We appreciate the comments of Burger and Gandhi on our article describing the resistance of chronic lymphocytic leukemia (CLL) cells to apoptosis induced by ABT-737, a specific BCL2 antagonist, after coculture with CD154-expressing fibroblasts in the presence of interleukin-4 (IL-4).1 The major question they raised is which model system may most faithfully reflect the in vivo CLL lymph node (LN) microenvironment. Based on previous studies2-4 and its simplicity, we chose to use the CD154/IL-4 system to mimic some aspects of the LN microenvironment. However, we agree that this model reflects mainly the T-cell–CLL interactions and might, as stated in our article, be an exaggeration of the LN microenvironment. Thus, we speculate that in the LN, only those CLL cells in direct contact with T cells will become dramatically resistant to ABT-737.
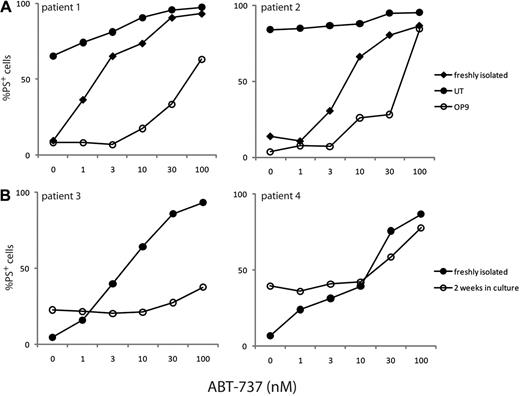
We also recognize that other model systems, including use of nurselike cells or marrow stromal cells, may more faithfully mimic other aspects of the in vivo microenvironment.5,6 It is perhaps noteworthy that immunohistochemical studies on CLL LN proliferation centers showed lower levels of BCL2 protein than in “regular” CLL cells.7 It is unclear which, if any, of these various model systems truly mimics the LN microenvironment in vivo. We have also investigated the sensitivity of CLL cells to ABT-737 after culture on either a marrow stromal cell line (OP-9) or primary autologous nurselike cells (Figure 1). Blood samples were obtained from CLL patients during routine diagnosis at the Leicester Royal Infirmary with patient consent in accordance with the Declaration of Helsinki and approval from the Leicestershire, Northampton, and Rutland ethics committee. Resistance to ABT-737 was observed using both these model systems (Figure 1), although the resistance was much more modest than observed in our previous study.1 Furthermore, unlike the CD154/IL-4 system, significant inter-individual variation was also observed in the degree of resistance observed (Figure 1). The resistance observed after culture on OP-9 cells corresponded with an increase in the up-regulation of antiapoptotic proteins. However, none of these systems is, in our view, an adequate substitute for studies on primary LN-derived and bone marrow CLL cells.
CLL cells cultured on marrow stromal cells or nurselike cells become partially resistant to ABT-737. (A) Bone marrow stromal cells secrete soluble factors and express matrix proteins that have the potential to traffic CLL to the bone marrow and promote their survival. CLL cells, from 2 representative different patients, cultured with the OP-9 bone marrow stromal cell line for 5 days (○) demonstrated a greater resistance to a range of ABT-737 concentrations than either freshly isolated CLL cells (♦) or CLL cells incubated for 5 days in the absence of OP-9 cells (●), although significant inter-individual variation in the degree of resistance was observed. Where the resistance to ABT-737 was greatest, as with cells from patient 1, there was substantial up-regulation of the prosurvival proteins, BCL-XL, BCL2A1, and MCL1, but no or lower expression of the prosurvival proteins in patients with lower resistance to ABT-737 (data not shown). (B) Similarly, nurselike cells, a subset of monocytes derived from the blood of CLL patients, attract CLL cells and render them less sensitive to drug-induced apoptosis. After 14 days in culture, CLL cells from some patients (patient 3) associated with their own nurselike cells and were markedly resistant to ABT-737 (○) compared with freshly isolated CLL cells from the same patient (●). However, significant variation was observed with nurselike cells from different patients (see patient 4).
CLL cells cultured on marrow stromal cells or nurselike cells become partially resistant to ABT-737. (A) Bone marrow stromal cells secrete soluble factors and express matrix proteins that have the potential to traffic CLL to the bone marrow and promote their survival. CLL cells, from 2 representative different patients, cultured with the OP-9 bone marrow stromal cell line for 5 days (○) demonstrated a greater resistance to a range of ABT-737 concentrations than either freshly isolated CLL cells (♦) or CLL cells incubated for 5 days in the absence of OP-9 cells (●), although significant inter-individual variation in the degree of resistance was observed. Where the resistance to ABT-737 was greatest, as with cells from patient 1, there was substantial up-regulation of the prosurvival proteins, BCL-XL, BCL2A1, and MCL1, but no or lower expression of the prosurvival proteins in patients with lower resistance to ABT-737 (data not shown). (B) Similarly, nurselike cells, a subset of monocytes derived from the blood of CLL patients, attract CLL cells and render them less sensitive to drug-induced apoptosis. After 14 days in culture, CLL cells from some patients (patient 3) associated with their own nurselike cells and were markedly resistant to ABT-737 (○) compared with freshly isolated CLL cells from the same patient (●). However, significant variation was observed with nurselike cells from different patients (see patient 4).
Greater understanding of the microenvironment-CLL interactions should enable improved targeted therapy of CLL and circumvent potential resistance problems to novel therapeutic agents, such as ABT-263, the orally active form of ABT-737, that is entering clinical trials in CLL. As ABT-737 and ABT-263 inhibit only BCL2, BCL-XL, and BCL-w, but do not inhibit MCL1 and BCL2A1, it may possibly be preferable to use a pan-antiapoptotic BCL2 family antagonist, although toxicities might be expected to be more severe with such antagonists. Several pan-BCL2 family antagonists have been proposed, including Obatoclax, gossypol, apogossypol, and chelerythrine. However, none of these compounds are BCL2 family–specific, and they appear to kill cells in a nonspecific manner.8-10 As Burger and Gandhi indicate, concurrent interference with microenvironment-CLL interactions by antagonizing interactions of CXCR4-CXCL12 and/or CD40-CD154 or use of inhibitors of BAFF, APRIL, or spleen tyrosine kinase may all be promising avenues to improve therapy of CLL either alone or in combination.1,9,11,12 However, all will require careful preclinical validation under situations that most closely mimic those in vivo to maximize clinical benefit and minimize possible toxicities.
Authorship
Contribution: M.B. and M.V. performed the experiments; and all authors wrote the response.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Professor Gerald M. Cohen, MRC Toxicology Unit, Hodgkin Bldg, PO Box 138, University of Leicester, Lancaster Rd, Leicester, LE1 9HN, United Kingdom; e-mail: gmc2@le.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal