Abstract
Phosphatidylinositol 3-kinase (PI3K) isoforms PI3Kβ and PI3Kγ are implicated in platelet adhesion, activation, and aggregation, but their relative contribution is still unclear or controversial. Here, we report the first comparative functional analysis of platelets from mice expressing a catalytically inactive form of PI3Kβ or PI3Kγ. We demonstrate that both isoforms were similarly required for maximal activation of the small GTPase Rap1b and for complete platelet aggregation upon stimulation of G protein–coupled receptors for adenosine 5′-diphosphate (ADP) or U46619. Their contribution to these events, however, was largely redundant and dispensable. However, PI3Kβ, but not PI3Kγ, enzymatic activity was absolutely required for Akt phosphorylation, Rap1 activation, and platelet aggregation downstream of the immunoreceptor tyrosine-based activation motif (ITAM)–bearing receptor glycoprotein VI (GPVI). Moreover, PI3Kβ was a major essential regulator of platelet adhesion to fibrinogen and of integrin αIIbβ3-mediated spreading. These results provide genetic evidence for a crucial and selective role of PI3Kβ in signaling through GPVI and integrin αIIbβ3.
Introduction
The phosphatidylinositol 3-kinase (PI3K) is implicated in platelet activation downstream of G protein–coupled receptors (GPCRs), immunoreceptor tyrosine-based activation motif (ITAM)–bearing receptors, as well as integrins.1 The different PI3K isoforms are grouped in 3 classes, I, II, and III. Class I PI3K includes the p85-associated members PI3Kα, PI3Kβ, and PI3Kδ (class IA), as well as the PI3Kγ isoform (class IB). Although all class I members are expressed in platelets, PI3Kγ and PI3Kβ are considered to play a major role in platelet physiology,1,2 but their relative contribution is still controversial.
PI3Kγ is activated by G protein βγ dimers, and, in platelets, it has been implicated in Rap1b activation and integrin αIIbβ3–supported aggregation downstream of the Gi-coupled P2Y12 adenosine 5′-diphosphate (ADP) receptor.3,4 This model has however been challenged by other studies, proposing a predominant role of PI3Kβ rather than PI3Kγ in ADP-induced platelet activation.5,6 In addition, the finding that PI3Kγ also functions as a scaffold protein7 supported a model for a kinase-independent contribution of PI3Kγ in ADP-mediated platelet activation.8
In platelets, PI3K is also activated upon stimulation of the ITAM-bearing collagen receptor glycoprotein VI (GPVI)/FcR γ-chain.9 Although there is evidence that a class IA member is involved,10 its identity is still unknown. Similarly, integrin αIIbβ3 recruitment activates PI3Ks. The involvement of a class II member has been hypothesized,11 but a possible role for class I isoforms clearly emerges from a more recent study.12
The specific contribution of PI3Kγ and PI3Kβ in platelet activation has remained largely elusive, mainly because of the early embryonic lethality caused by genetic ablation of PI3Kβ in mice,13 and because the interpretation of the analysis of PI3Kγ knockout mice turned out to be complicated by the kinase-independent action of this isoform.7
In this work we report the first comparative analysis of platelet activation in mice homozygous for the kinase dead forms of PI3Kβ (PI3KβKD) or PI3Kγ (PI3KγKD), in which lysine at positions 805 or 833, respectively, had been substituted with an arginine residue. Our results demonstrate that while both isoforms are implicated in signaling by GPCRs, PI3Kβ plays a unique role in platelet activation downstream of GPVI and integrin αIIbβ3.
Methods
Generation and characterization of PI3KβKD and PI3KγKD mice has been previously reported.7,14 Blood was withdrawn from the inferior vena cava of anesthetized animals, and washed platelets were prepared as described.15 Platelet aggregation was measured in a lumiaggregometer and followed for up to 5 minutes upon addition of ADP (in the presence of 200 μg/mL fibrinogen), U46619, or convulxin. Phosphorylation of Akt was evaluated on whole platelet lysates by immunoblotting with anti–phospho-Akt (Ser473) antibody, followed by reprobing of the membrane with anti-Akt antibody (Cell Signaling Technology). Rap1b activation was measured by a pulldown assay using glutathione S-transferase (GST)–tagged RalGDS-RBD. Analysis of platelet adhesion and spreading on immobilized fibrinogen was performed on coated glass coverslips, and results were quantified by fluorescence microscopy analysis upon staining with tetramethylrhodamine isothiocyanate (TRITC)–phalloidin (Sigma-Aldrich). Further details on the source of the reagents and on the experimental procedures adopted are reported in previous works.5,15,16 The use of mice for our experimental work was approved by the Ethics Committee of the University of Pavia.
Results and discussion
Tail bleeding time was not altered in both PI3KγKD and PI3KβKD mice (data not shown), indicating that activity of neither PI3K isoforms is essential for global hemostasis. Expression of p110γ and p85 was normal in PI3KβKD platelets, as was expression of p110β and p85 in PI3KγKD platelets (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, we observed that expression of p110γ in PI3KγKD platelets and expression of p110β in PI3KβKD platelets, albeit clearly evident, was reduced by approximately 50% to 60%. Although the reason for this enzyme instability is unclear, this finding is consistent with previous observations in other tissues.14
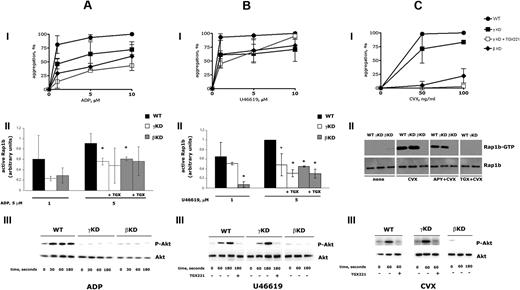
ADP-induced platelet aggregation was clearly impaired, but never suppressed, in both PI3KγKD and PI3KβKD mice (Figure 1Ai). The PI3Kβ inhibitor TGX221 further reduced, but did not abolish, ADP-induced aggregation of PI3KγKD platelets. Similarly, comparable defective ADP-induced Rap1b activation was observed in both genotypes, although Rap1b activation largely occurred independently of both PI3Kγ and PI3Kβ activity (Figure 1Aii). TGX221 did not further significantly reduce Rap1b activation in either ADP-stimulated PI3KγKD platelets or PI3KβKD platelets. Figure 1Aiii also shows that ADP-induced Akt phosphorylation was completely suppressed in both genotypes. This surprising and unexplained observation is actually in line with previous independent genetic and pharmacologic data,2,5,16,17 and it clearly indicates a complex interplay between the 2 isoforms in ADP signaling. These observations demonstrate that ADP-induced Rap1b activation and aggregation are only partially regulated by PI3Kγ and PI3Kβ kinase activity, and they question the role of Akt in these processes.
Analysis of platelet activation in PI3KγKD and PI3KβKD mice. ADP (A), U46619 (B), or convulxin (C) induced platelet aggregation (i), Rap1b activation (ii), and Akt phosphorylation (iii) in wild-type (WT), PI3KγKD (γKD), and PI3KβKD (βKD) mice. (i) Platelet aggregation was stimulated by 1, 5, or 10 μM ADP (in the presence of 200 μg/mL fibrinogen) or U46619, or with 50 and 100 ng/mL convulxin (CVX). The percentage of platelet aggregation was determined 5 minutes after addition of the agonist. Aggregation of PI3KγKD was also measured upon preincubation with 0.5 μM TGX221 (a gift from Dr Peter R. Shepherd, University of Auckland, Auckland, New Zealand) for 5 minutes. For each agonist, maximal aggregation induced by the highest dose of agonist was considered as 100%. In the case of CVX stimulation, the dose of 100 ng/mL was sufficient to trigger maximal platelet aggregation (light transmission higher than 95%) under our experimental conditions. Results are the mean ± SD of 3 (A-B) or 6 (C) different experiments (where not visible, SD bar is masked by the mark). Traces are as follows: ●, wild-type platelets; ■, PI3KγKD platelets; □, PI3KγKD platelets treated with TGX221; ♦, PI3KβKD platelets. Analysis with t test performed at each dose of agonists revealed that aggregation of both PI3KβKD and PI3KγKD platelets stimulated with ADP or U46619 was always significantly different from that of wild-type platelets (P < .01). Upon stimulation with CVX, PI3KβKD platelets showed significantly reduced aggregation relative to wild-type platelets at both concentrations tested, whereas aggregation of PI3KγKD platelets was significantly reduced relative to that of wild-type platelets only at 100 ng/mL (P < .05). (ii) Analysis of Rap1b activation was performed upon platelet stimulation with 1 or 5 μM ADP or U46619 or with 50 ng/mL CVX for 60 seconds. When indicated, stimulation of platelets was also performed upon preincubation with 0.5 μM TGX221 (+ TGX) for 5 minutes, while Rap1b activation induced by CVX was also analyzed in the presence of 2 U/mL apyrase (APY). Active Rap1b was precipitated with GST-tagged RalGDS-RBD and visualized by immunoblotting with a specific antibody. Identical aliquots of total cell lysates from each sample were also tested for the total Rap1b expression by immunoblotting. Quantitative analysis was performed by densitometric scanning of the immunoblots. For ADP and U46619, variable results were obtained, and thus histograms summarizing the quantitative analysis of 3 different experiments for wild-type (■), PI3KγKD (□), and PI3KβKD ( ) platelets are reported. Statistically significant differences in Rap1b activation in the PI3KγKD or PI3KβKD versus wild-type platelets (t test, P < .05) are indicated by *. For CVX-treated platelets, a representative immunoblot of 3 identical experiments is reported, as either a normal or a completely inhibited activation of Rap1b was constantly observed. (iii) Wild-type, PI3KγKD, and PI3KβKD platelets were stimulated with 5 μM ADP, 5 μM U46619, or 50 ng/mL CVX for the indicated times. When indicated, platelets were preincubated with 0.5 μM TGX221 for 5 minutes before stimulation. Proteins from identical aliquots of whole-cell lysates were separated by SDS-PAGE on a 10% acrylamide gel, transferred to polyvinylidene difluoride, and probed with anti–phospho-Akt (Ser473) antibody (top panels). Membranes were stripped and then reprobed with anti-Akt antibody (bottom panels). Immunoblots representative of 3 different experiments giving identical clearcut results are reported.
) platelets are reported. Statistically significant differences in Rap1b activation in the PI3KγKD or PI3KβKD versus wild-type platelets (t test, P < .05) are indicated by *. For CVX-treated platelets, a representative immunoblot of 3 identical experiments is reported, as either a normal or a completely inhibited activation of Rap1b was constantly observed. (iii) Wild-type, PI3KγKD, and PI3KβKD platelets were stimulated with 5 μM ADP, 5 μM U46619, or 50 ng/mL CVX for the indicated times. When indicated, platelets were preincubated with 0.5 μM TGX221 for 5 minutes before stimulation. Proteins from identical aliquots of whole-cell lysates were separated by SDS-PAGE on a 10% acrylamide gel, transferred to polyvinylidene difluoride, and probed with anti–phospho-Akt (Ser473) antibody (top panels). Membranes were stripped and then reprobed with anti-Akt antibody (bottom panels). Immunoblots representative of 3 different experiments giving identical clearcut results are reported.
Analysis of platelet activation in PI3KγKD and PI3KβKD mice. ADP (A), U46619 (B), or convulxin (C) induced platelet aggregation (i), Rap1b activation (ii), and Akt phosphorylation (iii) in wild-type (WT), PI3KγKD (γKD), and PI3KβKD (βKD) mice. (i) Platelet aggregation was stimulated by 1, 5, or 10 μM ADP (in the presence of 200 μg/mL fibrinogen) or U46619, or with 50 and 100 ng/mL convulxin (CVX). The percentage of platelet aggregation was determined 5 minutes after addition of the agonist. Aggregation of PI3KγKD was also measured upon preincubation with 0.5 μM TGX221 (a gift from Dr Peter R. Shepherd, University of Auckland, Auckland, New Zealand) for 5 minutes. For each agonist, maximal aggregation induced by the highest dose of agonist was considered as 100%. In the case of CVX stimulation, the dose of 100 ng/mL was sufficient to trigger maximal platelet aggregation (light transmission higher than 95%) under our experimental conditions. Results are the mean ± SD of 3 (A-B) or 6 (C) different experiments (where not visible, SD bar is masked by the mark). Traces are as follows: ●, wild-type platelets; ■, PI3KγKD platelets; □, PI3KγKD platelets treated with TGX221; ♦, PI3KβKD platelets. Analysis with t test performed at each dose of agonists revealed that aggregation of both PI3KβKD and PI3KγKD platelets stimulated with ADP or U46619 was always significantly different from that of wild-type platelets (P < .01). Upon stimulation with CVX, PI3KβKD platelets showed significantly reduced aggregation relative to wild-type platelets at both concentrations tested, whereas aggregation of PI3KγKD platelets was significantly reduced relative to that of wild-type platelets only at 100 ng/mL (P < .05). (ii) Analysis of Rap1b activation was performed upon platelet stimulation with 1 or 5 μM ADP or U46619 or with 50 ng/mL CVX for 60 seconds. When indicated, stimulation of platelets was also performed upon preincubation with 0.5 μM TGX221 (+ TGX) for 5 minutes, while Rap1b activation induced by CVX was also analyzed in the presence of 2 U/mL apyrase (APY). Active Rap1b was precipitated with GST-tagged RalGDS-RBD and visualized by immunoblotting with a specific antibody. Identical aliquots of total cell lysates from each sample were also tested for the total Rap1b expression by immunoblotting. Quantitative analysis was performed by densitometric scanning of the immunoblots. For ADP and U46619, variable results were obtained, and thus histograms summarizing the quantitative analysis of 3 different experiments for wild-type (■), PI3KγKD (□), and PI3KβKD ( ) platelets are reported. Statistically significant differences in Rap1b activation in the PI3KγKD or PI3KβKD versus wild-type platelets (t test, P < .05) are indicated by *. For CVX-treated platelets, a representative immunoblot of 3 identical experiments is reported, as either a normal or a completely inhibited activation of Rap1b was constantly observed. (iii) Wild-type, PI3KγKD, and PI3KβKD platelets were stimulated with 5 μM ADP, 5 μM U46619, or 50 ng/mL CVX for the indicated times. When indicated, platelets were preincubated with 0.5 μM TGX221 for 5 minutes before stimulation. Proteins from identical aliquots of whole-cell lysates were separated by SDS-PAGE on a 10% acrylamide gel, transferred to polyvinylidene difluoride, and probed with anti–phospho-Akt (Ser473) antibody (top panels). Membranes were stripped and then reprobed with anti-Akt antibody (bottom panels). Immunoblots representative of 3 different experiments giving identical clearcut results are reported.
) platelets are reported. Statistically significant differences in Rap1b activation in the PI3KγKD or PI3KβKD versus wild-type platelets (t test, P < .05) are indicated by *. For CVX-treated platelets, a representative immunoblot of 3 identical experiments is reported, as either a normal or a completely inhibited activation of Rap1b was constantly observed. (iii) Wild-type, PI3KγKD, and PI3KβKD platelets were stimulated with 5 μM ADP, 5 μM U46619, or 50 ng/mL CVX for the indicated times. When indicated, platelets were preincubated with 0.5 μM TGX221 for 5 minutes before stimulation. Proteins from identical aliquots of whole-cell lysates were separated by SDS-PAGE on a 10% acrylamide gel, transferred to polyvinylidene difluoride, and probed with anti–phospho-Akt (Ser473) antibody (top panels). Membranes were stripped and then reprobed with anti-Akt antibody (bottom panels). Immunoblots representative of 3 different experiments giving identical clearcut results are reported.
The limited and redundant contribution of PI3Kγ and PI3Kβ to platelet activation by GPCR stimulation was even more evident when U46619 was used as an agonist. In this case, inhibition of platelet aggregation was reduced only by approximately 30% in both genotypes, and there was no additional effect of TGX221 on either PI3KγKD platelets (Figure 1Bi) or PI3KβKD platelets (data not shown). The latter finding confirms the specificity of this inhibitor. U46619-induced activation of Rap1b was dependent on both PI3Kγ and PI3Kβ (Figure 1Bii). In fact, although at 1 μM U46619 activation of Rap1b was only minimally affected by inactivation of PI3Kγ, but was almost ablated in PI3KβKD platelets, upon stimulation with 5 μM approximately 50% of Rap1b activation was independent of either enzyme. In contrast, U46619-induced Akt phosphorylation was exclusively promoted by PI3Kβ, as it was undetectable in PI3KβKD platelets, but occurred normally in PI3KγKD platelets. Accordingly, TGX221 completely inhibited Akt phosphorylation in both wild-type and PI3KγKD platelets. Once again, these results indicate that Akt is not a necessary mediator of PI3K contribution to platelet activation.
Important differences between PI3KγKD and PI3KβKD mice were found upon stimulation of the ITAM-bearing receptor GPVI by convulxin. Aggregation of PI3KγKD platelets was only slightly reduced compared with wild type, but it was almost completely suppressed in PI3KβKD platelets (Figure 1Ci). Moreover, addition of TGX221 to PI3KγKD platelets completely abolished the residual convulxin-induced aggregation. The predominant contribution of PI3Kβ in convulxin-promoted platelet activation was confirmed by the analysis of Akt phosphorylation and Rap1b activation (Figure 1Cii-iii). Both of these events were completely undetectable in stimulated PI3KβKD platelets but occurred normally in PI3KγKD platelets. Moreover, convulxin-induced Akt and Rap1b activation in PI3KγKD platelets was completely suppressed by TGX221. GPVI-promoted Rap1b activation is partially dependent on secreted ADP,18 and actually it was decreased in the presence of apyrase. However, even in the presence of apyrase, convulxin-induced activation of Rap1b was still dependent exclusively on PI3Kβ but not on PI3Kγ enzymatic activity (Figure 1Cii). These results indicate that the catalytic activity of PI3Kβ, but not of PI3Kγ, is involved in GPVI signaling and is required for Akt phosphorylation, Rap1b activation, and platelet aggregation.
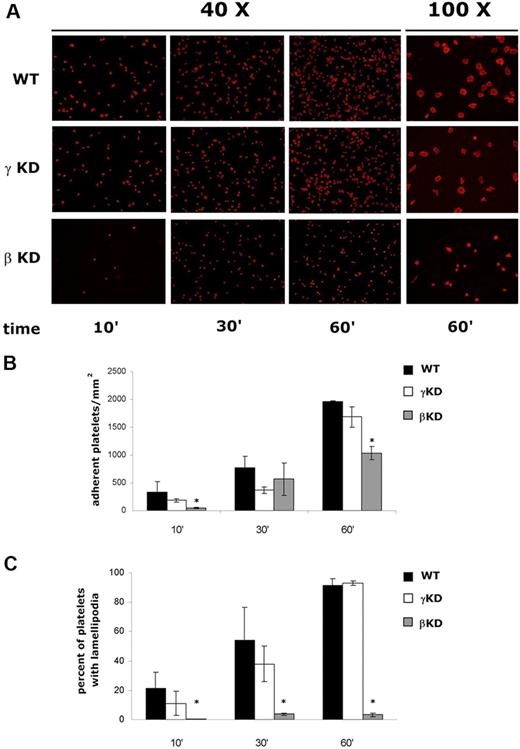
Finally, we analyzed the involvement of PI3Kβ and PI3Kγ in integrin αIIbβ3-mediated outside-in signaling. An evident and significant reduction of adhesion and spreading on immobilized fibrinogen was observed in the PI3KβKD but not in the PI3KγKD platelets (Figure 2A). Quantitative analysis confirmed that apparent reduction of PI3KγKD adhesion was not statistically significant, and that, in contrast, the defect of PI3KβKD platelet adhesion was more pronounced and statistically significant both at an early time point and late time points where a reduction of approximately 50% was measured (Figure 2B). According to previous findings,12 we found that catalytic inactivation of PI3Kγ slowed the initial rate of platelet spreading on fibrinogen, but this initial small defect, which was not statistically significant, was completely recovered within 60 minutes (Figure 1C). In contrast, PI3KβKD platelets were almost completely unable to spread on fibrinogen at every time point analyzed (Figure 1C).
Analysis of integrin αIIbβ3–mediated platelet adhesion and spreading. Platelets from wild type (WT), PI3KγKD (γKD), and PI3KβKD (βKD) mice were plated on glass coverslips coated with fibrinogen for 10, 30, and 60 minutes. Nonadherent cells were removed, and adherent platelets were fixed, permeabilized, and stained with TRITC-conjugated phalloidin. Both platelet adhesion, evaluated as number of counted adherent platelets, and platelet spreading, as number of adherent cells with lamellipodia extension were quantified by fluorescence microscopy analysis. Representative images at 40× magnification of adherent platelets from the 3 genotypes are reported in panel A. The column on the right reports enlarged images (100× magnification) of adherent platelets after 60 minutes to better evidentiate differences in the morphology of spreaded cells. Quantitative analysis of adhesion of wild type (■), PI3KγKD (□), and PI3KβKD ( ) platelets are reported in panels B and C, respectively. Platelet spreading is expressed as percentage of adherent platelets with lamellipodia. Data are the means ± SD of 5 different experiments, where adherent and spreaded cells in 5 different fields were counted in each experiment (*P < .05 vs wild-type platelets).
) platelets are reported in panels B and C, respectively. Platelet spreading is expressed as percentage of adherent platelets with lamellipodia. Data are the means ± SD of 5 different experiments, where adherent and spreaded cells in 5 different fields were counted in each experiment (*P < .05 vs wild-type platelets).
Analysis of integrin αIIbβ3–mediated platelet adhesion and spreading. Platelets from wild type (WT), PI3KγKD (γKD), and PI3KβKD (βKD) mice were plated on glass coverslips coated with fibrinogen for 10, 30, and 60 minutes. Nonadherent cells were removed, and adherent platelets were fixed, permeabilized, and stained with TRITC-conjugated phalloidin. Both platelet adhesion, evaluated as number of counted adherent platelets, and platelet spreading, as number of adherent cells with lamellipodia extension were quantified by fluorescence microscopy analysis. Representative images at 40× magnification of adherent platelets from the 3 genotypes are reported in panel A. The column on the right reports enlarged images (100× magnification) of adherent platelets after 60 minutes to better evidentiate differences in the morphology of spreaded cells. Quantitative analysis of adhesion of wild type (■), PI3KγKD (□), and PI3KβKD ( ) platelets are reported in panels B and C, respectively. Platelet spreading is expressed as percentage of adherent platelets with lamellipodia. Data are the means ± SD of 5 different experiments, where adherent and spreaded cells in 5 different fields were counted in each experiment (*P < .05 vs wild-type platelets).
) platelets are reported in panels B and C, respectively. Platelet spreading is expressed as percentage of adherent platelets with lamellipodia. Data are the means ± SD of 5 different experiments, where adherent and spreaded cells in 5 different fields were counted in each experiment (*P < .05 vs wild-type platelets).
In conclusion, our comparative analysis of murine platelets expressing catalytically inactive forms of PI3Kβ and PI3Kγ demonstrated that both of these isoforms are involved, at different extents and with a complex interplay, in GPCR-mediated platelet activation, suggesting the potential usefulness of double selective inhibitors. In contrast, we have demonstrated that PI3Kβ is essential for the tyrosine phosphorylation-based signaling pathways triggered by GPVI or αIIbβ3. PI3Kβ has been recently suggested as being mainly activated by GPCRs.19 Our data demonstrate that PI3Kβ is a common signaling platform shared by both ITAM signaling and GPCRs, but they also indicate that it plays a predominant role in ITAM- and integrin-mediated platelet activation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ministero dell'Istruzione, Università e Ricerca Scientifica (PRIN 2006; C.B.) and from the Regione Lombardia and the University of Pavia (project REGLOM16; I.C.).
Authorship
Contribution: I.C. and L.S. designed and performed experiments and analyzed data; L.C. and C.G. performed experiments; E.C. provided vital reagents and performed experiments; C.B. analyzed data and edited the manuscript; E.H. contributed vital new reagents, analyzed data, and edited the manuscript; and M.T. designed research, analyzed data, wrote the manuscript, and provided overall direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for Dr Stefanini is Department of Medicine, Thomas Jefferson University, Philadelphia, PA 19107.
Correspondence: Mauro Torti, Department of Biochemistry, University of Pavia, via Bassi 21, 27100 Pavia, Italy; e-mail: mtorti@unipv.it.
References
Author notes
I.C. and L.S. contributed equally to this work.
E.H. and M.T. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal