Abstract
Red blood cells deliver O2 from the lungs to every cell in the human body. Reduced tissue oxygenation triggers increased production of erythropoietin by hypoxia-inducible factor 1 (HIF-1), which is a transcriptional activator composed of an O2-regulated α subunit and a constitutively expressed β subunit. Hydroxylation of HIF-1α or HIF-2α by the asparaginyl hydroxylase FIH-1 blocks coactivator binding and transactivation. Hydroxylation of HIF-1α or HIF-2α by the prolyl hydroxylase PHD2 is required for binding of the von Hippel-Lindau protein (VHL), leading to ubiquitination and proteasomal degradation. Mutations in the genes encoding VHL, PHD2, and HIF-2α have been identified in patients with familial erythrocytosis. Patients with Chuvash polycythemia, who are homozygous for a missense mutation in the VHL gene, have multisystem pathology attributable to dysregulated oxygen homeostasis. Intense efforts are under way to identify small molecule hydroxylase inhibitors that can be administered chronically to selectively induce erythropoiesis without undesirable side effects.
Introduction
The evolution of vertebrate species of far greater mass and somatic complexity than invertebrates involved the appearance of specialized circulatory systems to insure that every cell received adequate O2. The most significant innovation within the circulatory system was the erythrocyte, a cell specialized for the transport of gases (O2, CO2, and NO) in and out of the organism. This review focuses on the molecular mechanisms that regulate red blood cell production as a function of tissue oxygenation and hereditable disorders of oxygen homeostasis that result in excess erythropoiesis.
Molecular mechanism of oxygen sensing
In mammals, O2 sensing occurs at many levels, leading to both acute and chronic adaptation. The carotid body, which is located at the bifurcation of the internal and external carotid arteries, contains highly specialized chemosensory cells. These glomus cells depolarize in response to reduction in arterial blood PO2 (hypoxemia), resulting in stimulation of the brain stem centers that control the respiratory and cardiovascular systems, which leads to rapid changes in ventilation, heart rate, and blood pressure that serve to increase O2 uptake in the lungs and O2 delivery to the tissues.1
The second major systemic O2-sensing system is located in the inner cortex and outer medulla of the kidney, where specialized interstitial cells respond to decreased tissue PO2 (hypoxia), resulting from, for example, hemorrhage or anemia, by producing and secreting into the circulation erythropoietin (EPO), a glycoprotein hormone that binds to erythroid progenitors in bone marrow and stimulates their survival, proliferation, and differentiation.2 In contrast to the rapidity of the responses mediated by the carotid body, the increase in blood O2-carrying capacity that is mediated by EPO occurs on the order of days rather than seconds, reflecting the developmental program of erythropoiesis.
Analysis of the human3,4 and mouse5 genes encoding EPO revealed the presence of a cis-acting hypoxia response element, which was located in the 3′-flanking region. Under hypoxic conditions, the hypoxia response element was occupied by a DNA-binding protein, which was designated hypoxia-inducible factor 1 (HIF-1).6 The purification of HIF-1 by DNA affinity chromatography revealed that it is a heterodimeric protein consisting of HIF-1α and HIF-1β subunits,7 and cDNA isolation revealed that both subunits were members of a subfamily of basic helix-loop-helix transcription factors that contain a PAS domain, which is an accessory dimerization domain that was originally identified as the Per, Arnt, and Sim proteins.8
Analysis of knockout mice revealed that expression of HIF-1α and HIF-1β is required for normal development of the heart, blood vessels, and blood cells; that is, all 3 components of the circulatory system are dependent on HIF-1.9-12 In addition to its broad effects on development, HIF-1 mediates a multitude of cellular and systemic physiologic and pathophysiologic responses to hypoxia/ischemia in postnatal life.13
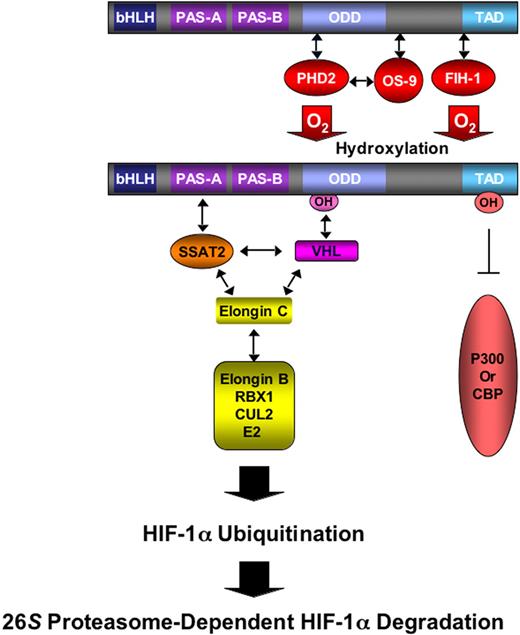
Levels of the HIF-1α subunit increase exponentially as O2 concentration declines.14 The induction of HIF-1α protein levels is the result of reduced ubiquitination and proteasomal degradation of the protein under hypoxic conditions.15 Under normoxic conditions, HIF-1α is hydroxylated at proline residue 402 or 564, by prolyl hydroxylase domain proteins 1 to 3,16,17 particularly PHD2.18 Prolyl hydroxylation is required for binding of the von Hippel-Lindau protein (VHL),19-22 which is the recognition subunit of an E3 ubiquitin-protein ligase consisting of Elongin C, Elongin B, RBX1, Cullin 2, and an E2 ubiquitin-conjugating enzyme (Figure 1). OS9 is a protein that binds to both HIF-1α and PHD2 and stabilizes their interaction, thereby facilitating prolyl hydroxylation.23 SSAT2 is a protein that binds to HIF-1α, VHL, and Elongin C and stabilizes their interaction, thereby facilitating ubiquitination.24 Under hypoxic conditions, hydroxylase activity is inhibited and HIF-1α accumulates, dimerizes with HIF-1β, and activates transcription of target genes. Factor inhibiting HIF-1 (FIH-1) binds to HIF-1α and inhibits its transactivation function25 under normoxic conditions by hydroxylating asparagine residue 803, which blocks interactions with coactivator proteins.26
O2-dependent hydroxylation regulates HIF-1α ubiquitination and transactivation. The prolyl hydroxylase PHD2 binds to the oxygen-dependent degradation domain (ODD) of HIF-1α and, in the presence of O2, hydroxylates proline residue 402 and/or 564, which is required for the binding of VHL, which then recruits Elongin C and its associated E3 ubiquitin-protein ligase complex, which consists of Elongin B, RBX1, Cullin 2 (CUL2), and an E2 ubiquitin-conjugating enzyme. The hydroxylation by FIH-1 of asparagine-803 in the carboxyl-terminal transactivation domain of HIF-1α blocks coactivator (p300 or CBP) binding and HIF-1 transcriptional activity.
O2-dependent hydroxylation regulates HIF-1α ubiquitination and transactivation. The prolyl hydroxylase PHD2 binds to the oxygen-dependent degradation domain (ODD) of HIF-1α and, in the presence of O2, hydroxylates proline residue 402 and/or 564, which is required for the binding of VHL, which then recruits Elongin C and its associated E3 ubiquitin-protein ligase complex, which consists of Elongin B, RBX1, Cullin 2 (CUL2), and an E2 ubiquitin-conjugating enzyme. The hydroxylation by FIH-1 of asparagine-803 in the carboxyl-terminal transactivation domain of HIF-1α blocks coactivator (p300 or CBP) binding and HIF-1 transcriptional activity.
The proline (PHD2) and asparagine (FIH-1) hydroxylases both use O2 and α-ketoglutarate (also known as 2-oxoglutarate) as substrates and generate CO2 and succinate as byproducts.27 The catalytic center of these dioxygenases contains nonheme Fe (II), which is oxidized to Fe (III) during the hydroxylation reaction, with ascorbate required for regeneration of Fe (II). As a result, the activity of the hydroxylases may be affected by changes in the concentration of O2, iron, and divalent metal ions, ascorbate, reactive oxygen species, nitric oxide, and tricarboxylic acid cycle intermediates,28 suggesting that they play a key role in the integration and maintenance of energy, oxygen, and redox homeostasis.
In addition to ubiquitination, another modification that regulates HIF-1α protein stability is covalent ligation of the small protein SUMO, which promotes the binding of VHL to HIF-1α in a hydroxylation-independent manner.29 Ligation of SUMO to HIF-1α is reversed by the SUMO-specific protease SENP1, deficiency of which results in deficient EPO production, severe anemia, and embryonic lethality of mice at midgestation.29
Using the HIF-1α cDNA sequence to search databases, cDNA sequences encoding a related protein, now designated HIF-2α, were identified.30-32 HIF-2α is also subject to O2-dependent hydroxylation, ubiquitination, and proteasomal degradation, whereas under hypoxic conditions it dimerizes with HIF-1β and activates the transcription of a set of target genes, which overlaps with the target genes regulated by HIF-1α/HIF-1β heterodimers.33 Whereas HIF-1α is expressed in all nucleated cells, the expression of HIF-2α is restricted to specific cell types, including vascular endothelial cells, renal interstitial cells, hepatocytes, cardiomyocytes, and astrocytes.34 HIF-2α appears to plays a key role in regulating EPO production and erythropoiesis in adult mice,35-37 although HIF-1α is also required for yolk sac erythropoiesis,12 renal EPO production in response to acute hypoxic episodes,38 and the development of polycythemia in response to chronic hypoxia.39 Whereas HIF-1α homologues are present in all metazoan species studied, it appears that HIF-2α arose coincident with the evolution of complex respiratory and circulatory systems in vertebrates.
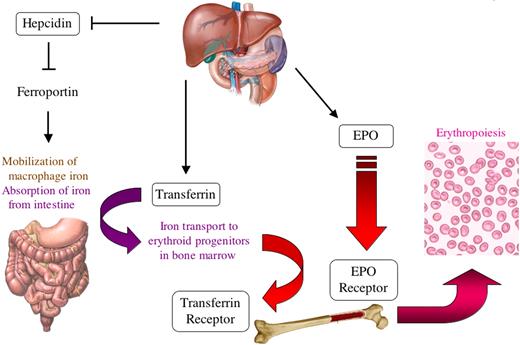
The effect of HIF-1 and HIF-2 (ie, HIF-1α/HIF-1β and HIF-2α/HIF-1β heterodimers, respectively) on erythropoiesis is profound and illustrates the concept that HIFs function as master regulators of oxygen homeostasis (Figure 2). In addition to the control of EPO production by HIF-1 and HIF-2, HIF-1 also regulates expression of the EPO receptor.12,40 Furthermore, HIF-1 controls the absorption and delivery of iron to the bone marrow for incorporation into the hemoglobin of red blood cells through its repression of the gene encoding hepcidin41 and activation of the genes encoding transferrin42 and transferrin receptor,43,44 which again illustrates the interplay between iron and oxygen homeostasis.
HIF-1 regulates the expression of multiple genes to stimulate erythropoiesis in response to hypoxia. HIF-1 stimulates production of the EPO in the kidney, which binds to its receptor (EPOR) on erythroid progenitors in the bone marrow (in the adult and yolk sac in the embryo) to stimulate their survival, proliferation, and differentiation. Erythropoiesis involves uptake by the marrow of large amounts of iron, which are used in the synthesis of hemoglobin. In the liver, HIF-1 stimulates iron uptake by repressing the gene encoding hepcidin, which is an inhibitor of ferroportin, the major protein responsible for intestinal iron uptake. HIF-1 also activates hepatic synthesis of transferrin, the major plasma protein responsible for transporting iron from the intestine to the bone marrow via the transferrin receptor. Thus, HIF-1 directly regulates the expression of 5 gene products (EPO, EPOR, hepcidin, transferrin, and transferrin receptor) involving 5 different organs (kidney, liver, intestine, blood, and bone marrow) to control erythropoiesis.
HIF-1 regulates the expression of multiple genes to stimulate erythropoiesis in response to hypoxia. HIF-1 stimulates production of the EPO in the kidney, which binds to its receptor (EPOR) on erythroid progenitors in the bone marrow (in the adult and yolk sac in the embryo) to stimulate their survival, proliferation, and differentiation. Erythropoiesis involves uptake by the marrow of large amounts of iron, which are used in the synthesis of hemoglobin. In the liver, HIF-1 stimulates iron uptake by repressing the gene encoding hepcidin, which is an inhibitor of ferroportin, the major protein responsible for intestinal iron uptake. HIF-1 also activates hepatic synthesis of transferrin, the major plasma protein responsible for transporting iron from the intestine to the bone marrow via the transferrin receptor. Thus, HIF-1 directly regulates the expression of 5 gene products (EPO, EPOR, hepcidin, transferrin, and transferrin receptor) involving 5 different organs (kidney, liver, intestine, blood, and bone marrow) to control erythropoiesis.
Hereditable disorders of oxygen sensing
The first hereditable disorder demonstrated to result from mutations affecting oxygen sensing is congenital Chuvash polycythemia (erythrocytosis, familial, type 2, Online Mendelian Inheritance in Man [OMIM] 263400), an autosomal-recessive disorder with high prevalence in the Chuvash population of the Russian Federation in which affected persons are homozygous for an arginine-to-tryptophan missense mutation at codon 200 (R200W) of the VHL gene that results in excessive red blood cell production.45 The R200W substitution reduces the interaction of VHL with hydroxylated HIF-1α, thereby reducing the O2-dependent ubiquitination and proteasomal degradation of HIF-1α.45 As a result, at any given O2 concentration, HIF-1 activity will be increased in cells expressing VHLR200W compared with cells expressing wild-type VHL. This is dramatically illustrated by the observation that, when partial exchange transfusion is performed to lower the hematocrit in patients with Chuvash polycythemia, EPO levels begin to rise even before a normal hematocrit is attained. Not surprisingly, mice homozygous for a targeted R200W mutation of the murine Vhl gene are also polycythemic.46
Although polycythemia is the most easily detected phenotypic manifestation of the VHLR200W/R200W genotype, recent studies indicate that oxygen sensing is globally dysregulated.47 Persons with Chuvash polycythemia were found to have striking abnormalities in respiratory and pulmonary vascular regulation. Basal ventilation and pulmonary vascular tone were elevated, and ventilatory, pulmonary vascular, and heart rate responses to acute hypoxia were greatly increased compared with controls. The increased magnitude of the physiologic responses to hypoxia in these patients, who have HIF-1 gain-of-function resulting from the hypomorphic VHLR200W alleles, mirror the decreased responses observed in Hif1a+/− mice, which have HIF-1 loss of function resulting from heterozygosity for a knockout allele at the locus encoding HIF-1α.39,48,49
Other abnormalities in persons with Chuvash polycythemia have been identified, including increased incidence of varicose veins, venous thrombosis, bleeding diatheses, vertebral hemangiomas, reduced systemic blood pressure, reduced white blood cell and platelet counts, pulmonary hypertension, cerebral vascular events, and premature mortality.50,51 These findings indicate that dysregulation of oxygen homeostasis has multiple, profound, systemic pathologic effects.
The molecular delineation of Chuvash polycythemia demonstrated that mutation of a component of the oxygen-sensing pathway causes congenital polycythemia/erythrocytosis. The intimate relationship between oxygen sensing and erythropoiesis was further underscored when an autosomal-dominant form of erythrocytosis (erythrocytosis, familial, type 3, OMIM 609820) was found to be the result of a proline-to-arginine missense mutation at codon 317 (P317R) in the EGLN1 gene encoding PHD2, which renders the prolyl hydroxylase protein defective in its ability to bind and hydroxylate HIF-1α.52 The substituted amino acid is only 2 residues away from aspartate-315, which binds Fe (II) directly, and close to the catalytic site entrance in the crystal structure, suggesting that the P317R mutation may affect both Fe (II) and substrate binding.53
Subsequently, a person with erythrocytosis was found to be heterozygous for an arginine-to-histidine missense mutation at codon 371 (R371H) of EGLN1.54 Residue 371 is 3 amino acids away from histidine-374, which is involved in iron binding. Thus, the R371H mutation is remarkably similar to the P317R mutation, which is also located close to an iron-binding residue, histidine-313. As expected, proline-317 and arginine-371 lie close to one another in the crystal structure of PHD2.52 Arginine-371 is conserved in all 3 mammalian PHD isoforms, as well as the single PHD isoform in Drosophila melanogaster and Caenorhabditis elegans.
Although conditional knockout of PHD2 in adult mice leads to massive and lethal erythrocytosis, heterozygosity for a complete loss-of-function allele does not result in erythrocytosis.55 It is possible that the erythrocytosis associated with partial PHD2 deficiency develops over a prolonged period of time and was not detected or that PHD2 deficiency was complemented by compensatory increases in PHD1 or PHD3 expression. Alternatively, the mutant human PHD2 proteins may have dominant-negative effects (ie, the mutant protein interferes with the function of the wild-type protein). The finding that the VHL missense mutation must be in the homozygous state for phenotypic manifestations also suggests the possibility that the PHD2 mutations have dominant-negative effects.
Finally, in addition to the loss-of-function mutations in VHL and PHD2, a gain-of-function mutation in the EPAS1 gene encoding HIF-2α was reported as another cause of familial erythrocytosis (type 4, OMIM 611783) with autosomal-dominant inheritance.56 The mutation changes glycine residue 537, which is conserved in human, mouse, chicken, frog, and zebrafish HIF-2α to tryptophan (G537W). Glycine-537 is located carboxy terminal to proline-531, which is the hydroxylated residue in HIF-2α that is analogous to proline-564 in HIF-1α. The G537W mutation impairs the interaction of HIF-2α with PHD2 and therefore reduces hydroxylation.56 Subsequently, additional cases of familial erythrocytosis were found to be the result of G537R, M535V, and M535I missense mutations at the EPAS1 locus.57,58 Unlike the mutations in VHL and PHD2, there is no evidence that the mutations in EPAS1 affect HIF-1α protein levels, and this finding therefore complements loss-of-function data from mice indicating that HIF-2α plays a critical role in regulating erythropoiesis. Thus far, no studies have reported whether patients with familial erythrocytosis resulting from missense mutations affecting PHD2 or HIF-2α manifest additional evidence of dysregulated oxygen homeostasis as described for persons with Chuvash polycythemia.
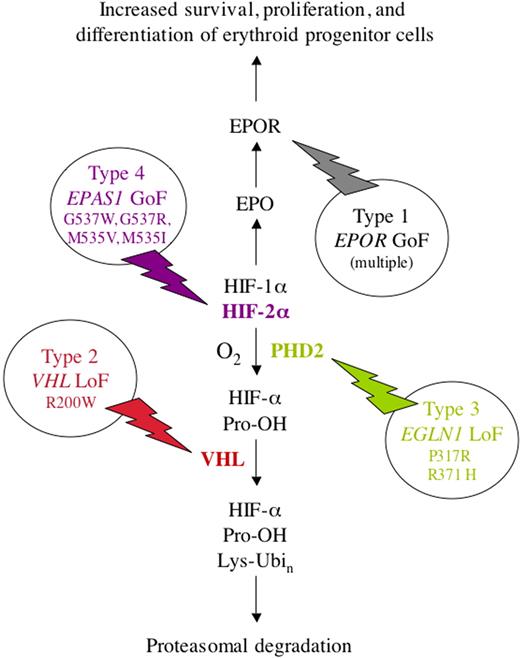
Thus, 15 years after analysis of EPO gene regulatory elements led to the discovery of HIF-1,6 which led in turn to the discovery of VHL19 and the PHDs,16,17 mutations in genes encoding all 3 of these essential components of the oxygen-sensing system have been identified in humans with familial erythrocytosis (Figure 3). Gain-of-function mutations in the EPO receptor also cause familial erythrocytosis (type 1, OMIM 133100).59 It is probable that additional patients with mutations in loci encoding other components of the O2-sensing pathway remain to be identified.
Molecular basis of familial erythrocytosis. Four types are shown, resulting from loss-of-function (LoF) or gain-of-function (GoF) missense mutations in the genes encoding EPOR (type 1), VHL (type 2), PHD2 (type 3), and HIF-2α (type 4), respectively. Note that the symbols for the genes encoding PHD2 and HIF-2α are EGLN1 and EPAS1, respectively. The familial erythrocytoses are inherited as autosomal-dominant traits, except for type 2 (Chuvash polycythemia), which displays autosomal-recessive inheritance. A variety of mutations that result in a truncated and constitutively active EPOR have been reported as the cause of type 1 familial erythrocytosis.
Molecular basis of familial erythrocytosis. Four types are shown, resulting from loss-of-function (LoF) or gain-of-function (GoF) missense mutations in the genes encoding EPOR (type 1), VHL (type 2), PHD2 (type 3), and HIF-2α (type 4), respectively. Note that the symbols for the genes encoding PHD2 and HIF-2α are EGLN1 and EPAS1, respectively. The familial erythrocytoses are inherited as autosomal-dominant traits, except for type 2 (Chuvash polycythemia), which displays autosomal-recessive inheritance. A variety of mutations that result in a truncated and constitutively active EPOR have been reported as the cause of type 1 familial erythrocytosis.
Pharmacologic inhibition of hydroxylases
The identification of chemical compounds that can inhibit the PHDs, such as the α-ketoglutarate (2-oxoglutarate) analog dimethyloxalylglycine,16,60 provides a potential novel oral therapy for stimulating erythropoiesis in patients with chronic kidney disease, which is currently achieved by subcutaneous injection of recombinant human EPO. Clinical trials of prolyl hydroxylase inhibitors are under way (eg, NCT00456053, www.clinicaltrials.gov). However, it is not clear whether it will be possible to selectively stimulate erythropoiesis on a chronic basis without undesired side effects associated with PHD inhibition, such as the pathologic findings associated with Chuvash polycythemia as well as additional side effects that may occur because of the inhibition of other iron- and oxoglutarate-dependent dioxygenases.
Note added in proof.
A novel H374R mutation in EGLN1 has been reported in a patient manifesting both erythrocytosis and a paraganglioma with loss of the wild-type EGLN1 allele in the tumor (Ladroue C, Carcenac R, Leporrier M, et al. PHD2 mutation and congenital erythrocyosis with paraganglioma. N Engl J Med. 2008;359[25]:2685-2692).
Authorship
Contribution: G.L.S. wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Gregg L. Semenza, 733 North Broadway, Broadway Research Bldg, Suite 671, Baltimore, MD 21205; e-mail: gsemenza@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal