Abstract
CD4+ T helper (Th) lymphocytes represent a heterogeneous population of cells. In addition to type 1 (Th1) and type 2 (Th2) cells, another subset of CD4+ effector Th cells has been discovered and named as Th17, because of its unique ability to produce interleukin (IL)–17. Studies in mice initially suggested that Th17 cells are the pathogenic cells in autoimmune disorders, whereas Th1 cells may behave rather as protective. Subsequent studies in humans demonstrated the plasticity of Th17 cells and their possibility to shift to Th1. The plasticity of Th17 to Th1 cells has recently been confirmed in mice, where it was found that Th17 cells seem to be pathogenic only when they shift to Th1 cells. Studies in humans also showed that Th17 cells are different than in mice because all of them express CD161 and exclusively originate from CD161+ precursors present in umbilical cord blood and newborn thymus. While murine Th17 cells develop in response to IL-6, IL-1, and transforming growth factor (TGF)–β, human Th17 cells originate from these CD161+ precursors in response to IL-1β and IL-23, the need for TGF-β being controversial. Thus, we believe that studies in humans have better depicted human Th17 cells than studies in mice.
Introduction
CD4+ T helper (Th) lymphocytes represent a heterogeneous population of cells that play an essential role in adaptive immunity. These cells include effector cells, which are devoted to protection against pathogens, and regulatory T cells (Treg), which protect against effector responses to autoantigens and also against responses to exogenous antigens when they may become dangerous for the host. Effector CD4+ Th cells are heterogeneous with regard to their protective function, enabling a type of response that is different according to the nature of the invading microorganism. More than 20 years ago, 2 main subsets of CD4+ Th cells with different functions and patterns of cytokine secretion were identified in both mice and humans, which were named as type 1 Th (Th1) and type 2 Th (Th2) lymphocytes, respectively.1,2 Th1 cells produce high levels of interferon-γ (IFN-γ) and are responsible for both phagocyte activation and the production of opsonizing and complement-fixing antibodies, thus playing an important role in the protection against intracellular pathogens. Th2 cells produce interleukin (IL)-4, IL-5, IL-9, and IL-13 and are mainly involved in the protection against parasitic helminths.3 In addition to their protective functions against invading pathogens, Th1 and Th2 cells contribute to the development of human disorders: Th1 cells have been thought to be involved in the pathogenesis of organ-specific autoimmune diseases, as well as other chronic inflammatory disorders, such as Crohn disease (CD), sarcoidosis, and atherosclerosis4 ; Th2 cells certainly play a central role in the development of allergic disorders.3 The Th1-Th2 paradigm was maintained until some years ago when a third subset of CD4+ effector Th cells, named Th17 cells, was identified.5,6
From mice to men: the discovery of Th17 cells
Murine Th17 cells
Although the existence of IL-17 as a product of activated CD4+ T cells has been known for more than 10 years, only recently was the existence of Th17 as a distinct cell subset recognized.5,6 The breakthrough leading to the discovery of the Th17 lineage came from murine models of autoimmunity. Experimental autoimmune encephalomyelitis (EAE), collagen-induce arthritis (CIA), and inflammatory bowel disorders have historically been associated with unchecked Th1 responses, based largely on studies in which disease development was abated by neutralizing the IL-12p40 chain (IL-12 being a powerful Th1-polarizing agent) or targeting the p40 or the IL-12Rβ1 genes.4 However, this initial concept of Th1 association to autoimmune disorders required an adjustment with the unexpected discovery that mice deficient in IFN-γ or IFN-γ receptor were not resistant to EAE but were actually more susceptible to central nervous system autoimmunity.7-9 Moreover, the link with IL-12 in these diseases was called into question by the discovery that a new IL-12 family member, IL-23, shares with IL-12 the p40 subunit, the heterodimer of IL-12 being composed of p40 and p35, and that of IL-23 being composed of p40 and p19.5 IL-23 shares with IL-12 also a chain of its receptor, the IL-12 receptor being composed of IL-12Rβ1 and IL-12Rβ2 chains, and that of IL-23 being composed of IL-12Rβ1 and IL-23 receptor (IL-23R) chains. After this discovery, it was found that EAE and CIA did not develop in mice deficient in IL-23p19 subunit or IL-23R chain, whereas they could develop in those deficient in IL-12p35 subunit or IL-12Rβ2 chain, suggesting that at least in these models IL-23, but not IL-12, is critically linked to autoimmunity.6,10,11 On the basis of these and other findings, a new role for Th17 cells in immunopathology and the distinct origin of Th1 and Th17 under differential IL-12 or IL-23 conditioning were proposed.6,10 More recently, however, a completely different pathway of murine Th17 origin has been described. Although IL-23 appeared to be required for Th17-induced immunopathology, different groups independently demonstrated that transforming growth factor-β (TGF-β) was required for initiation and that IL-6 was a critical cofactor for Th17 differentiation.12-14 Of note, the Th17-polarizing cytokine TGF-β was already known for its ability to promote the development of Foxp3+ Treg cells, but only in absence of IL-6.14 Moreover, IFN-regulatory factor 4 has been shown to be essential for the Th17 phenotype, by acting via the induction of IL-21, another cytokine that contributes to the induction, amplification, and stabilization of Th17 cells.15 Finally, the presence in culture medium of natural agonists for aryl hydrocarbon receptor seems also to be critical for optimal differentiation of Th17 cells.16 Murine Th17 express a master transcription factor different from Th1 and Th2 cells, an orphan receptor known as retinoic acid–related orphan receptor-γt (ROR-γt).17 A second orphan receptor, named as ROR-α, has also been found to contribute to the development of murine Th17 cells.18 The signal transducer and activator of transcription-3 (STAT3) transcription factor is also essential for the murine Th17 development, although whether it acts directly or through the activation of ROR-γt is still unclear.19 The distinctive cytokine of murine Th17 cells, IL-17A, is involved in the recruitment, activation, and migration of neutrophil granulocytes by inducing the production of colony-stimulatory factors and CXCL820 by both macrophages and tissue resident cells. The other cytokines produced by murine Th17 cells, such as IL-17F, IL-21, and IL-22, can also contribute to the activation of mononuclear and/or resident cells and therefore may induce and/or maintain a chronic inflammatory process. However, because of their unique ability to recruit neutrophils, the main protective function of Th17 cells appears to be the clearance of extracellular pathogens, including fungi.21 Nevertheless, the major emphasis on the pathophysiology of murine Th17 cells was placed on their determinant or even exclusive pathogenic role on models of autoimmunity. This concept was immediately extrapolated to human disorders that are considered as equivalent to the aforementioned murine models, such as multiple sclerosis, rheumatoid arthritis (RA), and inflammatory bowel disorder,22 but also to psoriasis and contact dermatitis.23 Therefore, Th17 cells were thought to be the pathogenic cells in virtually all chronic inflammatory disorders, where the effect Th1 cells, which had been shown to be important in hundreds of previous studies, was underscored or even seen as protective against the Th17-mediated inflammation.24 This sort of iconoclastic fury against the past dogma was well expressed in a commentary, which got the idea from the expression used in the age of monarchy “Le Roi est mort; vive le Roi,” to mark the passing of eras ushering out one regime while introducing another.25
Human Th17 cells
Several studies had already reported the expression of IL-17A mRNA and/or protein in biologic fluids or tissues of different human inflammatory disorders,26-31 but no clear evidence on the existence, phenotype, and functional activity of human Th17 cells was given until they were extensively investigated by different groups.32-35 Acosta-Rodriguez et al32,34 isolated human memory Th17 cells from the CD4+ T-cell fraction of peripheral blood of healthy subjects expressing the CC chemokine receptor-6 (CCR6). Among CCR6+ T cells, the CCR4+ subset had copious production of IL-17A, but not IFN-γ, whereas the CXCR3+ subset mainly produced IFN-γ and low IL-17A. CCR6+CCR4+ Th17 cells also expressed the human ortholog of mouse ROR-γt (RORC variant 2) and were found to contain the majority of Candida albicans–specific memory T cells, whereas the majority of Mycobacterium tuberculosis PPD-specific memory CD4+ T cells were found to be contained in the CCR6+CXCR3+ subset. Using a different experimental approach, Annunziato et al33 generated T-cell clones from the inflamed or apparently healthy gut of patients with CD as well as from the peripheral blood of CD patients or healthy subjects. The Th17 clones that were generated in addition to Th1 and Th2 clones from both peripheral blood and gut tissue were found to express RORC, CCR6, CCR4, and the IL-23R. When CCR6+ cells were isolated from either peripheral blood or tonsil of healthy subjects, virtually all Th17 cells were found to be contained in the CCR6+ fraction, whereas Th1 cells could be recovered from either the CCR6+ or the CCR6− fraction. The CCR6+ fraction also contained significantly higher amounts of RORC and IL-23R mRNA, supporting the concept that CCR6 is an important marker for human Th17 cells. Finally, Wilson et al35 demonstrated that human Th17 cells express IL-17A, IL-17F, IL-22, IL-26, IFN-γ, the chemokine CCL20, the transcription factor ROR-γt, and the IL-23R.
From men to mice: Th17 plasticity to Th1 cells and its reflection on pathogenicity
Human Th17 cells can be induced in vitro to produce IFN-γ
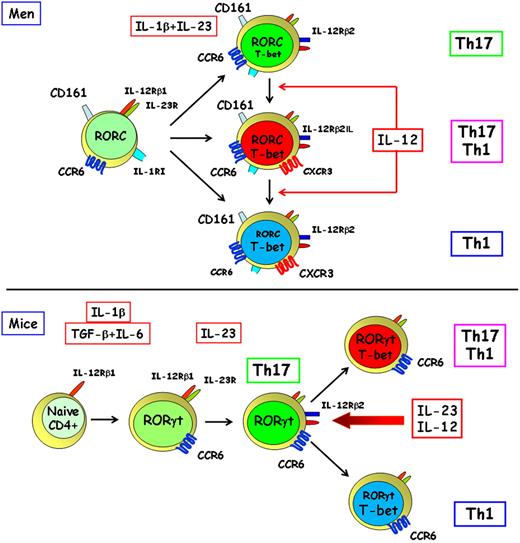
After the discovery of CCR6 as a major chemokine receptor of human Th17 cells,32,33 the demonstration of CCR6 expression even on murine Th17 cells was established in an animal model of RA.36 In this study, it was also shown that synoviocytes produce CCL20 and that CCR6 blocking substantially inhibited mouse arthritis,36 thus supporting the concept of a similarity between murine and human Th17 cells. However, in our study on human Th17 clones mentioned in “Human Th17 cells,”33 some features clearly different from, or even incompatible with, the Th17 paradigm established in mice were reported.12-14 For example, many human Th17 clones also produced IFN-γ, which were named as Th17/Th1. This discrepancy could obviously be the result of the different experimental conditions used to investigate Th17 cells in mice and humans. However, even ex vivo studies performed in humans clearly demonstrated the existence in both peripheral blood and gut tissue of Th17/Th1 cells.33 Second, both Th17 and Th17/Th1 clones not only expressed the IL-23R and RORC, but also the IL-12Rβ2 chain and the Th1-related transcription factor T-box expressed in T cells (T-bet).33 Finally and most importantly, stimulation of human Th17 clones in the presence of IL-12 down-regulated RORC and up-regulated T-bet and enabled these cells to produce IFN-γ in addition to IL-17A. By contrast, human Th1 clones could never be induced to produce IL-17A. These findings allowed us to suggest the existence of a developmental relationship between human Th17 and Th1 cells and, more importantly, to demonstrate the plasticity of human Th17 cells (Figure 1), thus providing the novel concept that these cells can shift to the Th1 profile, whereas Th1 cells seem unable to shift to Th17.33
Origin and plasticity of human and murine Th17 cells. Human Th17 cells originate from a small subset of CD4+CD161+ T-cell precursors present in newborn thymus and UCB, which already express RORC, IL-23R, IL-1RI, and CCR6.37 (This account of the origin of human Th17 cells mainly reflects a personal view and does not take into account all the data presently available in the literature.) These cells differentiate in vitro in response to the combined activity of IL-1β and IL-23 into mature Th17 cells, which express RORC, T-bet, IL-12Rβ2, CD161,37 and CCR6,32,33 but under the same conditions can also give rise to Th17/Th1 and Th1 cells, which express T-bet33 and CXCR3.32,38 In the presence of IL-12, T-bet expression is up-regulated in Th17 cells, which shift to the production of IFN-γ.33 Murine Th17 cells originate from a naive Th cell in response to the combined activity of TGF-β and IL-6,12-14 which induce ROR-γt,17,18 IL-23R,12-14,17,18 and CCR636 expression, but the early IL-1 signaling seems also to be critical for their differentiation.39 IL-23 appears to be required for the survival and/or expansion of murine Th17 cells.12-14,17,18 However, in response to IL-12 or of the prolonged activity of IL-23, these cells up-regulate T-bet and shift to cells producing IFN-γ both in vitro and in vivo.40
Origin and plasticity of human and murine Th17 cells. Human Th17 cells originate from a small subset of CD4+CD161+ T-cell precursors present in newborn thymus and UCB, which already express RORC, IL-23R, IL-1RI, and CCR6.37 (This account of the origin of human Th17 cells mainly reflects a personal view and does not take into account all the data presently available in the literature.) These cells differentiate in vitro in response to the combined activity of IL-1β and IL-23 into mature Th17 cells, which express RORC, T-bet, IL-12Rβ2, CD161,37 and CCR6,32,33 but under the same conditions can also give rise to Th17/Th1 and Th1 cells, which express T-bet33 and CXCR3.32,38 In the presence of IL-12, T-bet expression is up-regulated in Th17 cells, which shift to the production of IFN-γ.33 Murine Th17 cells originate from a naive Th cell in response to the combined activity of TGF-β and IL-6,12-14 which induce ROR-γt,17,18 IL-23R,12-14,17,18 and CCR636 expression, but the early IL-1 signaling seems also to be critical for their differentiation.39 IL-23 appears to be required for the survival and/or expansion of murine Th17 cells.12-14,17,18 However, in response to IL-12 or of the prolonged activity of IL-23, these cells up-regulate T-bet and shift to cells producing IFN-γ both in vitro and in vivo.40
Either murine Th17 or Th1 cells can exhibit pathogenicity
The demonstration of a potential plasticity of human Th17 cells to Th1 cells was of great importance with regard to the controversial issue of the respective role of the 2 effector cell types in the pathogenesis of murine and human autoimmune, as well as of other chronic inflammatory, disorders. Indeed, after the initial substitution of Th1 cells with Th17 cells as responsible for pathogenicity, mainly based on the results obtained in a murine model of autoimmunity in gene-deficient animals,6,10 a series of well-performed studies has strongly challenged this possibility. Therapeutic administration of a small interfering RNA specific for T-bet significantly improved the clinical course of established EAE by limiting the differentiation of autoreactive Th1 and inhibiting pathogenic Th17 cells through the regulation of IL-23R.41 In Helicobacter hepaticus–induced colitis, IFN-γ, but not IL-17, was the crucial T-cell effector cytokine when Treg cells were absent.42 Th17 can be pathogenic in certain forms of EAE (those characterized by granulocyte infiltration), whereas Th1 cells are mainly responsible for the EAE models characterized by prevalent mononuclear cell infiltration, where Th17 cells do not seem to play any pathogenic role.43 In the murine model of autoimmune disorder known as proteoglycan-induced arthritis, Th1 cells, but not Th17 cells, are pathogenic.44 Either Th1 or Th17 cells have been found to be pathogenic in experimental autoimmune uveitis.45 Neither IL-17A nor IL-17F contributes vitally to autoimmune neuroinflammation in mice.46 Obviously, it should be reminded that Th17 cells do not produce only IL-17A and IL-17F but also other cytokines, such as IL-22, which has been found to be responsible for tissue damage.47 The contrast between these findings and those derived from genetically deficient mice may probably be explained by the heterodimeric structure of the proteins of the IL-12 family.48 Indeed, the recent discovery of IL-35, a cytokine with immunosuppressive activity produced by Treg cells, which is composed by the association of p35 and EBI3 subunits, allows the suggestion that p35−/− mice lack not only IL-12 but also IL-35.49,50 Thus, the opposing activities of these cytokines (IL-12 vs IL-35) further complicate the analysis, as the loss of one may mitigate the loss of the other and therefore explain the higher EAE and CIA susceptibility of p35−/− mice.
Even murine Th17 cells exhibit plasticity to Th1 cells both in vitro and in vivo
Based on this impressive series of new data,41-46 a need for clarification on the concept that Th17 cells are the master mediators of tissue damage in a variety of pathologic conditions was invoked.51 This clarification recently came from 4 independent studies. First, not only the propagation of committed Th17 precursors in the presence of IL-23 without TGF-β resulted in a progressive extinction of IL-17A and IL-17F and promoted the emergence of IFN-γ–producing cells that lacked IL-17 expression, but their stimulation with IL-12 induced a rapid, STAT4- and T-bet–dependent transition marked by an extinction of ROR-γt, ROR-α, IL-17A, and IL-17F and an induction of a Th1-like expression signature.40 Moreover, the potential plasticity of Th17 to Th1, but not of Th1 to Th17, cells has been reported.52 These findings support the substantial developmental plasticity of the Th17 lineage already observed in humans,33 which identified a mechanism for latent Th1-like responsiveness of Th17 cells and provided the basis for understanding the relationship between Th17- and Th1-mediated pathophysiology. Indeed, it has recently been shown that Th17 cells can promote pancreatic inflammation but only induce type 1 (insulin-dependent) diabetes mellitus efficiently in lymphopenic mice after conversion into Th1 cells.53 Accordingly, highly purified Th17 cells from BDC2.5NOD mice shift into Th1-like cells in nonobese diabetic/severe combined immunodeficiency recipient mice. The transferred Th17 cells, completely devoid of IFN-γ at the time of transfer, rapidly converted to secrete IFN-γ in the nonobese diabetic/severe combined immunodeficiency recipients. More importantly, the development of insulin-dependent diabetes mellitus was prevented by the treatment with anti–IFN-γ, but not with anti-IL17A, specific antibody.54 Thus, the plasticity of Th17 cells into Th1 cells initially observed in humans33 is now confirmed in mice (Figure 1) and provides the basis for supporting again the major pathogenic role of Th17-derived Th1 cells in murine autoimmune or other chronic inflammatory disorders. Obviously, a pathogenic role for these cells in mice does not mean that they are also pathogenic in humans. Moreover, it is not yet clear the relationship between the Th17-derived Th1 cells and the classic Th1 cells obtained in response to IL-12–mediated polarization. Interestingly, we have recently observed that Th1 clones derived from circulating CCR6+CD161+ CD4+ T cells express higher RORC mRNA levels than Th1 clones derived from CCR6−CD161− CD4+ T cells (F.A., S.R., manuscript in preparation), supporting the possibility of a distinct origin of these 2 types of Th1 cells. In conclusion, it was certainly not correct to say, “Le Roi est mort; vive le Roi”25 ; at the most, we can accept the formula: “Le Roi est encore vivant; vive le nouveau Prince.”
Of mice and men: their Th17 cells have a different origin
The role of TGF-β in human Th17 differentiation is still controversial
Although it is widely accepted that murine Th17 cells originate from naive CD4+ T cells in the presence of IL-6 and TGF-β, and their development is then stabilized and/or amplified by IL-23 and IL-21 (mentioned in “Murine Th17 cells”), several studies have denied the role of TGF-β in human Th17 cell differentiation.34,35,55 The differences between humans and mice were attributed to the difficulty to ensure a truly naive T-cell population in humans. Accordingly, van Beelen et al56 found that the combined activity of IL-1α or IL-1β and IL-23 was required for the enhancement of human Th17 memory T cells, whereas under these or even other conditions the differentiation of human naive T cells from adult subjects into Th17 cells could not be achieved. These findings were extrapolated to suggest that mice are of limited use as models for the development of such cells in the immune system.57 More recently, however, 3 independent studies showed that, in contrast to the previous observations, even the differentiation of human Th17 cells requires the activity of TGF-β.58-60 All the 3 reports suggested that in previous studies performed in humans the role of TGF-β had been underevaluated because CD4+ T cells were cultured in media composed of human or bovine serum, which usually contains TGF-β. The new findings elicited new enthusiasm in the community of “mouse” immunologists61 because the possibility of a completely different development between murine and human Th17 cells was certainly unfortunate to the biomedical research community, as it is essential to have preclinical data obtained from in vitro and in vivo mouse models before progressing to clinical trials in humans. However, the results of these latter studies are somehow contradictory. Manel et al58 found that TGF-β, IL-1β, and IL-6, IL-21, or IL-23 were necessary and sufficient to induce IL-17 expression in naive umbilical cord blood (UCB) human CD4+ T cells. TGF-β up-regulated RORC expression but simultaneously inhibited its ability to induce IL-17 expression, an activity that was antagonized by the inflammatory cytokines. Volpe et al59 found that TGF-β, IL-23, IL-1β, and IL-6 were all essential for human Th17 differentiation, but they differentially modulated the cytokines produced by Th17 cells. More importantly, the absence of TGF-β induced a shift from the Th17 profile to a Th1-like profile. By contrast, Yang et al60 found that TGF-β and IL-21 uniquely promoted the differentiation of human naive CD4+ T cells into Th17 cells accompanied by expression of RORC.
All human memory Th17 cells express CD161, whereas murine Th17 cells do not express NK1.1
In the same time, we performed a second study on human Th17, Th1, Th17/Th1, and Th2 clones based on the microarray analysis. The results showed that, among genes that were up-regulated in Th17 (or Th17/Th1) in comparison with Th1 or Th2 clones, there was CD161.37 CD161 is the equivalent of murine NK1.1,62 a molecule that is expressed on the surface of natural killer (NK), NKT, and on a variable proportion of both CD4+ and CD8+ T cells. IL-17A and IFN-γ production was then evaluated by flow cytometry on CD161+ and CD161− CD4+ T cells from peripheral blood of healthy adult subjects, and virtually all Th17 cells were found to be contained into the CD161+ fraction, whereas Th1 cells could be demonstrated in both the CD161+ and the CD161− fraction. Of note, all CD161+CD4+ T cells were CD45RA−CD45RO+ (memory) cells, whereas there were virtually no CD161+ cells in the CD45RA+RO− (naive) population of CD4+ circulating T cells of adult subjects.37 Accordingly, CD161 expression was found in a remarkable proportion (∼ 60%) of CD3+ cells present in the inflamed skin of patients with psoriasis or the gut mucosa of patients with CD. Virtually no Th17 cells were observed in the CD161− fraction of both peripheral blood of healthy subjects or the inflamed tissues of patients with CD or psoriasis.37 The possibility that CD161+ Th17 cells were NKT cells, which are also able to produce IL-17A,63,64 was excluded by the observation that they had a broad T-cell receptor repertoire and the restriction molecule used in antigen recognition was major histocompatibility complex class II,37 whereas NKT cells are known to have a limited T-cell receptor and their antigen recognition is restricted by CD1.65 Recently, Kleinschek et al66 confirmed our data identifying CD161 as a marker of both circulating and gut resident T cells able to produce IL-17A and IL-22. Moreover, they demonstrated that circulating CD161+ cells are imprinted for gut homing, as indicated by high levels of CCR6 and β7 integrin expression. This phenotype may explain why CD161+ Th17 cells are increased in the inflammatory infiltrate of CD lesions.33,37,66 Therefore, 2 independent studies in humans have demonstrated that all memory Th17 cells are contained into the CD161+ fraction.37,66 thus identifying CD161 as a second marker (besides CCR6) of Th17 cells, a feature that has never been reported in mice.
Human Th17 cells originate exclusively from CD161+ T-cell precursors
The subsequent search for CD4+CD161+ T cells in UCB demonstrated that a very small proportion of UCB CD4+ T cells expressed CD161. Surprisingly, these CD161+, but not the CD161−, cells ex vivo expressed RORC, IL-23R, IL-1RI, and CCR6, but not IL-17, mRNA.37,38 The addition of IL-1β or IL-23 up-regulated RORC, T-bet, IL-23R, and IL-12Rβ2, but only the combination of IL-1β and IL-23 allowed mRNA IL-17A expression and induced the appearance of Th17 and Th17/Th1 cells in stimulated cultures of cells from the CD161+ fraction (Figure 1). Like CD161+, CD161− cells from UCB CD4+ T cells could be induced to differentiate into Th1 or Th2 cells under the appropriate polarizing conditions (IL-12 for Th1 cells and IL-4 for Th2 cells), but CD161− cells never differentiated into Th17 cells.37 In the presence of IL-1β plus IL-23, a strong increase in the proportions of Th1 cells was observed in both the CD161+ and the CD161− fractions. No other cytokine or cytokine combinations (including TGF-β, IL-6, and IL-21) were able to induce IL-17A mRNA expression and IL-17A production in any of the 2 cell fractions. Because these experiments were initially performed by culturing UCB cells in fetal calf serum–containing medium, the possibility that TGF-β eventually present in this serum may have biased our results was considered. However, this possibility is unlikely for 2 main reasons: (1) naive CD161+, but not CD161−, CD4+ T cells from UCB were found to express constitutively RORC and IL-23R even before their culturing, suggesting that RORC expression by these cells was not dependent on the activity of exogenously added TGF-β; and (2) the addition in culture of an anti–TGF-β antibody did not induce any change in the effects induced by the combination of IL-1β and IL-23. Moreover, even in serum-free cultures, the development of Th17 cells could be observed only by CD161+ CD4+ T cells activated in presence of IL-1β plus IL-23.67 Obviously, we cannot exclude that ex vivo RORC expression by the small subset of UCB CD4+CD161+ T cells reflects an in vivo activity of TGF-β. However, it is of note that, in a very recent report, it has been shown that IL-1 signaling is required for early murine Th17 differentiation, which can occur in the absence of exogenous TGF-β,39 suggesting that even in mice IL-1 is a critical cytokine for Th17 differentiation, whereas the addition of exogenous TGF-β may not be required.39
UCB CD4+CD161+ cells that give rise to Th17 cells are naive T cells
The demonstration that human Th17 cells not only express CD161, but also that they can exclusively originate from naive CD4+CD161+ T-cell precursors was intriguing. Our results were in agreement with those previously reported by van Beelen et al,56 who were unable to induce the development of Th17 cells from purified CD45RA+CD45RO− (naive) circulating CD4+ T cells of adult subjects in response to TGF-β plus IL-6 or IL-1 plus IL-23,37 a finding that is consistent with the apparent lack of CD161+ cells within the CD45RA+CD45RO− population of adult circulating CD4+ T cells (mentioned in “All human memory Th17 cells express CD161, whereas murine Th17 cells do not express NK1.1”). The possibility that UCB CD161+ cells giving rise to Th17 cells represents the expansion of a small population of contaminating memory T cells resulting from fetal immune system stimulation during pregnancy can be reasonably excluded on the basis of 3 main observations. First, neither CD161+ nor CD161− UBC CD4+ T cells produced any cytokine in response to stimulation with PMA plus ionomycin, except IL-2, which is a functional property of naive T cells.37 Second, both CD161+ and CD161− cells showed comparable levels of T-cell receptor rearrangement excision circles, which were significantly higher than those of CD4+CD45RA−CD45RO+ (memory) adult circulating T cells. Finally and more importantly, identical results were obtained when the small population of CD161+ cells present among single-positive CD4+CD8− T cells of newborn human thymi was assessed.37 Obviously, why CD161+ UCB or thymic naive T cells can differentiate into Th17, whereas purified CD45RA+RO− circulating CD4+ T cells from adult subjects do not, remains unclear. One might speculate that Th17 responses are mainly mounted during the prenatal period. However, this would imply that for an effective Th17 response all possible bacterial pathogens should present itself during the prenatal period, which is doubtful. Another possibility may be that these cells rapidly migrate during pregnancy and shortly after birth to mucosal lymphoid tissue where they can undergo the differentiation into Th17 cells in response to inflammatory cytokines. This possibility seems to be in keeping with recent observations showing the existence of human gut-resident “precommitted” CD161+ CD4+ T cells, which in healthy subjects become fully differentiated Th17 cells in response to IL-1β and IL-23, whereas in patients with CD the same cells require IL-23 alone.66 Another important question is the meaning of CD161+ cells, which behave as precursors of Th17 cells. These cells are not multipotent hemopoietic precursors because they are already differentiated into CD4+ T cells; on the other hand, they are not determined to become Th17 cells alone because they can also give rise to Th1 or Th2 cells under appropriate polarizing conditions. However, it is clear that, whereas Th1 or Th2 cells can originate from either CD161− or CD161+ CD4+ T cells, human Th17 cells seem to exclusively originate from the small CD161+CD4+ T-cell fraction present in both UCB and newborn thymus.
TGF-β may indirectly favor the development of Th17 cells by suppressing the proliferation of Th1 cells
Interestingly, we also found that, under the conditions that favored the development of both Th1 and Th17 cells (IL-1β + IL-23) from UCB CD4+CD161+ T cells, the presence of TGF-β strongly inhibited the expression of T-bet and the development of Th1 cells, without having the same inhibitory effect on the expression of RORC and on the development of Th17 cells because of the different susceptibility of Th1 and Th17 cells to the suppressive effect of TGF-β on their proliferation. This finding may be in keeping with the observation67 that human Th17 clones express significantly lower levels of mRNA for clusterin (a proapoptotic molecule) and significantly higher levels of mRNA for Bcl-2 (an antiapoptotic molecule).67 Accordingly, human Th17 cells appeared to be much less susceptible than Th1 cells to the proapoptotic activity of TGF-β, which may account for the different effect of TGF-β on the growth of Th1 and Th17 cells.67 Thus, without having any direct effect on the expression of RORC and on the development of Th17 cells, the addition in vitro of TGF-β may indirectly favor their expansion by strongly inhibiting the development of Th1 cells.
Th17 cells: teachings and future perspectives
The story of Th17 cells is still complex and controversial, but some major teachings seem to be worthy of mention.
Th17 cells exhibit plasticity and can shift to Th1 cells in both humans and mice
As often occurs in immunology, after Th17 cells were discovered in mice as a novel subset of effector T cells and found to be responsible for all inflammatory damages previously attributed to Th1 cells in murine models of autoimmunity, this new dogma was immediately extrapolated to human autoimmune as well as to all other chronic inflammatory disorders, and the role of Th1 cells was rapidly changed from pathogenic to protective. However, when the properties of Th17 cells were directly investigated in humans, it appeared that these cells are not an established, but rather a flexible, population that can be induced to produce IFN-γ.33 Although this finding, being in contrast with the new dogma established in mice, was underscored and rarely mentioned in the current literature, the Th17 plasticity to Th1 cells has now been recognized even in murine Th17 cells,40 where it seems to be even obligatory for the induction of tissue damage.53,54 Thus, the first teaching from the Th17 story is that the initial extrapolation of the results obtained in mice to humans was only partially right and that the different results obtained in humans, which suggested the Th17 plasticity to Th1 cells,37 was true and it has now been confirmed in mice.40,52-54 Thus, we can say that murine Th17 cells can be considered as similar to human Th17 cells, whereas it was not right to consider human Th17 cells equivalent to murine Th17 cells, at least as these latter were initially described.
Human and murine Th17 cells seem to have different cell origins
The second teaching is that murine and human Th17 cells seem to have different origins. Indeed, all murine studies agree that Th17 cells share a common origin with Treg cells. When the naive T cell is activated in the presence of TGF-β alone, it expresses Foxp3 and differentiates into a Treg cell; whereas in the presence of TGF-β plus IL-6, the same cell expresses ROR-γt and differentiates to Th17.12-14 Apart from the still controversial issue on the need of in vitro addition of TGF-β for the development of human Th17 cells, which has been extensively discussed (mentioned in “The role of TGF-β in human Th17 differentiation is still controversial” and “TGF-β may indirectly favor the development of Th17 cells by suppressing the proliferation of Th1 cells”), it is clear that human Th17 cells clearly differ from murine Th17 cells because they all express CD161, whereas the CD161 equivalent, NK1.1, has never been reported on murine Th17 cells. Moreover, human Th17 cells exclusively originate from CD161+ precursors present in both UCB and thymus, which constitutively express both RORC and the IL-23R, but not IL-17 mRNA, and seem to be already planned to become Th17 cells only in response to a combination of IL-1β and IL-23. Intriguingly, it has recently been shown that human fetal lymphoid tissue inducer (LTi)-like cells are RORC+ IL-17–producing precursors of NK-like cells68 and that in vitro stimulation with IL-23 induced murine LTi cells, which constitutively expressed the IL-23R, CCR6 and, part of them, CD161, to produce IL-17 and IL-22.69 However, the relationship between these and our findings37 is presently unclear. What is clear is that, based on our data,37 2 major issues should now be pursued, even if they cannot be explored in mice. The first issue is to understand the meaning of CD161 on human CD4+ T cells planned to become IL-17A-producing cells. CD161 is able to interact with at least 2 different ligands. One is known as lectin-like transcript 1, which belongs to the C-type lectin domain family 2.70,71 Thus, it is possible that CD161 expression by Th17 cells allows their transendothelial migration of Th17 effectors into tissues. More recently, a second CD161 ligand has been identified and named as proliferation-induced lymphocyte-associated receptor (PILAR).72 PILAR signaling through CD161 supports T-cell proliferation by increasing the expression of the antiapoptotic Bcl-xL and induces secretion of Th1 cytokines. If CD161 engagement is blocked, PILAR induces apoptotic death on naive and early activated T cells. Thus, this molecule suggests the existence of very interesting scenery on the regulation of both Th1 and Th17 cells, which certainly requires careful investigation. Another important issue is the question of why human Th17 precursors cannot be found in adult peripheral blood,37,56 but they are present as CD161+RORC+IL-23R+CCR6+ cells in both UCB and thymus.37
Studies in humans have been critical for dissecting the features and origin of Th17 cells
All these findings allow the suggestion of a final major teaching that has also been proposed in a recent article,73 where it was stressed that “although the mouse model has been spectacularly successful in advancing our understanding of basic immunologic mechanisms, its record in formulating clinically useful protocols is much less impressive. Thus to fully realize the potential benefits of immunology for human health, we need to place more emphasis on human studies and make greater efforts to allow it to flourish.”73 The field of Th17 cells seems to be one of the clearest examples of how studies in humans are critical for dissecting the behavior and origin of this cell subset. Therefore, they cannot be disregarded or underevaluated, because they seem to be very useful for correcting wrong views derived from murine models or even for opening completely new avenues.
Acknowledgments
This work was supported by the European Community (project LSHB-CT-2005-518167, INNOCHEM, FP6), the Italian Ministry of University and Research (Programmi dí Ricerca Scientifica dí Rilevante Interesse Nazionale [PRIN] prot. 20077NFBH8), and the Associazione Italiana per la Ricerca sul Cancro AIRC.
Authorship
Contribution: F.A. and S.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sergio Romagnani, Dipartimento di medicina Interna, Università di Firenze, Viale Morgagni, 85, Firenze-50134 Italy; e-mail: s.romagnani@dmi.unifi.it.