Abstract
Angiostatin was first discovered as a plasminogen fragment with antitumor/antiangiogenic property. One of the angiostatin isoforms, that is, angiostatin 4.5 (AS4.5), consisting of plasminogen kringle 1 to 4 and a most part of kringle 5, is produced by autoproteolysis and present in human plasma. β2-glycoprotein I (β2GPI) is proteolytically cleaved by plasmin in its domain V (nicked β2GPI), resulting in binding to plasminogen. Antiangiogenic properties have been recently reported in nicked β2GPI as well as in intact β2GPI at higher concentrations. In the present study, we found significant binding of nicked β2GPI to AS4.5 (KD = 3.27 × 106 M−1). Via this binding, nicked β2GPI attenuates the antiangiogenic functions of AS4.5 in the proliferation of arterial/venous endothelial cells, in the extracellular matrix invasion and the tube formation of venous endothelial cells, and in vivo angiogenesis. In contrast, intact β2GPI does not bind to AS4.5 or inhibit its antiangiogenic activity. Thus, nicked β2GPI exerts dual effects on angiogenesis, that is, nicked β2GPI promotes angiogenesis in the presence of AS4.5, whereas nicked β2GPI inhibits angiogenesis at concentrations high enough to neutralize AS4.5. Our data suggest that plasmin-nicked β2GPI promotes angiogenesis by interacting with plasmin-generated AS4.5 in sites of increased fibrinolysis such as thrombus.
Introduction
Angiogenesis is the formation of a new capillary network from preexisting vessels and is essential in many physiologic and pathologic states, such as reproduction, development, wound healing, tumorigenesis, rheumatoid arthritis, diabetic retinopathy, and thrombosis.1,2 Angiogenesis is tightly controlled by activators, such as fibroblast growth factors (FGFs) and vascular endothelial growth factor (VEGF), and by inhibitors, such as thrombospondin-1, interferon-α/β, platelet factor-4, and angiostatin.3 Angiostatin was discovered in urine from mice with low-metastatic Lewis lung carcinoma as a kringle-containing fragment of plasminogen.4 This first-reported angiostatin consists of 4 kringle domains (K1-K4) and possesses antitumor/antiangiogenic properties. Later, K1 to K3 was revealed to be a more potent inhibitor of angiogenesis.5 Although several isoforms have been reported, angiostatin 4.5 (AS4.5, also referred to as K1-K5) is the only naturally occurring isoform identified in human plasma that consists of kringles 1 to 4 and approximately 85% of kringle 5.6-8 Plasma concentrations of in vivo–generated AS4.5 were measured in cancer patients who received tissue plasminogen activator and mesna in a clinical trial.9 In this study, generation of 2 isoforms of AS4.5 in human plasma was observed, namely, Lys-AS4.5 and Glu-AS4.5. Whereas Lys-AS4.5 is the originally reported natural AS4.5, Glu-AS4.5 is a larger form that obtains intact N-terminal domain of the precursor protein. Approximately 20 nM Lys-AS4.5 was detected even in the patients before treatment. After infusion of tissue plasminogen activator with mesna, Lys-AS4.5 levels increased to approximately 40 nM.
β2-glycoprotein-I (β2GPI), also known as apolipoprotein H, is a phospholipid-binding plasma protein that is one of the major autoantigens in patients with antiphospholipid syndrome, an autoimmune disorder characterized by thrombosis and pregnancy morbidity.10-13 β2GPI is a single chain plasma glycoprotein at a concentration of approximately 4 μM composed of 5 homologous short consensus repeats, designated as domains I to V. β2GPI is cleaved by plasmin between Lys-317 and Thr-318 in domain V (nicked β2GPI), being unable to bind phospholipids.14 This cleavage was first observed in vivo by Horbach et al15 in plasma of patients with disseminated intravascular coagulation and in plasma with patients treated with streptokinase. In these cases, up to 12 μg/mL nicked β2GPI (∼ 6% of intact β2GPI) was present. In other pathologic contexts, approximately 0.1% and approximately 1.5% of intact β2GPI are cleaved to nicked β2GPI in patients with leukemia and in patients with lupus anticoagulant, respectively.16 In addition, in our previous study in which patients with history of stroke were investigated, up to 0.5%, mostly from 0.1% to 0.2% of intact β2GPI, was converted to nicked β2GPI in the stable state of their disease.17 Instead of losing phospholipid-binding properties, nicked β2GPI gains the binding capacity to plasminogen and mildly suppresses the plasmin generation in the presence of tissue plasminogen activator and fibrin.17 Because this binding is mediated by the interaction between lysine binding site on K5 of plasminogen and the lysine cluster on domain V of nicked β2GPI, we speculated on the interaction between nicked β2GPI and AS4.5, which still possesses the most part of kringle 5.
In the present study, we show that nicked β2GPI does bind AS4.5 and attenuates its antiangiogenic property in an endothelial cell proliferation assay, in an invasion assay, in a tube-formation assay, and in an in vivo angiogenesis assay.
Methods
Proteins
β2GPI and nicked β2GPI were prepared as previously reported.17 AS4.5 (K1-K5) was prepared from Glu-plasminogen (Technoclone GmbH) treated with plasmin (Calbiochem Novabiochem Corp), followed by purification steps using a lysine-Sepharose column and a Sephadex G-75 column (GE Healthcare), as previously reported.8 Purified AS4.5 was reduced using 2-mercaptoethanol and subjected to polyacrylamide gel electrophoresis (PAGE). In some experiments, AS4.5 was treated with PNGase F or Endo H (New England Biolabs Inc) to determine whether our preparation of AS4.5 undergoes glycosylation. Purified materials were tested to exclude the possibility of lipopolysaccharide contamination using Limulus ES II Single Test (Wako).
Cells
Human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs) were obtained from Kurabo. These cells were cultured in the moisturized chamber at 37°C with 5% CO2, using provided cell-culture medium “EGM2” which include 2% fetal calf serum (FCS), human epidermal growth factor (10 ng/mL), human FGF-B (5 ng/mL), heparin (10 μg/mL), hydrocortisone (1 μg/mL), amphotericin B (50 ng/mL), and gentamicin (50 μg/mL). HAECs/HUVECs from 2 to 6 passages were used in the following experiments.
Kinetic assay for molecular interaction between nicked β2GPI and AS4.5
Real-time analysis for molecular interaction between intact/nicked β2GPI and AS4.5 was performed using an optical biosensor, BIACORE X (Biacore AB). Biotinylated AS4.5 was immobilized onto the streptavidin-coupled sensor chip (Biacore AB). After blocking, various concentrations (0.125, 0.25, 0.5, 1.0, and 2.0 μM) of intact or nicked β2GPI were injected and ligands bound to the surface were detected. Obtained data were used to determine the association rate constant (kass) and dissociation rate constant (kdiss). KD and KA were determined as follows: KD = kdiss/kass and KA = kass/kdiss.
Inhibition ELISA
To see the fluid-phase binding between AS4.5 and nicked β2GPI, enzyme-linked immunosorbent assay (ELISA) was performed in a similar way that we previously reported.17 Briefly, Glu-plasminogen was immobilized onto a Sumilon Type S microtiter plate (Sumitomo Bakelite). After blocking, 0.25 μM nicked β2GPI with or without AS4.5 (0.4, 0.8, or 1.6 μM) dissolved in 1% bovine serum albumin–phosphate-buffered saline (PBS) was added to the wells and plasminogen-bound nicked β2GPI molecules were detected by Cof-22 mouse monoclonal anti-β2GPI antibody.
Cell proliferation assay
To see the effect of nicked β2GPI on the proliferation of aortic endothelial cells in the presence of AS4.5, which is a potent inhibitor of endothelial cell growth, we used tetrazolium/formazan assay. After wash with PBS, 5000 HAECs in 50 μL Opti-MEM I medium (Invitrogen) were added to each wells of Celltiter 96 proliferation Assay Kit (Promega). Different concentrations of intact/nicked β2GPI with or without approximately 50 nM AS4.5 were added to the medium. After a 72-hour incubation at 37°C, 100 μL Dye Solution was added to each well and incubated for another 4 hours at 37°C. Reaction was terminated using Stop Solution and optical density at 570 nm was measured. Cell proliferation assays were also performed using HUVECs in the same manner with or without 2.5 ng/mL human recombinant VEGF (Kurabo). These assays were done in a triplicate manner for 3 times. Because the effects of intact/nicked β2GPI on HAEC proliferation and HUVEC proliferation were similar regardless of the presence or absence of AS4.5, we performed the following in vitro angiogenesis experiments using HUVECs.
Matrigel cell-invasion assay
Biocoat invasion chambers containing Matrigel-coated membranes with 8-μm pores (BD Biosciences) were used to evaluate the effect of intact/nicked β2GPI on HUVECs to migrate through a basement membrane-like extracellular matrix, in the presence or absence of AS4.5. This assay was done as previously reported, with some alterations.18 Briefly, after removal of FCS and growth factors by washing with PBS, 5 × 104 of HUVECs in 0.5 mL Opti-MEM I medium were placed in the top chamber of each well of a 24-well culture dish. Different concentrations of intact or nicked β2GPI were applied to the top chamber in the presence or absence of AS4.5 (final concentration, ∼ 50 nM). Opti-MEM I medium containing 10% FCS was added to the lower chamber of each well as chemo-attractant. After an 8-hour incubation at 37°C, the noninvading cells were removed from the top chamber and the cells that extravasated through the extracellular matrix were stained with Diff-Quick (Kokusai Shiyaku). The number of cells that migrated through the membrane's 8-μm pores were counted under Olympus IX71 inverted microscope (Olympus) equipped with a 10×/0.30 ph1 objective at a final magnification of 100×, using WinROOF image processing software (Mitani Corp). Each assay was performed in a triplicate manner 4 times.
Capillary tube formation of HUVECs cocultured with fibroblasts
The capillary tube formation of HUVECs was evaluated using an angiogenesis kit (Kurabo), according to the manufacturer's instructions. In vitro HUVEC cord formation can be observed when cocultured with human primary fibroblasts, being suitable for quantification of cumulative sprout length.19 Evaluation of HUVEC tube formation using this coculture system is reproducible using in vitro angiogenesis kits from Kurabo or TCScell-works.20 The culture medium in each well of a 24-well cluster dish seeded with HUVECs and human skin fibroblasts was replaced by the fresh medium containing 2.5 ng/mL human recombinant VEGF at days 1, 4, 7, and 9. We reduced the concentration of VEGF to maximize the antiangiogenic effect of AS4.5, although the recommended concentration of VEGF for the positive growth control was 10 ng/mL. At day 11, the capillary tubes formed were detected by immunostaining using anti–human CD31 antibody supplied by the manufacturer. For scoring the capillary tube formation, tube length was measured quantitatively using an Olympus IX71 inverted microscope equipped with a 4×/0.13 PhL objective and angiogenesis measuring software (KURABO Angiogenesis Image Analyzer, Version 2; Kurabo).21 This experiment was done in a duplicate manner 3 times.
Directed in vivo angiogenesis assay
The directed in vivo angiogenesis assay was obtained from Trevigen Inc and performed as previously described,22,23 with modifications. Briefly, sterile 0.15-cm × 1-cm-long semiclosed surgical silicone tubing (angioreactors) was filled with 18-μL high-concentration basement membrane extract, including VEGF and FGF (Trevigen Inc). Various concentrations of nicked β2GPI (0-0.4 μM) were added to angioreactors containing AS4.5. The angioreactors were then inverted and incubated at 37°C for 1 hour to allow gel formation. The angioreactors were then implanted subcutaneously into the dorsal flank of 6- to 8-week-old athymic nude female mice. Blood vessels generated in the angioreactors were quantified by staining of the recovered cell pellets with fluorescein isothiocyanate-lectin on day 10. This experiment was performed in a triplicate manner.
Statistical analysis
Statistical evaluation was performed by Student t test. P values less than .05 were considered statistically significant.
Results
Purification of nicked β2GPI and AS4.5
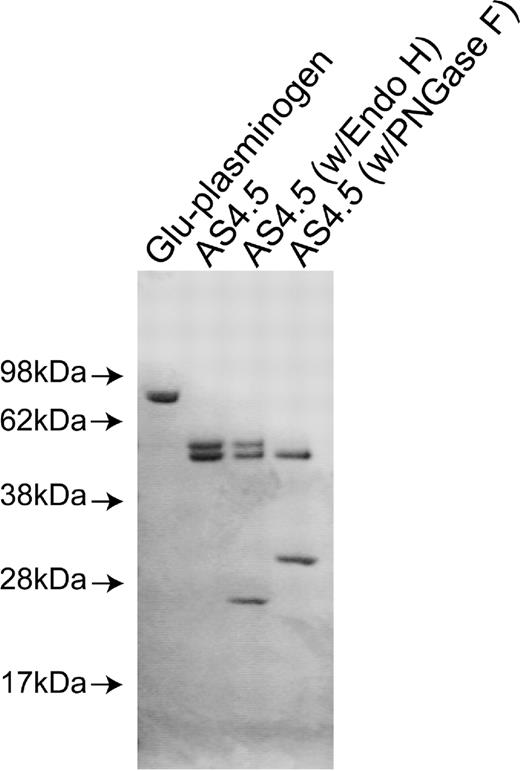
Purified nicked β2GPI and intact β2GPI appeared as a single band with appropriate size under sodium dodecyl sulfate-PAGE with reduced conditions. Purified AS4.5 showed clear main band at expected size (52-55 kDa) with slightly larger minor band (Figure 1A). Treatment of purified AS4.5 with PNGase F resulted in the reduction of molecular weight to that of the main band, whereas treatment with Endo H had scarce effect on the size of AS4.5 (Figure 1B). According to the susceptibility to PNGase F and resistance to Endo H, at least some portion of purified AS4.5 is suggested to undergo glycosylation with complex oligosaccharides. Plasminogen undergoes glycosylation at Asn-289 and Thr-346, and additional site Leu-532, such glycosylation, can alter interaction between plasmin kringle domains and integrin αVβ3 expressed on endothelial cells.24
Preparation of nicked β2GPI and AS4.5. AS4.5 was prepared from Glu-plasminogen by plasmin digestion followed by purification using lysine-Sepharose column and Sephadex G-75 column. Purified AS4.5 was treated with PNGase F or Endo H to determine whether AS4.5 undergoes glycosylation. Glu-plasminogen, purified product (AS4.5), AS4.5 treated with PNGase F, and AS4.5 treated with Endo H were subjected to sodium dodecyl sulfate-PAGE under reduced conditions.
Preparation of nicked β2GPI and AS4.5. AS4.5 was prepared from Glu-plasminogen by plasmin digestion followed by purification using lysine-Sepharose column and Sephadex G-75 column. Purified AS4.5 was treated with PNGase F or Endo H to determine whether AS4.5 undergoes glycosylation. Glu-plasminogen, purified product (AS4.5), AS4.5 treated with PNGase F, and AS4.5 treated with Endo H were subjected to sodium dodecyl sulfate-PAGE under reduced conditions.
Binding of nicked β2GPI to AS4.5
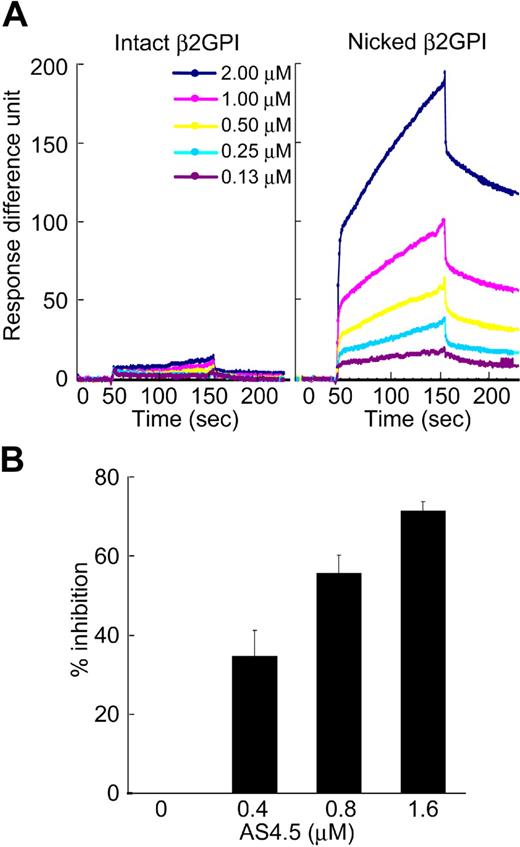
Molecular interaction between intact or nicked β2GPI and AS4.5 was analyzed using an optical biosensor. Nicked β2GPI showed a large extent of binding to immobilized AS4.5, whereas intact β2GPI did not show any specific binding (Figure 2A). The data at different concentrations of nicked β2GPI were regressed, determining kass as 2.99 × 103 M−1s−1, and kdiss as 9.14 × 10−4s−1. Accordingly, KD and KA were determined as 3.05 × 10−7 M and 3.27 × 106 M−1, respectively. To confirm this binding in the fluid phase, we performed inhibition ELISA. In this system, AS4.5 inhibited the binding of nicked β2GPI to immobilized Glu-plasminogen, even in the presence of excess amount of albumin (Figure 2B).
Binding of intact/nicked β2GPI to AS4.5. (A) Kinetic curves showing molecular interaction between AS4.5 and intact or nicked β2GPI. Intact β2GPI or nicked β2GPI binding to immobilized AS4.5 was detected using Biacore X, an optical biosensor as described in “Kinetic assay for molecular interaction between nicked B2GPI and AS4.5.” Binding curve was compared between intact (left panel) and nicked β2GPI (right panel). Binding constants (KD and KA) between AS4.5 and nicked β2GPI were determined. (B) Binding of nicked β2GPI to immobilized Glu-plasminogen was tested in the presence or absence of AS4.5 in the fluid, using ELISA. The abilities of AS4.5 to inhibit the binding between fluid-phase nicked β2GPI and solid-phase Glu-plasminogen were shown as percentage inhibition. Error bars represent SE.
Binding of intact/nicked β2GPI to AS4.5. (A) Kinetic curves showing molecular interaction between AS4.5 and intact or nicked β2GPI. Intact β2GPI or nicked β2GPI binding to immobilized AS4.5 was detected using Biacore X, an optical biosensor as described in “Kinetic assay for molecular interaction between nicked B2GPI and AS4.5.” Binding curve was compared between intact (left panel) and nicked β2GPI (right panel). Binding constants (KD and KA) between AS4.5 and nicked β2GPI were determined. (B) Binding of nicked β2GPI to immobilized Glu-plasminogen was tested in the presence or absence of AS4.5 in the fluid, using ELISA. The abilities of AS4.5 to inhibit the binding between fluid-phase nicked β2GPI and solid-phase Glu-plasminogen were shown as percentage inhibition. Error bars represent SE.
Effect of intact/nicked β2GPI on the proliferation of HAECs/HUVECs in the presence or absence of AS4.5
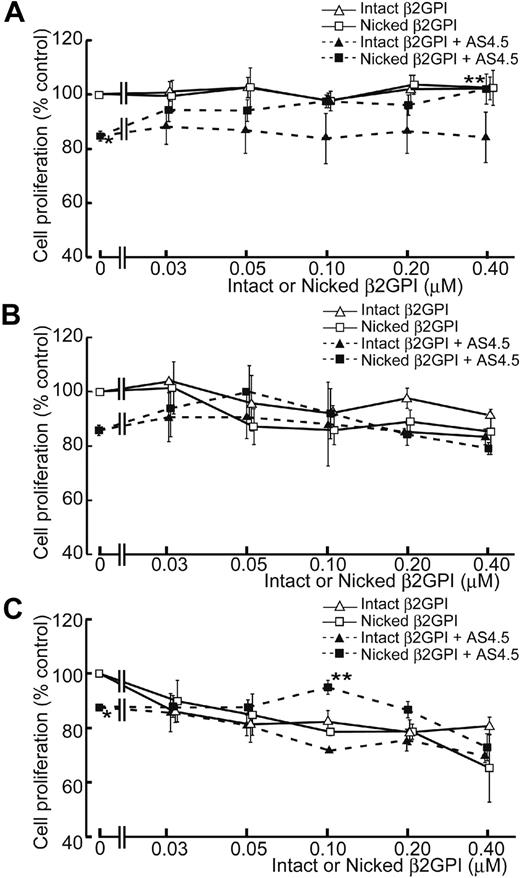
AS4.5 exhibited suppressive effect on the proliferation of HAECs to approximately 15% inhibition at the concentration of 50 nM, compared with the HAEC proliferation in the absence of AS4.5 (Figure 3A). In this growth factor-removed system, intact β2GPI up to the final concentration of 0.4 μM did not have any effect on the proliferation of HAECs both in the presence and absence of AS4.5. In contrast, nicked β2GPI reversed the suppressive effect on HAEC proliferation by AS4.5 (P = .021 at 0.4 μM of nicked β2GPI**, compared with the point without nicked β2GPI*). However, this form of β2GPI again had no effect on proliferation in the absence of AS4.5 at concentrations up to 0.4 μM. When HUVEC proliferation was examined in the same system, intact/nicked β2GPI exerted similar effect both in the presence and in the absence of AS4.5 (Figure 3B). When VEGF was added to this proliferation system, HUVEC proliferation was suppressed by lower concentrations of intact/nicked β2GPI in the absence of AS4.5 (Figure 3C). Suppressive effect of 50 nM AS4.5 on HUVEC proliferation was neutralized by approximately 0.1 μM nicked β2GPI. Higher concentrations (1.0-4.0 μM) of intact/nicked β2GPI suppressed HAEC/HUVEC proliferation regardless of the existence of VEGF/AS4.5 (data not shown).
Effect of intact/nicked β2GPI on the proliferation of HAECs in the presence or absence of AS4.5. (A) HAECs were subjected to cell-proliferation assay using tetrazolium/formazan-based method. A total of 5000 HAECs were placed onto each wells of 96-well plate and incubated for 72 hours. The effect of serial concentrations of intact or nicked β2GPI was tested in the presence or absence of 50 nM AS4.5. HAEC proliferation in the presence of AS4.5 alone (50 nM)* was compared with that in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.4 μM; **P = .021; Student t test). (B) Proliferations of HUVECs were tested using the same proliferation assay. (C) HUVEC proliferation was tested in the presence of VEGF. HUVEC proliferation in the presence of AS4.5 alone (50 nM)* was compared with that in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.1 μM; **P = .030; Student t test). Error bars represent SE.
Effect of intact/nicked β2GPI on the proliferation of HAECs in the presence or absence of AS4.5. (A) HAECs were subjected to cell-proliferation assay using tetrazolium/formazan-based method. A total of 5000 HAECs were placed onto each wells of 96-well plate and incubated for 72 hours. The effect of serial concentrations of intact or nicked β2GPI was tested in the presence or absence of 50 nM AS4.5. HAEC proliferation in the presence of AS4.5 alone (50 nM)* was compared with that in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.4 μM; **P = .021; Student t test). (B) Proliferations of HUVECs were tested using the same proliferation assay. (C) HUVEC proliferation was tested in the presence of VEGF. HUVEC proliferation in the presence of AS4.5 alone (50 nM)* was compared with that in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.1 μM; **P = .030; Student t test). Error bars represent SE.
Effect of intact/nicked β2GPI on the invasion of HUVECs in the presence or absence of AS4.5
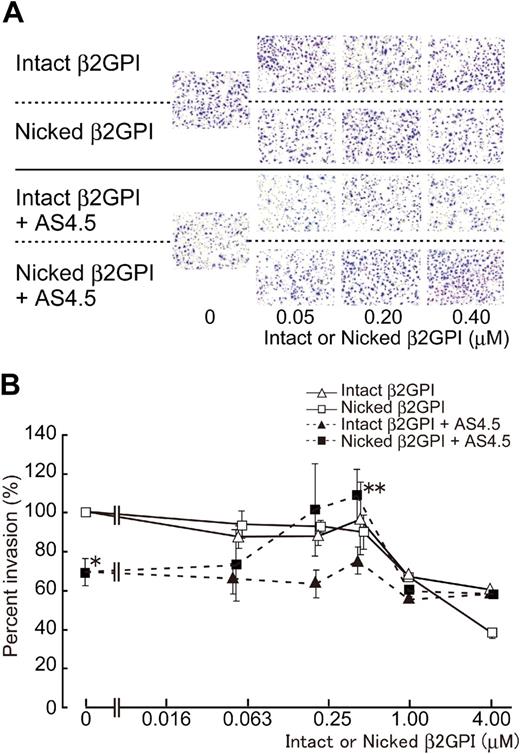
Without AS4.5, intact or nicked β2GPI had no effect on the migration of HUVECs in concentrations up to 0.4 μM (Figure 4), whereas both forms of β2GPI significantly suppressed HUVEC migration in higher concentrations (from 1 to 4 μM) with dose dependency (∼ 40% and 60% inhibition by 4 μM of intact and nicked β2GPI, respectively). Migration of HUVECs was down-regulated by 50 nM of AS4.5 to approximately 30% inhibition. Nicked β2GPI reversed this suppressive effect of AS4.5 at lower concentrations from 0.2 to 0.4 μM (P = .027 at 0.4 μM of nicked β2GPI**, compared with the point without nicked β2GPI*), although increment of concentration diminished this reverse effect and, in turn, resulted in inhibition of HUVEC migration (∼ 40% inhibition by 4 μM nicked β2GPI). Thus, depending on the concentration, nicked β2GPI shows dual effect on the mobility of HUVECs in the presence of AS4.5. Intact β2GPI had no additional or reverse effect on HUVEC migration in the presence of AS4.5 at concentrations up to 0.4 μM.
Effect of intact/nicked β2GPI on extravasation of HUVECs using a Matrigel-cell invasion assay in the presence or absence of AS4.5. Effect of intact or nicked β2GPI on the ability of HUVECs to migrate through a basement membrane-like extracellular matrix was evaluated in the presence or absence of AS4.5. HUVECs were added to the top wells of each chamber, and 10% FCS-enriched culture medium was added to each bottom chamber as a source of chemotactic factors. (A) In the top 2 lines of the panels, assays were done without AS4.5. AS4.5 was added to the wells in the bottom 2 lines of the panels. Serial concentrations of intact β2GPI were added in lines 1 and 3, whereas nicked β2GPI was added in lines 2 and 4. Similar results were obtained in other 3 experiments (original magnification, ×100). (B) HUVECs migrated through the Matrigel, and 8-μM pores on the membrane were stained and counted using image processing software. Ratios of the numbers of the HUVECs migrated under treatment with reagents against the number of those cells without any additional reagents were plotted on the graph. Concentrations of intact/nicked β2GPI were as follows: 0.05, 0.2, 0.4, 1.0, or 4.0 μM. Error bars represent SE. Invaded cell counts in the presence of AS4.5 alone (50 nM)* were compared with those in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.4 μM; **P = .027; Student t test).
Effect of intact/nicked β2GPI on extravasation of HUVECs using a Matrigel-cell invasion assay in the presence or absence of AS4.5. Effect of intact or nicked β2GPI on the ability of HUVECs to migrate through a basement membrane-like extracellular matrix was evaluated in the presence or absence of AS4.5. HUVECs were added to the top wells of each chamber, and 10% FCS-enriched culture medium was added to each bottom chamber as a source of chemotactic factors. (A) In the top 2 lines of the panels, assays were done without AS4.5. AS4.5 was added to the wells in the bottom 2 lines of the panels. Serial concentrations of intact β2GPI were added in lines 1 and 3, whereas nicked β2GPI was added in lines 2 and 4. Similar results were obtained in other 3 experiments (original magnification, ×100). (B) HUVECs migrated through the Matrigel, and 8-μM pores on the membrane were stained and counted using image processing software. Ratios of the numbers of the HUVECs migrated under treatment with reagents against the number of those cells without any additional reagents were plotted on the graph. Concentrations of intact/nicked β2GPI were as follows: 0.05, 0.2, 0.4, 1.0, or 4.0 μM. Error bars represent SE. Invaded cell counts in the presence of AS4.5 alone (50 nM)* were compared with those in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.4 μM; **P = .027; Student t test).
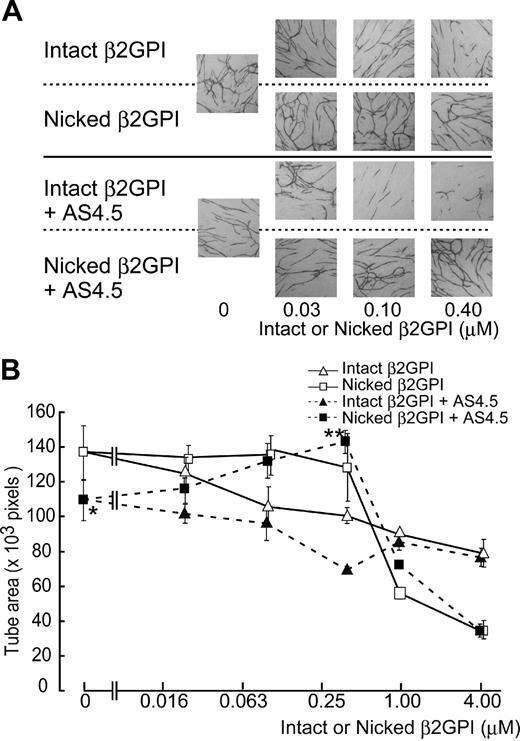
Effects of nicked β2GPI on angiogenesis (in vitro tube formation assay)
Formation of capillary-like structure by HUVECs was evaluated in the VEGF-dependent tube formation assay system. Fifty nanomolar of AS4.5 suppressed the function of HUVECs in this assay (Figure 5). Capillary tube formation was disrupted by intact β2GPI in a dose-dependent manner, in the presence or absence of AS4.5. Nicked β2GPI again reversed the inhibitory effect of AS4.5 on tube formation by HUVECs in a dose-dependent manner at concentrations up to 0.4 μM (P = .044 at 0.4 μM nicked β2GPI**, compared with the point without nicked β2GPI*). In the absence of AS4.5, nicked β2GPI had no significant effect on tube formation at concentrations less than 0.4 μM. In this assay, higher concentrations of intact/nicked β2GPI suppressed tube formation, the latter being more potent (4 μM of nicked β2GPI suppressed tube area to ∼ 40 × 103 pixels).
Effect of intact/nicked β2GPI on the VEGF-dependent tube formation of HUVECs cocultured with fibroblasts in the presence or in the absence of AS4.5. VEGF-dependent tube formation of HUVECs cocultured with primary human fibroblasts was evaluated in the presence or absence of AS4.5. (A) HUVECs were visualized by immunostaining with anti–human CD31 antibodies. In the top 2 lines of the panels, the assay was done without AS4.5. AS4.5 was added in the bottom 2 lines of the panels. Serial concentrations of intact β2GPI were added in lines 1 and 3, whereas nicked β2GPI was added in lines 2 and 4. Similar results were obtained in the second and third experiments (original magnification, ×40). (B) Capillary tube formation was quantified using KURABO Angiogenesis Image Analyzer, Version 2. Obtained data (pixels) were plotted onto the graph. Concentrations of intact/nicked β2GPI were as follows: 0.025, 0.1, 0.4, 1.0, or 4.0 μM. Error bars represent SE. Tube areas in the presence of AS4.5 alone (50 nM)* were compared with those in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.4 μM; **P = .044; Student t test).
Effect of intact/nicked β2GPI on the VEGF-dependent tube formation of HUVECs cocultured with fibroblasts in the presence or in the absence of AS4.5. VEGF-dependent tube formation of HUVECs cocultured with primary human fibroblasts was evaluated in the presence or absence of AS4.5. (A) HUVECs were visualized by immunostaining with anti–human CD31 antibodies. In the top 2 lines of the panels, the assay was done without AS4.5. AS4.5 was added in the bottom 2 lines of the panels. Serial concentrations of intact β2GPI were added in lines 1 and 3, whereas nicked β2GPI was added in lines 2 and 4. Similar results were obtained in the second and third experiments (original magnification, ×40). (B) Capillary tube formation was quantified using KURABO Angiogenesis Image Analyzer, Version 2. Obtained data (pixels) were plotted onto the graph. Concentrations of intact/nicked β2GPI were as follows: 0.025, 0.1, 0.4, 1.0, or 4.0 μM. Error bars represent SE. Tube areas in the presence of AS4.5 alone (50 nM)* were compared with those in the presence of both AS4.5 (50 nM) and nicked β2GPI (0.4 μM; **P = .044; Student t test).
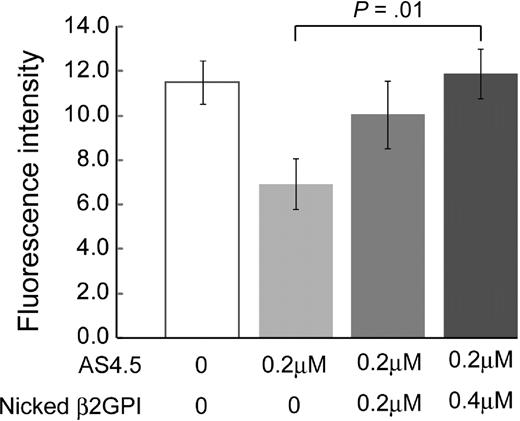
Effects of nicked β2GPI on in vivo angiogenesis
Generation of blood vessels into angioreactors was suppressed by 0.2 μM AS4.5 (Figure 6). Addition of nicked β2GPI to this system up to 0.4 μM significantly recovered angiogenesis in a dose-dependent manner (P = .01).
Nicked β2GPI suppresses the antiangiogenesis effect of AS4.5 in in vivo angiogenesis assay. Directed in vivo angiogenesis assay was performed. Semiclosed surgical silicone tubings (angioreactors) were prefilled with extracellular matrices containing VEGF and FGF alone, VEGF, FGF, and AS4.5, or VEGF, FGF, AS4.5, plus various concentrations of nicked β2GPI, then implanted subcutaneously into the dorsal flank of athymic nude mice. Blood vessels generated in the angioreactors were quantified by staining of the recovered cell pellets with fluorescein isothiocyanate-lectin. Error bars represent SE. *Fluorescence values in the presence of AS4.5 (0.2 μM) alone were compared with those in the presence of both AS4.5 and nicked β2GPI (0.4 μM) by Student t test (P = .01).
Nicked β2GPI suppresses the antiangiogenesis effect of AS4.5 in in vivo angiogenesis assay. Directed in vivo angiogenesis assay was performed. Semiclosed surgical silicone tubings (angioreactors) were prefilled with extracellular matrices containing VEGF and FGF alone, VEGF, FGF, and AS4.5, or VEGF, FGF, AS4.5, plus various concentrations of nicked β2GPI, then implanted subcutaneously into the dorsal flank of athymic nude mice. Blood vessels generated in the angioreactors were quantified by staining of the recovered cell pellets with fluorescein isothiocyanate-lectin. Error bars represent SE. *Fluorescence values in the presence of AS4.5 (0.2 μM) alone were compared with those in the presence of both AS4.5 and nicked β2GPI (0.4 μM) by Student t test (P = .01).
Discussion
In the first part of this study, we demonstrated that nicked β2GPI binds AS4.5 with similar kinetics found in the interaction between nicked β2GPI and plasminogen.17 Whereas intact β2GPI does not show any binding to plasminogen, the binding between nicked β2GPI and plasminogen was mediated via interaction between the lysine cluster of β2GPI domain V and the lysine binding site on the plasminogen K5.17 In addition, in the present study, intact β2GPI did not show any specific binding to AS4.5. This phenomenon indicates that 85% of K5 still functions enough for the binding with nicked β2GPI or that nicked β2GPI gains accessibility to other kringle domain(s) even when 15% of K5 is lost, the latter being less probable because neither K1 to K3 nor K4 disrupted the binding between nicked β2GPI and plasminogen in our previous inhibition assay.17
Next, we investigated the functional aspect of the interaction between nicked β2GPI and AS4.5 on the vascular endothelial cell biology. The antiangiogenic function of angiostatin is mediated by its binding onto the endothelial cell surface. Angiostatin inhibits adenosine triphosphate synthase F1F0 by direct binding to the extracellular portion of this enzyme, resulting in caspase-mediated apoptosis of endothelial cells.25,26 Angiostatin also binds other endothelial cell surface molecules, such as integrin αVβ3 and angiomotin, although the latter binding was shown using original angiostatin K1 to K4.27 Thus, it is speculated that nicked β2GPI interferes with the binding of AS4.5 onto the endothelial cells, resulting in attenuation of the antiangiogenic function of AS4.5. Indeed, 25 nM nicked β2GPI starts to compete with 50 nM AS4.5 and 0.1 to 0.2 μM nicked β2GPI completely abolished the antiangiogenic effect of the given AS4.5 in our HAEC proliferation assay (Figure 3A). In the HUVEC proliferation assay, 50 nM nicked β2GPI neutralized the same molar of AS4.5 (Figure 3B). In the HUVEC invasion assay and tube-formation assay, 0.1 to 0.2 μM nicked β2GPI abolished antiangiogenic properties of 50 nM AS4.5 (Figures 4,5). Based on the previous studies,9,15,16 plasma concentrations of both nicked β2GPI and AS4.5 used in this study are physiologically available at least in thrombotic status. Moreover, it is predicted that local concentrations of nicked β2GPI and AS4.5 increase at sites of thrombosis where plasmin generation is up-regulated.
To make the situation complex, intact/nicked β2GPI itself has been reported as an inhibitor of angiogenesis. In the first report, Beecken et al28 identified β2GPI from transitional cell carcinoma cell line, which inhibits the growth of a tumor implant in severe combined immunodeficiency mice. They demonstrated that β2GPI together with plasmin, but neither β2GPI alone nor plasmin alone, inhibits proliferation and tube formation of HUVECs, concluding that the nicked form of β2GPI is an inhibitor of angiogenesis. However, lack of using purified material makes it difficult to assume the concentration of nicked β2GPI generated in this study. Sakai et al29 reported that 4 μM nicked β2GPI inhibited endothelial cell migration, proliferation, and neovascularization into subcutaneous implants that contain VEGF. Intraperitoneal injection of nicked β2GPI also inhibited growth of orthotopically injected tumors in a murine prostate cancer model. Only in the neovascularization study, which is a VEGF-dependent system, the same amount of intact β2GPI had similar antiangiogenic properties. Although the authors found no binding between nicked β2GPI and commercially available angiostatin (Sigma-Aldrich) using immunoprecipitation, this preparation of angiostatin does not include any portion of kringle 5. Recently, Yu et al30 have shown that β2GPI inhibits VEGF- and FGF-induced proliferation, migration, and tube formation of HUVECs. This antiangiogenic property was found in intact β2GPI, nicked β2GPI, and also in deletion mutant lacking domain V of β2GPI, but not in β2GPI lacking domain I, concluding that this antiangiogenic property is mediated via domain I of β2GPI. This group also demonstrated that 0.5 to 2 μM intact β2GPI down-regulates mRNA expression of the VEGF receptor.
In our HAEC/HUVEC proliferation assay in which EGF and FGF were removed (Figure 3A-B), intact/nicked β2GPI had almost no effect on the proliferation of arterial endothelial cells in lower concentrations, up to 0.4 μM. Our result does not contradict that from the Yu et al study25 in which the antiangiogenic property of intact/nicked β2GPI was dependent on VEGF and FGF. Indeed, in our HUVEC proliferation assay with VEGF (Figure 3C) and in our VEGF-dependent tube formation assay (Figure 5), both intact and nicked β2GPI exerted antiangiogenic properties at lower concentrations. At relatively high concentrations more than 1 μM, both intact and nicked β2GPI inhibited HUVEC migration and tube formation (Figures 4B,5B), being relevant to the previous reports.29,30 In these functional assays, nicked β2GPI exerted dual effect in the presence of AS4.5; at the lower concentrations, nicked β2GPI works as an AS4.5 inhibitor, whereas it works as an angiogenesis inhibitor at the higher concentrations. In the last part of this study, we confirmed that nicked β2GPI suppresses the antiangiogenic effect of AS4.5 in vivo (Figure 6).
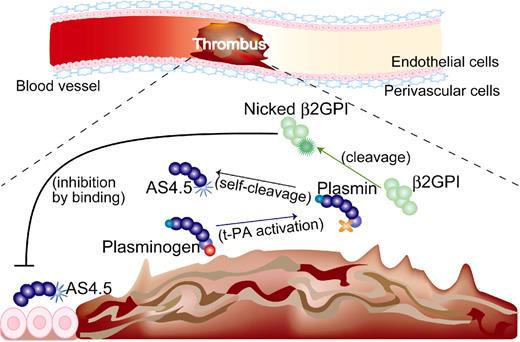
Although intact β2GPI in human plasma is abundant, plasma levels of nicked β2GPI in steady-state human is up to 0.5%, 1.5% of intact form even in patients with a history of stroke17 and those with lupus anticoagulant,16 respectively. Thus, the antiangiogenic/antitumor effect of β2GPI in steady state is probably dependent on the intact form. On the other hand, both nicked β2GPI and angiostatin are proteolytically processed by plasmin, thus being produced at sites where fibrinolysis is up-regulated. When ischemic thrombus is formed in the artery, collateral blood flow needs to be generated by angiogenesis. In such situations, plasmin is generated, leading to the production of both nicked β2GPI and angiostatin. Those products might be stuck near the lesion resulting from the stagnant blood flow. Nicked β2GPI may exert its property as antiangiostatin in such situations, resulting in promoted angiogenesis (Figure 7). In other situations, such as tumor and diabetic retinopathy where angiostatin works beneficially, however, nicked β2GPI might be a disease-worsening factor, being a candidate for the treatment target.
Beneficial effect of nicked β2GPI on thrombotic ischemia (hypothesis). (1) If thrombus is formed in the artery, up-regulation of fibrinolysis occurs and plasminogen is converted into plasmin. (2) Plasmin cleaves β2GPI into nicked β2GPI, whereas angiostatin is generated via autoproteolysis of plasminogen. (3) Nicked β2GPI binds angiostatin and attenuates its antiangiogenic property, resulting in promoted angiogenesis. tPA indicates tissue plasminogen activator.
Beneficial effect of nicked β2GPI on thrombotic ischemia (hypothesis). (1) If thrombus is formed in the artery, up-regulation of fibrinolysis occurs and plasminogen is converted into plasmin. (2) Plasmin cleaves β2GPI into nicked β2GPI, whereas angiostatin is generated via autoproteolysis of plasminogen. (3) Nicked β2GPI binds angiostatin and attenuates its antiangiogenic property, resulting in promoted angiogenesis. tPA indicates tissue plasminogen activator.
In conclusion, (1) we have demonstrated the binding between nicked β2GPI and AS4.5; and (2) we propose that nicked β2GPI is a physiologic inhibitor of angiostatin both of which are produced only when fibrinolytic system is activated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Hiroko Ogawa and Satoshi Hirohata (Department of Molecular Biology and Biochemistry, Okayama University School of Medicine) for great suggestions and discussions.
This work was supported in part by grants from the Japanese Ministry of Health, Labor and Welfare and the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Authorship
Contribution: H.N. performed the research and analyzed data; S.Y. designed and performed the research and wrote the paper; E.M. and K.K. contributed analytical tools; M.I. contributed vital reagents; H.K. and T.H. analyzed data; and T.A. and T.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shinsuke Yasuda, Department of Medicine II, Hokkaido University Graduate School of Medicine, N15, W7, Kita-ku, Sapporo 060-8638, Japan; e-mail: syasuda@med.hokudai.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal