest one-third of the entire cohort). Table 1 shows the composition of these age groups. This comparison revealed that 477 probe sets were higher expressed in the older age group (“Up-with-Age” group) and that 492 probe sets were lower expressed in the older age group (“Down-with-Age” group) at the significance level of P less than .00001 (supplemental Table 1).
Validation of age-dependent differences in gene expression profiles
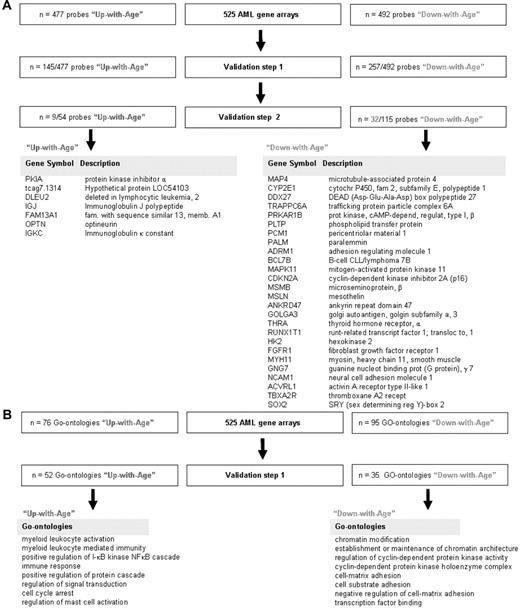
To validate the list of differently expressed genes between AML samples of younger and older patients, 2 smaller publicly available independent gene expression profiling cohorts were used.26,27 The median age at diagnosis in the first validation cohort described by Tomasson et al26 (n = 180) was 48 years (range, 18-81 years). All AML samples in this cohort were also analyzed with the use of Affymetrix U133A 2.0 GeneChips. All identified probe sets could thus be evaluated. In addition to the use of independent cohorts of AML, to account for the problem of multiple testing, the 477 “Up-with-Age” probe sets and the 492 “Down-with-Age” probe sets were individually validated as a continuous variable dependent on age. Hence, 145 of 477 (32%) “Up-with-Age” as well as 257 of 492 (52%) “Down-with-Age” probe sets could be confirmed (ie, validation step 1 in Figure 1A). Subsequently, the cohort described by Wilson et al27 (n = 170), with a median age at diagnosis of 65 years (range, 20-84 years), was used for the second validation. As the AML samples of the Wilson et al study27 had been analyzed with Affymetrix HG_U95Av2 GeneChips, only 54 of 145 “Up-with-Age” and 115 of 257 “Down-with-Age” genes were available on this chip and thus could be used for confirmation as continuous variable dependent on age. In sum, of the differentially expressed probe sets on aging in the original dataset, 7 “Up-with-Age” genes and 26 “Down-with-Age” genes could be confirmed to be significantly differently expressed as a continuous variable depending on age in 2 independent AML cohorts (Figure 1A).
Probes and GO ontologies differently expressed between AML samples of young and old patients. (A) The transcriptome of AML samples of the 175 oldest patients was compared with the transcriptome of AML samples of the 175 youngest patients. This comparison revealed 477 probe sets that were higher expressed in the older age group (“Up-with-Age” group) and 492 that were lower expressed in the older age group (“Down-with-Age” group) at the significance level of P < .00001. An independent publicly available gene expression profiling cohort of 180 samples26 was used to validate the differentially expressed probe sets between younger and older AML patients (validation step 1). Hereafter another publicly available cohort of 170 samples was used (validation step 2).27 After 2 validation steps, 9 probe sets, representing 7 unique genes, were found to be higher expressed in older AML patients compared with younger AML patients; and 32 probe sets, representing 26 unique genes, were found to be lower expressed in older AML patients compared with younger AML patients. (B) Biologic processes (represented by GO ontologies) enriched among the 477 (up) and 492 (down) differentially expressed probe sets revealed 76 significantly “Up-with-Age” and 95 significantly “Down-with-Age” GO ontologies (P < .01). Next, GO ontologies enriched among the 145 significantly “Up-with-Age” and 257 significantly “Down-with-Age” probe sets were identified (ie, for the genes present after validation step 1). This analysis revealed that 52 “Up-with-Age” and 35 “Down-with-Age” GO ontologies were significantly enriched at the significance level of P < .01 (supplemental Table 2).
Probes and GO ontologies differently expressed between AML samples of young and old patients. (A) The transcriptome of AML samples of the 175 oldest patients was compared with the transcriptome of AML samples of the 175 youngest patients. This comparison revealed 477 probe sets that were higher expressed in the older age group (“Up-with-Age” group) and 492 that were lower expressed in the older age group (“Down-with-Age” group) at the significance level of P < .00001. An independent publicly available gene expression profiling cohort of 180 samples26 was used to validate the differentially expressed probe sets between younger and older AML patients (validation step 1). Hereafter another publicly available cohort of 170 samples was used (validation step 2).27 After 2 validation steps, 9 probe sets, representing 7 unique genes, were found to be higher expressed in older AML patients compared with younger AML patients; and 32 probe sets, representing 26 unique genes, were found to be lower expressed in older AML patients compared with younger AML patients. (B) Biologic processes (represented by GO ontologies) enriched among the 477 (up) and 492 (down) differentially expressed probe sets revealed 76 significantly “Up-with-Age” and 95 significantly “Down-with-Age” GO ontologies (P < .01). Next, GO ontologies enriched among the 145 significantly “Up-with-Age” and 257 significantly “Down-with-Age” probe sets were identified (ie, for the genes present after validation step 1). This analysis revealed that 52 “Up-with-Age” and 35 “Down-with-Age” GO ontologies were significantly enriched at the significance level of P < .01 (supplemental Table 2).
GO categories enriched for “Up-with-Age” and “Down-with-Age” genes
Then biologic processes (represented by GO ontologies) enriched among AML samples of older patients were analyzed. For this analysis, those genes that were significantly up-regulated or down-regulated after the first validation step were used. We have chosen to run the GO analysis on the gene set found to be differentially expressed after the first validation step (instead of using the genes after the second validation step) because identical gene array platforms had been used in these 2 cohorts (ie, Affymetrix U133A 2.0 GeneChips) and because the number of genes was large enough (145 probes up and and 257 probes down) for further GO analysis. These 145 (up) and 257 (down) differentially expressed probe sets consisted of 52 significantly “Up-with-Age” and 35 significantly “Down-with-Age” GO ontologies at the significance level of P less than .01 (supplemental Table 2). This analysis revealed that genes involved in biologic processes as regulation of I-κB kinase nuclear factor-κB cascade and immune response were up-regulated with increasing age. Biologic processes as establishment or maintenance of chromatin architecture, regulation of cyclin-dependent protein kinase activity, and cell-matrix and cell-substrate adhesion were found to be down-regulated with increasing age (Figure 1B).
Age-related or leukemia-specific differences?
The final list of validated genes that were differently expressed depending on age in 3 independent AML cohorts yielded several interesting genes, including RUNX1T1, FGFR1, and SOX2 (Figure 1A). Remarkable was the finding that the expression of p16INK4A was down-regulated in all 3 independent AML gene array profiles. This observation is in contrast with observations in various studies, which have demonstrated (in various tissues) a progressively moderate increasing expression of the tumor suppressor gene p16INK4A during physiologic aging.9,10 In the original cohort of 525 AML samples, the expression of p16INK4A declined significantly with increasing age (P < .0001, ρ = −0.202, n = 525; supplemental Figure 3A). Thus, the level of expression of p16INK4A in AML samples during aging was reciprocal to the usual trend of an increased expression at higher age.
p16INK4A expression in CD34+ normal hematopoietic cells versus CD34+ AML cells
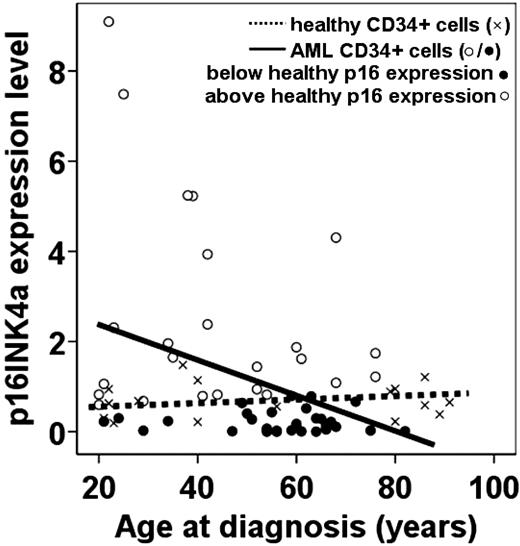
To verify the gene expression profiles, quantitative RT-PCR studies were performed using CD34+ cells derived from healthy bone marrow and AML samples of persons of various ages. These quantitative RT-PCR studies confirmed the positive correlation between increased expression of p16INK4A and physiologic aging in the human hematopoietic system (supplemental Figure 3B, Figure 2). Furthermore, the level of expression of p16INK4A was determined with quantitative RT-PCR on isolated CD34+ cells derived from an independent set of 53 AML samples (supplemental Table 3 shows patient characteristics of this independent AML cohort). Again, also in this AML cohort, p16INK4A was inversely correlated with increasing age (P = .025, ρ = − 0.308, n = 53; Figure 2). Of note, the AML samples of younger patients with high p16INK4A expression do not represent a certain risk group. Subsequently, the expression levels of p16INK4A in CD34+ AML cells were compared with the expression levels of p16INK4A in CD34+ cells of the adult healthy controls (n = 16). Although hampered by low numbers, the linear regression coefficients of the AML samples and the adult healthy controls were borderline significantly different (P = .05), indicating an interaction between the expression of p16INK4A in CD34+ cells in AML samples and healthy controls during aging (Figure 2).
Expression levels of p16INK4A as a function of age in healthy and leukemic CD34+ cells. The expression levels of p16INK4A mRNA of CD34+ cells of adult healthy controls and CD34+ cells of adult AML samples is shown. × indicates healthy adult sorted CD34+ cells; and ○/●, sorted CD34+ AML cells. Spearman rank correlations were calculated between age at diagnosis and p16INK4A mRNA expression levels in AML samples (P = .025, ρ = −0.308, n = 53). The dotted line represents the regression line for the adult healthy sorted CD34+ cells (n = 16); and solid line, the regression line for the sorted CD34+ AML cells. We investigated whether the expression level of p16INK4A mRNA in the sorted CD34+ cells of 53 AML samples was above or below the expected p16INK4A mRNA expression in the sorted CD34+ cells of the adult healthy controls (n = 16). ● represents a p16INK4A expression lower than the age-matched p16INK4A expression in healthy controls; and ○ represents a p16INK4A expression higher than the age-matched p16INK4A expression in healthy controls. The majority of patients with low p16INK4A expression (●) were older than 55 years.
Expression levels of p16INK4A as a function of age in healthy and leukemic CD34+ cells. The expression levels of p16INK4A mRNA of CD34+ cells of adult healthy controls and CD34+ cells of adult AML samples is shown. × indicates healthy adult sorted CD34+ cells; and ○/●, sorted CD34+ AML cells. Spearman rank correlations were calculated between age at diagnosis and p16INK4A mRNA expression levels in AML samples (P = .025, ρ = −0.308, n = 53). The dotted line represents the regression line for the adult healthy sorted CD34+ cells (n = 16); and solid line, the regression line for the sorted CD34+ AML cells. We investigated whether the expression level of p16INK4A mRNA in the sorted CD34+ cells of 53 AML samples was above or below the expected p16INK4A mRNA expression in the sorted CD34+ cells of the adult healthy controls (n = 16). ● represents a p16INK4A expression lower than the age-matched p16INK4A expression in healthy controls; and ○ represents a p16INK4A expression higher than the age-matched p16INK4A expression in healthy controls. The majority of patients with low p16INK4A expression (●) were older than 55 years.
To investigate whether the lower expression of p16INK4A is skewed to the older patients with AML, we marked the expression of all 53 AML samples as below or above expected (based on the p16INK4A expression in CD34+ healthy cells). This revealed that, in younger AML patients, the expression of p16INK4A was not different compared with normal controls (P = .87, Student t test; Figure 2). In contrast, although not significant, older AML patients (> 55 years) tend to express lower p16INK4A compared with healthy age-matched controls (P = .11, Student t test; Figure 2).
We then examined the expression of p16INK4A as a continuous variable in relation to age in the different AML cytogenetic subgroups in the original cohort of 525 AML samples. In the favorable-risk group, comparable p16INK4A expression levels were observed between the AML samples of younger and older patients. In the intermediate- and unfavorable-risk groups, however, the expression of p16INK4A significantly decreased in older patients (Table 2). Similarly, in the molecularly defined subsets “NPM1 wild-type with or without FLT3-ITD” or “NPM1 mutant with FLT3-ITD” (among the intermediate-risk subgroup) it was also shown that p16INK4A expression decreased with increasing age. However, of note, the favorable subgroup “NPM1 mutant without FLT3-ITD,” like the cytogenetic favorable-risk subgroup, did not show a decrease of p16INK4A with increasing age (Table 2).
Correlation between the expression of p16INK4A with age at diagnosis in leukemic subgroups
| AML subgroup . | n . | P . | ρ . |
|---|---|---|---|
| All patients | 525 | < .001 | −0.202 |
| Favorable risk | 89 | .749 | 0.034 |
| Intermediate risk | 331 | < .001 | −0.243 |
| Unfavorable risk | 85 | < .001 | −0.397 |
| NPM1 mutation | 159 | .125 | −0.122 |
| NPM1 wild type | 366 | < .001 | −0.227 |
| FLT-3 with ITD | 143 | < .001 | −0.328 |
| FLT-3 without ITD | 382 | .003 | −0.154 |
| NPM1 mutation with FLT-3 ITD | 82 | .009 | −0.289 |
| NPM1 mutation without FLT-3 ITD | 77 | .529 | 0.073 |
| NPM1 wild type with FLT-3 ITD | 61 | .009 | −0.334 |
| NPM1 wild type without FLT-3 ITD | 305 | < .001 | −0.206 |
| AML subgroup . | n . | P . | ρ . |
|---|---|---|---|
| All patients | 525 | < .001 | −0.202 |
| Favorable risk | 89 | .749 | 0.034 |
| Intermediate risk | 331 | < .001 | −0.243 |
| Unfavorable risk | 85 | < .001 | −0.397 |
| NPM1 mutation | 159 | .125 | −0.122 |
| NPM1 wild type | 366 | < .001 | −0.227 |
| FLT-3 with ITD | 143 | < .001 | −0.328 |
| FLT-3 without ITD | 382 | .003 | −0.154 |
| NPM1 mutation with FLT-3 ITD | 82 | .009 | −0.289 |
| NPM1 mutation without FLT-3 ITD | 77 | .529 | 0.073 |
| NPM1 wild type with FLT-3 ITD | 61 | .009 | −0.334 |
| NPM1 wild type without FLT-3 ITD | 305 | < .001 | −0.206 |
Spearman rank correlation coefficients between the continuous variables age and the averaged p16INK4A probe sets (n = 3) were calculated.
Clinical significance of p16INK4A expression
Because these observations suggest a specific decline of p16INK4A expression in AML samples of older patients, we analyzed the effect of p16INK4A expression on OS in different age cohorts of 525 AML samples. Because no biologic thresholds are available for p16 expression, we used a priori quartiles to analyze the effect of the level of p16 expression in AML samples of younger (ie, 175 youngest) as well as older (ie, 175 oldest) patients. The OS of patients with lower p16INK4A (first, second, and third quartiles) was compared with the OS of patients with the highest p16INK4A expression (fourth quartile), both within the youngest one-third (n = 175) as well as the oldest one-third (n = 175) of patients. No difference in OS was observed between patients with higher versus lower p16INK4A expression in the youngest one-third of patients (Figure 3, P = .57). On the other hand, in the oldest one-third of patients, those patients with lowest expression levels of p16INK4A (ie, first, second, and third quartiles) had a significantly worse OS compared with the older patients with highest expression of p16INK4A (ie, fourth quartile; Figure 3, P = .04). Of note, the number of patients who had received less intensive chemotherapy or an allogeneic HCT was not different in the fourth quartile from that among the other 3 quartiles. The number of patients who received intensive chemotherapy and allogeneic HCT, respectively, were: n = 20 and n = 8 (first quartile), n = 16 and n = 3 (second quartile), n = 16 and n = 6 (third quartile), and n = 21 and n = 6 (fourth quartile). Univariate analysis demonstrated that p16INK4A expression (ie, fourth quartile), allogeneic HCT, and cytogenetic risk group (ie, unfavorable risk) significantly affected OS. In univariate analysis, NPM1 mutation (HR = 1.29; 95% CI, 0.87-1.92; P = .19) and FLT3-ITD (HR = 0.74; 95% CI, 0.50-1.09; P = .14) were not significantly affecting OS in the oldest one-third (n = 175) of patients. Subsequently, the significantly different variables in the univariate analysis (ie, cytogenetic risk group, allogeneic HCT, and p16INK4A expression) were included in a multivariate analysis. This analysis established high p16INK4A expression (ie, fourth quartile) (HR = 1.79; 95% CI, 1.11-2.89; P = .02), unfavorable-risk cytogenetics (compared with intermediate-risk cytogenetics) (HR = 0.33; 95% CI, 0.21-0.51; P < .001) as well as allogeneic HCT (HR = 0.36; 95% CI, 0.19-0.71; P = .003) as prognostic factors for OS. Thus, lower p16INK4A expression in AML samples of patients of older age appears to be an independent prognostic indicator and predicts for reduced OS. Furthermore, p16INK4A expression was analyzed as a continuous variable with age in the various age groups. In the youngest age group (n = 175), no significant correlation between p16INK4A expression and OS existed (ρ of −0.049, P = .522). In the middle age group (n = 175), the correlation between p16INK4A expression and OS was just significant (ρ = 0.151, P = .046); and, interestingly, in the oldest age group (n = 175), the correlation between p16INK4A expression and OS was certainly significant with an estimated ρ of 0.236 (P = .002).
A different effect of the level of p16INK4A expression on OS in young versus old AML patients. (A) Kaplan-Meier analysis depicted for AML samples with higher p16INK4A mRNA expression levels versus AML samples with lower p16INK4A mRNA expression levels within the youngest one-third of patients of the cohort of 525 AMLs (n = 175). No effect of the level of expression of p16INK4A mRNA on OS could be observed (P = .57). (B) Kaplan-Meier analysis depicted for AML samples with high p16INK4A mRNA expression levels versus AML samples with lower p16INK4A mRNA expression levels within the oldest one-third of patients of the cohort of 525 AMLs (n = 175). Patients with high p16INK4A mRNA expression levels (ie, fourth quartile) showed a significantly better OS compared with patients with lower p16INK4A mRNA expression levels (ie, first, second, and third quartiles) in this cohort of 175 oldest AML patients (P = .04).
A different effect of the level of p16INK4A expression on OS in young versus old AML patients. (A) Kaplan-Meier analysis depicted for AML samples with higher p16INK4A mRNA expression levels versus AML samples with lower p16INK4A mRNA expression levels within the youngest one-third of patients of the cohort of 525 AMLs (n = 175). No effect of the level of expression of p16INK4A mRNA on OS could be observed (P = .57). (B) Kaplan-Meier analysis depicted for AML samples with high p16INK4A mRNA expression levels versus AML samples with lower p16INK4A mRNA expression levels within the oldest one-third of patients of the cohort of 525 AMLs (n = 175). Patients with high p16INK4A mRNA expression levels (ie, fourth quartile) showed a significantly better OS compared with patients with lower p16INK4A mRNA expression levels (ie, first, second, and third quartiles) in this cohort of 175 oldest AML patients (P = .04).
Finally, comparable results were found for other clinical endpoints (CR and EFS). In the 175 oldest patients, those with lowest p16INK4A expression levels (ie, first, second, and third quartiles) had a significantly reduced EFS compared with patients with highest p16INK4A expression levels (ie, fourth quartile; P = .03; data not shown). No difference in EFS was observed between patients with higher versus lower p16INK4A expression in the youngest one-third of patients (P = .89). Furthermore, in the 175 oldest patients, those patients who obtained a CR showed a significantly higher p16INK4A expression level compared with patients who did not obtain a CR (P = .046). This difference was not found in the 175 youngest patients (P = .677; data not shown).
Discussion
It is generally accepted, and confirmed in this study, that AML at older age has different clinical and biologic characteristics.12,,,–16,28,–30 To further improve the insight into the molecular mechanisms associated and possibly responsible for the differences between AML in persons of younger and older age, we have analyzed the expression profiles of 54 675 probe sets in 525 AML samples. This analysis revealed distinct gene expression profiles in AML samples of older patients compared with AML samples of younger patients. However, the question is whether these differences in gene expression profiles are caused by age-related alterations or by leukemia-related differences in relation to aging.
Aging is generally considered to be the consequence of stem cell attrition caused by the activity of tumor suppressor pathways (eg, p16INK4A) that censor potentially malignant clones by eliciting apoptosis or senescence.7,8 Indeed, p16INK4A is not only a suppressor of cancer but also a major effector of mammalian aging. The tumor-suppressive activity of p16INK4A is based on its ability to bind and inhibit the cyclin-D–dependent kinases CDK4 and CDK6. These kinases are known to have oncogenic potential and phosphorylate the retinoblastoma family of tumor suppressors, which in turn are main negative regulators of the cell cycle.31 The expression of p16INK4A rises with increasing age and induces an age-dependent decrease in the proliferative capacity of certain tissue-specific stem cells and progenitor cells.9,10 Accordingly, we also observed an increased expression of p16INK4A during aging in human healthy CD34+ hematopoietic cells.
In contrast, the inverse pattern of p16INK4A expression during aging was observed in 4 independent cohorts of AML patients. However, it appeared not to be a general phenomenon in AML samples of older age. Only AML samples belonging to the intermediate- and unfavorable-risk groups demonstrate lower expression of p16INK4A at older compared with younger age. The level of p16INK4A expression was independent of age in AML samples of patients belonging to the favorable-risk group and the molecularly defined subset “NPM1 mutant without FLT3-ITD.” This is consistent with earlier observations indicating that differences in gene expression profiles in AML reflect the abnormal genotypes of the disease22 and the argument that differences in gene expression between AML samples of younger and older age are not merely a reflection of the age of the person. Another argument for this is the fact that none of 3 genes (IRF8, NDRG1, and NEO1) for which expression was recently shown to be age dependent in both human and murine hematopoietic stem/progenitor cells was present in our list of genes significantly associated with aging in AML.32 All 3 genes had at least one representative probe on the Affymetrix U133A 2.0 GeneChip. These findings suggest that differences in gene expression profiles between AML samples of younger and older age are at least partly related to leukemogenesis in relation to the aging process itself (ie, specific for leukemogenesis in older patients).
Nevertheless, some general age-related effects were observed in AML samples. Comparison of 87 GO ontologies associated with aging in AML with a published list of significantly differentially expressed GO ontologies between young and old purified murine long-term HSCs revealed that in both sets the nuclear factor-κB cascade was up-regulated and that maintenance of chromatin architecture, chromatin modification, and organelle organization were down-regulated.6
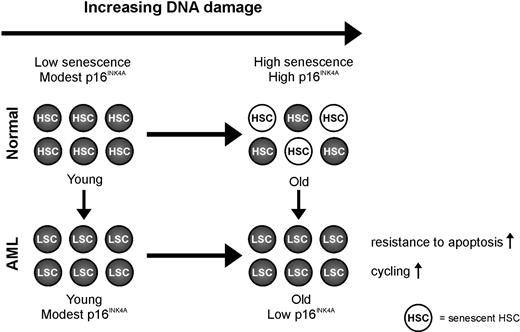
It is intriguing that the cyclin-dependent kinase inhibitor p16INK4A, which has emerged as an important effector in aging and a potent tumor suppressor, is frequently down-regulated in older AML samples. This observation provides an interesting new link between aging and cancer. It suggests that suppression of defense mechanisms, which protect older stem cells or progenitor cells against cellular and DNA damage, facilitate leukemogenesis in older stem cells or progenitor cells (Figure 4). In line with this role of p16INK4A is the fact that the level of p16INK4A expression is an independent predictor for OS in older but not in younger patients.
Model of p16INK4A in aging in AML. The expression of p16INK4A mRNA increases with advancing age to ensure that potentially dangerous lesions, resulting from accumulated DNA damage, do not lead to malignancy. The increased expression of p16INK4A mRNA has the potential to negatively modulate stem cell function through the induction of apoptosis or senescence. This concept, a decline in regenerative potential of tissues caused by a decline in functional stem cells mediated by stress-induced senescence, is a leading dogma in aging. Our data illustrate the importance of this p16INK4A-dependent mechanism because AML samples of older patients have a lower instead of higher expression of p16INK4A mRNA compared with AML samples of younger patients and compared with CD34+ cells of healthy controls. So we hypothesize that suppression of defense mechanisms, which protect older stem cells against accumulated cellular and DNA damage, facilitates the development of AML in older persons.
Model of p16INK4A in aging in AML. The expression of p16INK4A mRNA increases with advancing age to ensure that potentially dangerous lesions, resulting from accumulated DNA damage, do not lead to malignancy. The increased expression of p16INK4A mRNA has the potential to negatively modulate stem cell function through the induction of apoptosis or senescence. This concept, a decline in regenerative potential of tissues caused by a decline in functional stem cells mediated by stress-induced senescence, is a leading dogma in aging. Our data illustrate the importance of this p16INK4A-dependent mechanism because AML samples of older patients have a lower instead of higher expression of p16INK4A mRNA compared with AML samples of younger patients and compared with CD34+ cells of healthy controls. So we hypothesize that suppression of defense mechanisms, which protect older stem cells against accumulated cellular and DNA damage, facilitates the development of AML in older persons.
The regulation of p16INK4A is complex and only incompletely understood.33 Three transcriptional regulators, namely, the positive effectors ETS1 and JUNB, and the member of the polycomb family of repressors BMI1, have received particular attention.9,34,–36 Mice lacking Bmi1 displayed impaired proliferative potential of normal and leukemic stem cells.37,38 In these mice, loss of hematopoietic function was correlated with an increase in p16INK4A expression. Further, gain-of-function studies with BMI1 demonstrate enhanced self-renewal of murine and human HSCs in conjunction with reduced p16INK4A expression.39,–41
The cause of the reduced expression of p16INK4A in AML samples of older patients has not been resolved. The main genetic alterations involving p16 are deletions (bi- or monoallelic) or 5′ CpG island methylation. However, deletions of p16INK4a are uncommon in AML.42 Data on the rate of hypermethylation of the p16INK4a promoter are not consistent and, depending on the method used, vary between 17% and 38%.43,,–46 Nevertheless, the methylation pattern of the p16INK4a promoter was clearly different in leukemic versus normal bone marrow cells.45 Further, a recent histologic study showed that p16INK4A protein expression was only detected in a small number (17%) of AML cases, suggesting that p16INK4A may be the target for inactivation.47 Interestingly, the expression of p16INK4a and BMI1 was significantly inversely correlated in our cohort of 525 AML samples (ρ = −0.237; P < .001). Similar results were obtained in the cohort of 53 AML patients (ρ = −0.298; P = .034), suggesting that BMI1 might play a role in the down-regulation of the expression of p16INK4A on aging.
Remarkable is the observation that the decreased expression of p16INK4a was not observed in older AML patients with favorable-risk cytogenetics. This suggests that, besides the age of the stem/progenitor cells, also concurrent leukemic hits determine the formatting of the AML cells in older patients. This interconnection might dictate that certain sets of genes are activated in AML cells of older patients and not in AML cells of younger patients. Ultimately, this might translate in a reduced susceptibility for chemotherapy agents.
In conclusion, our study suggests that AML presenting at older age shows different gene expression profiles in addition to altered clinical and biologic characteristics. That these differences in gene expression profiles not simply reflect a general aging phenomenon is illustrated by the down-regulation of p16INK4A. The observed down-regulation of p16INK4A suggests that suppression of defense mechanisms, which protect older stem cells against accumulated cellular and DNA damage, facilitates the development of AML in older persons.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.J.M.d.J. collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; E.S.J.M.d.B. and J.J.S. analyzed and interpreted data; P.J.M.V. and R.D. collected, analyzed, and interpreted data; M.K. and C.M.W. performed research; N.J.G.M.V. performed statistical analysis; E.V. and G.H. designed research, analyzed and interpreted data, and wrote the manuscript; and B.L. analyzed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerwin Huls, Department of Hematology, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: g.huls@int.umcg.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal