Abstract
This article discusses how we approach medical decision making in the treatment of the various facets of the antiphospholipid syndrome (APS), including secondary prophylaxis in the setting of venous and arterial thrombosis, as well as treatment for the prevention of recurrent miscarriages and fetal death. The role of primary thromboprophylaxis is also discussed in depth. Great emphasis is given to incorporating the most up-to-date and relevant evidence base both from the APS literature, and from large, recent, randomized controlled trials (RCTs) of primary and secondary thrombotic prophylaxis in the general population setting (ie, the population that has not been specifically investigated for APS).
Introduction
The antiphospholipid syndrome (APS) is characterized clinically by the occurrence of either venous or arterial thrombosis in diverse vascular beds, or recurrent miscarriages in the first trimester, or fetal death in the second or third trimesters, or severe pre-eclampsia necessitating delivery of a premature infant before 34 weeks of gestation.1 It is an important cause of acquired thrombophilia, with the diagnosis being particularly considered and made in younger age groups relative to the average age at which thrombosis occurs in the general population.2 Within the arterial circulation, cerebrovascular infarction is a prominent event, whereas lower limb deep venous thrombosis and pulmonary embolus are important locations in which venous pathology occurs.2
Three prominent features of the updated APS classification criteria are (1) the incorporation of the beta 2-glycoprotein I (β2GPI) enzyme-linked immunosorbent assay (ELISA) as a diagnostic test in addition to the cardiolipin ELISA (CL-ELISA) and the lupus anticoagulant (LA) assay(s), (2) the recommendation that classification of APS requires the persistence of antibodies for at least 12 weeks, and (3) the setting of definitive cutoff values for the CL-ELISA and the β2GPI-ELISA (see details in Miyakis et al1 regarding a detailed description of the current APS classification criteria, including cutoff values for the ELISAs).
The current classification criteria do not exclude the diagnosis of thrombotic APS being made in the presence of concurrent arterial and/or venous prothrombotic risk factors, such as the presence of atherosclerotic risk factors in the former instance. In the context of obstetric APS, the situation is distinct as the diagnosis is excluded if there is an identifiable alternate explanation for either recurrent first-trimester miscarriages or fetal death (second and third trimesters).
In 2 previous review articles published in this journal we have presented evidence for considering the importance of the antibodies, in particular anti-β2GPI antibodies with LA activity as having diagnostic (ie, etiopathogenic) significance.3,4 The concept of prognosis involves consideration of whether the detection of the autoantibodies alters the probability of thrombotic recurrence or death compared with not having the antibodies in patients who have otherwise had a similar initial thrombotic event and have received identical secondary prophylaxis. This is a theme explored in this review (see supplemental material for the literature search strategy used).
Venous thrombosis
Overview
A physician confronted with the management of a patient with venous thromboembolism (VTE) who satisfies the laboratory criteria of APS has to consider several issues. First, what is the optimal intensity of anticoagulation to use? The second issue is what is the optimal duration of therapy?
Intensity of anticoagulation
Crowther et al5 and Finazzi et al6 in their respective RCTs examined the question whether high-intensity anticoagulation (INR 3.1 to 4) is superior to moderate intensity (international normalized ratio [INR] 2-3) for secondary prophylaxis in patients who had an initial thrombotic event and satisfy the laboratory criteria for APS. The initial events included VTE and arterial thrombosis, though approximately 70% involved the venous circulation.
Both studies found that high-intensity anticoagulation was not superior to moderate-intensity therapy. The rates of major bleeding were similar between the 2 intensities in both studies. When the data from both studies were pooled, they suggested that high-intensity treatment is associated with an increased risk of minor bleeding complications.6
Both these RCTs were critical in our understanding of treating APS because they emphasized the limitations of using retrospective studies to guide management issues, such as the intensity of anticoagulation that should be used in thrombotic APS. A previous retrospective study had indicated that high intensity (INR ≥ 3) was superior to moderate-intensity anticoagulation (INR 2-3) for arterial and venous events.7 Hence in our opinion, we do not feel it is prudent to make definitive conclusions based on retrospective studies, such as specifically advocating high-intensity over moderate-intensity anticoagulation or antiplatelet therapy in the arterial thrombosis setting,8 pending an RCT specifically addressing this issue.
Duration of treatment
The next question a physician has to address in the management of venous APS is, what is the optimal duration of anticoagulation? It is relevant to acknowledge that RCTs in which the patients satisfy the APS classification criteria have not been undertaken to date to address this issue. Hence, in this context, a clinician has to incorporate other lines of evidence in their decision making.
Schulman et al9 in an RCT noted that patients with VTE who stopped anticoagulants at 6 months and were positive on the IgG CL-ELISA (LA was not tested) had higher rates of recurrence and death compared with patients who were treated for the same duration but who tested negative. Most recurrences tended to occur within the first 6 months of cessation of anticoagulation. Low and high titers of antibodies detected by the CL-ELISA were associated with increased risk, though it was greater with the latter.
Kearon et al10 performed an RCT study looking at duration of anticoagulation treatment (3 months vs prolonged) in patients who had a first VTE that occurred in the absence of cancer or an identifiable, reversible, provoking risk factor. On subgroup analysis, it was found that patients positive for LA at the time of randomization had an increased risk of recurrent VTE upon cessation of anticoagulation at 3 months from the incident event, compared with patients who were LA negative. They suggested that LA-positive patients may particularly benefit from extended anticoagulation beyond 3 months. Patients were followed up for an average of 10 months.
Findings from large RCTs that have attempted to delineate the optimal intensity of long-term anticoagulation in patients with idiopathic VTE are relevant to note. Ridker et al11 found that prolonged (mean follow-up: 2.1 years, maximum: 4.3 years), low-intensity anticoagulation (INR 1.5 to 2.0) was associated with superior clinical outcomes compared with placebo in patients who had initially received standard anticoagulation (INR 2-3) for the first 6 months. Kearon et al12 demonstrated in a similar group of patients (idiopathic VTE) that prolonged anticoagulation (mean time of follow-up: 2.4 years) with conventional intensity therapy (INR 2 to 3) is superior to low-intensity therapy (INR 1.5-1.9) in terms of preventing VTE recurrence, without increasing the bleeding risk. In both studies, patients with LA and anticardiolipin antibodies were excluded, whereas patients with certain genetic thrombophilias were included in the analysis.11,12
A meta-analysis of 8 RCTs (2994 patients)13 addressed the question of duration of anticoagulation after a VTE. The conclusion was that patients with symptomatic VTE are protected against recurrence while they continue to receive anticoagulation. However, the absolute risk of VTE recurrence declines over time since the incident event, whereas the risk of bleeding related to anticoagulation remains.
In the most recent American College of Chest Physicians VTE treatment guidelines,14 patients with VTE are broadly divided into 3 major categories for the purposes of treatment considerations: (a) those with cancer, (b) those in whom the VTE occurred in the setting of a reversible, provoking risk factor (ie, surgery, plaster cast immobilization, estrogen therapy, pregnancy, prolonged [> 8 hours] travel), and (c) unprovoked (ie, absence of factors included in group b, and includes patients with clearly delineated genetic thrombophilias, acquired thrombophilia [such as APS], and those designated as having an idiopathic VTE). It is recommended in the guidelines that patients who have had a first unprovoked proximal deep vein thrombosis (DVT) or pulmonary embolism (PE; ie, group c) be considered for long-term anticoagulation, independent of considerations such as the presence or absence of an identifiable genetic or acquired thrombophilia.14
This approach certainly seems reasonable on the basis of the evidence we have reviewed.11-13 However, it is also appropriate to note that there are alternate perspectives in the literature regarding the management of an unprovoked first VTE.15 Some advocate that the process of risk stratification undertaken by clinicians to guide duration of treatment not be based solely on whether the VTE was “provoked” or “unprovoked.”15 This complex issue has recently been debated in the literature,15,16 and a detailed analysis is beyond the scope of this review article. What can perhaps be agreed on is that on the basis of the evidence presented herein, if one were to take a stratified approach to guide which patients with an unprovoked VTE should be considered for long-term anticoagulation, one group of patients who should be strongly considered are those positive for antiphospholipid antibodies.9,10
As has been shown by Galli et al17 in their systematic review, LA associates more strongly with the risk of thrombosis than positivity on the CL-ELISA, hence greater weighting in the stratification process for duration of anticoagulation treatment would have to be given to patients persistently positive for LA. Emerging evidence, predominantly from retrospective studies, seems to suggest that the risk of thrombosis may be higher in patients who are positive for LA plus the CL and β2GPI ELISAs.18,19 With further prospective studies, antibody profiling may allow for a greater degree of stratification of APS patients with an initial unprovoked VTE.
Residual blood clot on lower limb deep-vein ultrasound has been shown to be useful for predicting an increased risk for VTE recurrence,20,21 as has an elevated D-DIMER22,23 1 month after cessation of anticoagulation. The utility of ultrasound in guiding treatment duration for proximal DVT has been shown in a recent randomized study, though the trial lacked a double-blind design and patients with APS were not included.24 Whether cessation of anticoagulation in an APS patient with an unprovoked proximal DVT (ie, absence of a concurrent, reversible, transient risk factor) is appropriate upon demonstrating clot resolution on ultrasound or the normalization of D-DIMER levels needs to be determined, as it may potentially allow avoiding bleeding risks related to prolonged, indefinite anticoagulation in patients who may be at low risk of recurrence.
As has been emphasized by others, it is important to be cognizant of the fact that patients with VTE in RCTs that have assessed prolonged duration anticoagulation are a select population, tending to exclude those deemed to be at an increased risk of hemorrhage or who are poorly compliant.25,26 Hence, within the “real-world” clinical setting it is important that the risks and benefits of prolonged anticoagulation be reassessed at regular intervals. A lower risk of recurrence exists if the incident event was provoked by a temporary risk factor (ie, surgery, trauma, or exposure to estrogen therapy).27 Other variables include location of the incident event—higher risk of recurrence with proximal DVT or PE versus distal DVT—and a higher risk exists in the setting of malignancy, male sex, older age (up to the age of 70 years), and obesity.28 A variable that has been suggested to predict an increased likelihood of VTE recurrence in the general population is clinical evidence of venous insufficiency.27
Variables that may be associated with bleeding having been extensively reviewed by Schulman et al29 and include anticoagulation intensity (risk of intracerebral hemorrhage increases dramatically with an INR > 4, the frequency of major bleeds doubles with an INR > 3 compared with an INR of 2-3), age (> 75 years), medical comorbidities (eg, prior bleeding, uncontrolled hypertension, cerebrovascular disease, at risk of falls, renal impairment, liver disease, alcohol or drug abuse, diabetes, anemia, low platelet counts, malignancy), and concurrent medication use (eg, aspirin and nonsteroidal anti-inflammatory drugs). Several bleeding prediction models have been developed to assist in assessing bleeding risk29 (see references therein).
For patients who have had an initial VTE that was specifically associated with a transient, reversible risk factor such as surgery, and who are known to satisfy the laboratory criteria for APS, the optimal duration of anticoagulation has not been determined with an RCT. In an RCT study by Kearon et al30 comparing 1 month with 3 months of anticoagulation for a proximal DVT or PE provoked by a transient, reversible risk factor, it was noted on subgroup analysis that testing positive for antiphospholipid antibodies (IgG and/or IgM anticardiolipin, and/or LA) at the time of randomization was not associated with an increased risk of VTE recurrence compared with patients who were antibody negative.
Our interpretation is that on the basis of the current, limited available evidence a reasonable argument can be made for considering a finite duration of anticoagulation for a first VTE in a patient who satisfies the laboratory criteria of APS if it occurs in the context of a transient, reversible provoking risk factor. This is similar to the strategy undertaken for a proximal DVT and PE associated with a reversible, transient risk factor in the general population (ie, treatment for a duration of 3 months).14 An alternate strategy, if the appropriate radiologic expertise is available, is to assess for residual blood clot in the lower limb veins with Doppler ultrasound at 3 months, continuing with anticoagulation for a further finite period if residual clot is found.24 This may be prudent in view of findings that suggest that APS autoantibodies may impair fibrinolysis,31 and may theoretically at least delay clot resolution, as suggested in a murine APS thrombosis model.32
Arterial thrombosis
Overview
Stroke is a major arterial manifestation of APS. The important questions confronting the physician managing such a patient are, should they choose an antiplatelet agent such as aspirin, or should an oral vitamin-K antagonist such as warfarin be used? If the latter is chosen, what should the intensity of anticoagulation be?
Antiplatelet agent or vitamin-K antagonist?
In the absence of a potential cardiac source for the stroke (ie, valve thrombi or mural thrombus on the atrial or ventricle wall), the question is raised of using aspirin or warfarin for secondary thromboprophylaxis. The Antiphospholipid Antibodies and Stroke Study (APASS)33 is the only RCT examining the significance of antiphospholipid antibodies in the specific setting of noncardioembolic stroke. It showed that patients who have sustained an initial stroke, and who test positive for LA, the CL-ELISA, or both, within 30 days of the event, do not appear to have a different prognosis than those who test negative when treated either with aspirin (325 mg/d) or low-moderate intensity anticoagulation (INR 1.4-2.8; mean INR achieved in the study: 1.9) when followed up for 2 years. Aspirin and low-moderate intensity anticoagulation were of equal efficacy in stroke prevention. Patients with low and high titers on the CL-ELISA were included in the APASS. Antibodies were measured only once, and so the prognostic significance of persistence was not assessed. Pertinent to this, a small retrospective analysis of 8 patients with a history of noncardioembolic stroke who satisfied the laboratory criteria for APS had rates of thrombotic recurrence similar to the general stroke population when treated with aspirin.34
In view of the similar prognosis between antibody-positive and antibody-negative patients who have sustained a noncardioembolic stroke, it is relevant to consider data from large RCTs assessing secondary thromboprophylaxis after such an event in the general population. It has been shown that high-intensity warfarin (INR 3-4.5) is not superior to aspirin (30 mg), and is in fact associated with a significantly increased risk of major bleeding, particularly in the older age groups.35 Another study comparing warfarin at a moderate intensity (INR 2-3) and aspirin (30-325 mg) after a transient ischemic attack (TIA) or minor stroke noted that a possible protective effect of anticoagulation against further ischemic events is offset by increased bleeding complications, concluding that oral anticoagulation is not more effective than aspirin.36 Hence this set of studies, at the present time, is used by us to justify the use of either aspirin or moderate intensity (INR 2-3) warfarin for the treatment of noncardioembolic APS stroke or TIA.
If the decision is made to treat the patient with an antiplatelet agent rather than moderate-intensity warfarin for noncardioembolic stroke, then we consider adding a second antiplatelet agent such as dipyridamole. There are data from the general population that demonstrate that aspirin (30-325 mg) plus extended-release dipyridamole (200 mg twice daily) is superior to aspirin alone for secondary prophylaxis in the setting of noncardioembolic stroke.37 Recently, clopidogrel (75 mg) has been shown to be similar in efficacy to aspirin plus dipyridamole in the setting of secondary prophylaxis after a noncardioembolic stroke in the general population, and is an alternate option we consider in the context of noncardioembolic APS stroke.38 Clopidogrel (75 mg) alone is more effective secondary prophylaxis than aspirin (325 mg) alone in patients who have had a previous myocardial infarction or a stroke.39
The specific indications for using the combination of aspirin (75-100 mg) plus clopidogrel (75 mg) for preventing stroke have been recently delineated. This combination is superior to aspirin alone in preventing major vascular events in the setting of atrial fibrillation (AF),40 though warfarin (INR 2-3) is superior in this regard compared with the combination of the 2 antiplatelet agents.41 In the setting of secondary prevention for noncardioembolic ischemic stroke or TIA, the combination of aspirin (75 mg) and clopidogrel (75 mg) is not superior to clopidogrel alone.42
The annualized rates of major bleeding with the various agents in the setting of secondary stroke prevention have recently been determined by pooling multiple trials43 : aspirin alone (< 325 mg): 1%, clopidogrel: 0.85%, aspirin plus extended release dipyridamole: 0.93%, aspirin plus clopidogrel: 1.7%, and anticoagulation (INR 2-3): 2.5%. Combining aspirin with warfarin increases the risk of major bleeding to 3.9%, without improving protection against stroke in the setting of AF.44 Doses of aspirin 100 mg or greater when used in combination with clopidogrel may increase the bleeding risk, without improving protection.45
If a decision is made to treat noncardioembolic stroke with oral anticoagulants rather than an antiplatelet agent for secondary thromboprophylaxis, then the question arises as to what is the optimal time and manner to commence? Definitive guidelines are currently lacking.46 Hence several lines of evidence (from the noncardioembolic and cardioembolic stroke literature) need to be considered. These observations are pertinent in the setting in which the acute stroke will not be treated with thrombolysis, as distinct guidelines need to be followed in this situation, and readers are referred to expert consensus guidelines.46 In our unit, decisions to implement thrombolysis are made by the neurologists who routinely manage thrombolysis in acute stroke patients in our hospital stroke unit.
It is relevant to note that within the first 48 hours after an acute (noncardioembolic or cardioembolic) stroke in the general population, aspirin alone compared with placebo provides an advantage.47 Anticoagulation, either with unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), heparinoids, oral anticoagulants, or thrombin inhibitors, within the first 48 hours of an acute (noncardioembolic or cardioembolic) stroke was not found to reduce the odds of death or dependence in 2 meta-analyses.48,49 Although anticoagulant therapy was associated with fewer recurrent ischemic strokes, it was also associated with an increase in symptomatic intracranial hemorrhages.48,49 It is pertinent to contrast this finding with the clear advantage of oral anticoagulation (started within 3 months) over aspirin for long-term secondary stroke prophylaxis after a minor stroke or TIA associated with AF.50
These findings suggest that if a decision to implement secondary thromboprophylaxis with oral anticoagulation after a stroke (noncardioembolic or cardioembolic) is made, it is perhaps reasonable to consider commencing it, at some time after the acute period (at least 48 hours).48,49 Aspirin should be used in the interim.47 If the stroke is large (ie, there is evidence of a substantial mass effect or well-defined hypodensity involving greater than one-third of the middle cerebral artery territory on computed tomography scanning), then it may be prudent to delay the commencement of oral anticoagulation for perhaps 2 weeks or more46 in view of the elevated risk of spontaneous hemorrhagic transformation associated with large infarcts.51
Prophylactic, low doses of heparin (in contrast to weight-adjusted doses) have a role in preventing VTE in acute stroke.46 A meta-analysis has suggested that LMWH may be more effective than UFH for preventing VTE in this setting.52 Current consensus guidelines recommend either low-dose UFH or LMWH for VTE prophylaxis in acute stroke.46
A definitive role for bridging therapy with full-anticoagulant doses of heparin while commencing oral anticoagulation remains uncertain in the stroke setting, with retrospective studies suggesting an increased risk of serious bleeding.53 Bridging with heparin is not something that is routinely used in our stroke unit for general stroke patients being commenced on oral anticoagulation. There may be a risk of inducing warfarin-associated skin necrosis54 and a transient hypercoagulable state55 if bridging with heparin is not used, though in the general stroke population this risk seems to be very small.53,56
The risk of aggravating a hypercoagulable state may theoretically be greater in APS patients in view of the noted functional impairment of the activated protein C system,57 likely related to anti-β2GPI autoantibodies with LA activity.57,58 Hence our threshold for considering bridging therapy with full-dose heparin is decreased if the stroke patient is positive on the β2GPI ELISA and for LA, though we avoid this strategy during the first few weeks of the acute stroke period, using aspirin and prophylactic doses of heparin in the interim. Clearly, further clinical studies on the optimal manner and timing for commencing oral anticoagulation in stroke patients who are found to be positive on the β2GPI ELISA and for LA are warranted.
The use of early anticoagulation with full therapeutic doses of heparin has been recommended by some experts in certain specific subgroups of cardioembolic stroke patients such as atrial fibrillation with rheumatic heart disease, prosthetic heart valves, or intracardiac thrombus.46 However, it has been noted that clinical trials have not as yet adequately evaluated the use of early, full therapeutic doses of heparin in these specific stroke subgroups, and the risk of hemorrhage may outweigh the benefits.46 In close consultation with the neurologists from the acute stroke unit we factor into our decision making the following: the size and stability of the intracardiac thrombus (advised by the cardiologist), the size of the cerebral infarct (advised by the neuroradiologist), and the presence or absence of uncontrolled hypertension.51,59 A large, unstable intracardiac thrombus on the echocardiogram, a small stroke or TIA (and exclusion of hemorrhage) on computed tomography scanning or magnetic resonance imaging, and well-controlled blood pressure tends to influence our decision toward considering using adjusted-dose heparin early, followed by long-term, oral anticoagulation.
One subgroup of noncardioembolic stroke patients who we feel warrant further study of their prognosis in the prospective setting are patients who are noted to have high and persistent titers of antibodies on the CL-ELISA and the β2GPI-ELISA and are positive for LA, patients who are so-called “triple positive.” These patients are likely to have high titers of anti-β2GPI Abs with LA activity, and retrospective studies suggest they may have a worst clinical course.18 Supporting this possibility, a subgroup of patients in the APASS study who were positive for LA and the CL-ELISA, and thus were likely to be the group containing patients who are triple positive (the β2GPI-ELISA was not used in the APASS study), had a trend toward an increased risk of recurrent stroke and death in the aspirin and low-moderate range INR groups compared with patients who were antibody negative, though the results did not achieve statistical significance.33 Hence, it is not possible to justify a distinct, more intense course of antithrombotic treatment for this APS stroke subgroup compared with the general stroke population at the current time.
We tend to treat noncerebral artery thrombosis that may occur in locations such as the renal arteries with moderate-intensity oral anticoagulation, and treatment tends to be indefinite. Acute coronary artery syndromes and myocardial infarction are treated according to the evidence base for the general population60 (see references therein).
The treatment of concomitant risk factors such as hypertension and hypercholesterolemia is relevant for secondary thromboprophylaxis in view of their concurrence in a significant portion of patients with APS.61 The importance of treating these variables derives from trials of secondary thromboprophylaxis undertaken in the general stroke population, which have demonstrated the benefits of blood pressure (BP) control,62 and the lowering of LDL-cholesterol levels using a statin.63,64 Lowering BP by at least 9/4 mmHg and cholesterol levels by 1.0 mM was significantly beneficial even in patients who were not hypertensive (mean BP at study entry: 136/79 mmHg),62 and whose pretreatment LDL-cholesterol levels were below 3.0 mM,64 respectively. It is relevant to note that a small increase in risk for symptomatic hemorrhagic stroke was seen with the aggressive LDL-cholesterol lowering strategy compared with placebo in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) RCT study.63 Observational studies have suggested that total cholesterol is negatively associated with hemorrhagic stroke in people with high BP,65 emphasizing the likely importance of BP control if an aggressive LDL-cholesterol lowering strategy is adopted.
Treatment failures
The recurrence of VTE upon cessation of anticoagulation, in particular if it was not associated with a transient risk factor, tends to be subsequently treated with indefinite anticoagulation (INR 2-3) provided that the patient is not deemed to be at an increased risk of bleeding due to associated comorbidities. This is reassessed on a regular basis. This approach is supported by a study undertaken in the general population that found that 6 months of treatment (INR 2-2.8) after a second VTE was associated with an increased rate of VTE recurrences compared with indefinite anticoagulation, though there was a trend toward an increased risk of major bleeding complications with the prolonged regimen over the 4-year period.66
If the venous recurrence occurred while the patient was on an oral anticoagulant such as warfarin at a moderate intensity (INR 2-3), then we first assess whether the patient's INR has been in the therapeutic range in the time interval preceding the recurrence. If it has been in the subtherapeutic range, then we attempt to assess why, so we can rectify the situation, and our management continues with moderate-intensity anticoagulation. If the INR was in the therapeutic range when the venous recurrence occurred, then one option we consider is aiming for a higher intensity of anticoagulation (INR 3 to 3.5). We acknowledge that an evidence base to definitively inform what should be done in the situation of “VTE treatment failure” does not exist as the available RCTs of venous APS tended to exclude patients with a previous recurrence.5,6
If an arterial thrombotic event occurs while the patient is on moderate-intensity anticoagulation (and is in the therapeutic range), then an alternate strategy is to consider adding low-dose aspirin. Once again, we acknowledge that a definitive standard of care does not exist in view of the absence of RCTs addressing the issue of “arterial treatment failure” in APS. There is currently debate in the literature on the merit of combining aspirin with warfarin in the general stroke population, with some advocating its use67 and others its avoidance.68 As has been emphasized previously, the risk of bleeding increases when the intensity of anticoagulation is increased, or when anticoagulation is combined with an antiplatelet agent, and when certain combinations of antiplatelet agents are used together.43 Hence, these variables need to be factored into the risk-benefit decision-making process.
In the situation where we are finding it difficult to stabilize the patient's INR within the therapeutic range, in particular if there has been thrombotic recurrences because it has been subtherapeutic, we consider switching to therapeutic LMWH. A comparable efficacy and safety profile of long-term LMWH compared with oral anticoagulation for preventing VTE recurrences has been demonstrated in a meta-analysis.69 There are case reports of the utility of this strategy in APS patients.70
The importance of maintaining good control of the INR within the therapeutic range to prevent thrombotic recurrences and bleeding complications has been demonstrated in the setting of treatment for stroke71 and VTE.72 A consensus statement from the Anticoagulation Forum on the delivery of optimized anticoagulant care has identified and made recommendations on the following 9 key areas: (1) qualifications of personnel, (2) supervision, (3) care management and coordination, (4) documentation, (5) patient education, (6) patient selection and assessment, (7) laboratory monitoring, (8) initiation and stabilization of warfarin therapy, and (9) maintenance therapy.73
It has been noted, though it seems to be very uncommon, that a small proportion of commercial preparations of thromboplastins may be affected by LA from certain patients, prolonging the prothrombin time (PT), leading to an overestimation of the actual degree of anticoagulation when PT-INR is used for monitoring.74-76 Thromboplastins, especially those made of relipidated tissue factor, should be checked for their responsiveness to LA before being used to monitor anticoagulation (PT-INR) in APS patients.76
Pharmacogenetic testing, specifically for CYP2C19 loss-of-function alleles, may help identify individuals at risk of treatment failure if clopidogrel is being considered.77
The role of the emerging novel anticoagulants in preventing thrombotic recurrence in the context of APS awaits to be determined in formal clinical trials. The key questions that will need to be answered are, are the novel anticoagulants more effective than standard anticoagulant therapy in preventing recurrences, and do they have a better side effect profile? Recent trials in the general population have shown some promising results. Idraparinux, the factor Xa inhibitor, has shown promise in the treatment of venous thromboembolism.78 Its role in preventing stroke in association with AF has been assessed, and though it was equivalent to vitamin-K antagonists in preventing stroke and systemic embolus, its use was associated with significantly more bleeding complications.79
The role of immunomodulation (ie, with corticosteroids and/or intravenous immunoglobulins) in the treatment of APS-related venous and arterial thrombosis has not been established, and is not a strategy that we adopt outside the specific setting of catastrophic APS (“Catastrophic APS”).
Primary thromboprophylaxis
Erkan et al80 have demonstrated in an RCT setting that treating asymptomatic individuals who are persistently positive on either the CL-ELISA and/or the LA assay(s) with long-term low-dose aspirin (81 mg) for primary thrombotic prophylaxis is not associated with an improved clinical outcome compared with placebo. Hence, in our practice we do not tend to give prophylactic aspirin on the basis of the patient's antibody status alone, however we do treat concomitant cardiovascular risk factors such as hypertension, hypercholesterolemia, smoking, and obesity aggressively.
Recently it has been demonstrated in large RCTs that statins significantly lower the risk of developing either a VTE81 or having a major arterial cardiovascular event82 in the apparently healthy population (men > 50 years old, and women > 60 years old) who have an elevated high-sensitivity CRP (hsCRP; 2.0 mg/L or higher) and LDL-cholesterol levels less than 3.4 mM81 at the time of randomization. Protection against cardiovascular events using statins was more likely to be seen in individuals who achieved either an LDL-cholesterol level less than 1.8 mM or a hsCRP less than 2 mg/L, with the greatest protection seen in individuals who achieved low levels in both parameters.83 The mechanism of these protective effects by statins is beyond the scope of this article. It is pertinent to note that a sedentary lifestyle, poor fitness level, abdominal obesity, insulin resistance, and smoking are all predictive of raised hsCRP,84 and as noted by Ridker at al83 lifestyle recommendations for dietary restriction, exercise, and smoking cessation are reasonable initial management strategies before considering pharmacologic intervention. The rates of hemorrhagic stroke did not differ between the statin-treated group and the placebo group.82
The use of statins for primary thromboprophylaxis in the setting of pretreatment LDL-cholesterol levels less than 3.4 mM may potentially have relevance in patients with systemic lupus erythematosus (SLE) who are persistently positive on antiphospholipid antibody testing. It has been noted that SLE patients have an increased risk of thrombotic events compared with the age-matched general population.85,86 Positivity for LA further increases the risk for arterial and venous thrombosis in SLE patients.87-89 In a cross-sectional study of SLE patients, it has been noted that an elevated hsCRP is significantly associated with LA positivity, though an association between elevated hsCRP and thrombosis was not seen.90
The potential use of statins as a treatment strategy in antiphospholipid antibody–positive patients is supported by experimental work.91,92 Using in vitro and in vivo murine APS thrombosis models it has been demonstrated that statins may protect against the direct prothrombotic effects of the pathogenic antibodies. Hence, prospective RCTs are required to determine whether statins in the setting of relatively “normal” pretreatment LDL-cholesterol levels have a role in primary thromboprophylaxis in SLE patients, in particular those who are LA positive in association with elevated hsCRP levels. In view of the potential difficulty in recruiting adequate numbers of patients to assess primary thromboprophylactic measures in SLE,80 it may need to be part of a larger investigation assessing the role of statins for primary thromboprophylaxis in diverse chronic inflammatory states, including for example patients with rheumatoid arthritis.93
It is important to note that the safety of statins in pregnancy is subject to ongoing investigations,94 with concerns having been raised by some studies regarding a possible association with birth defects.95 Hence their use in women intending to get pregnant should be avoided pending clarification of the risks. At the moment, we take a stratified approach for considering statins for primary thromboprophylaxis in the setting of relatively “normal” pretreatment LDL-cholesterol levels (< 3.4 mM) in SLE patients. Our threshold for statin use is lowered if the patient (1) has SLE which is difficult to control, characterized by persistently elevated hsCRP in the absence of infection, (2) is in the older age bracket, (3) is persistently triple positive for LA, CL, and β2GPI-ELISA, (4) has abdominal obesity or insulin resistance, (5) has failed a trial of lifestyle modifications to lose weight, and (6) continues to smoke. We avoid an aggressive LDL-cholesterol lowering strategy in patients with difficult to control BP in view of the potential risk for hemorrhagic stroke.
Hydroxychloroquine is an agent that is used in the treatment of SLE.96 Several observational studies have suggested that its use may be associated with a reduction in the risk of thrombosis in SLE patients.61,97 These observations are supported by in vivo studies using the murine APS thrombosis model,98 as well as by in vitro studies demonstrating blocking of antibody-mediated pathogenic effects on platelets by hydroxychloroquine.99 Hence our threshold for treating a patient who has SLE with hydroxychloroquine is lowered if the patient is also persistently positive for antiphospholipid antibodies.
Obstetric APS
Management of obstetric APS without a history of thrombosis
The trials in the area of prevention of recurrent miscarriages or fetal loss associated with the persistent presence of antibodies detected either by the CL-ELISA or the various LA assays have been extensively reviewed in a meta-analysis by Empson et al100 (see references contained therein). Unfractionated heparin (UFH) plus aspirin is beneficial compared with aspirin alone, reducing pregnancy loss by 54%.100 This is based on 2 small trials,101,102 and one of these lacked adequate allocation concealment.102 There does not appear to be an advantage in using high- over low-dose UFH.100 Aspirin alone compared with placebo or standard care does not reduce the risk of miscarriage.100 There does not appear to be a role for prednisone or intravenous immunoglobulin for the prevention of recurrent miscarriages in APS patients.100
A key question that has not been directly addressed with an RCT is whether UFH alone is superior or equivalent to aspirin plus UFH in obstetric APS. Pending these types of studies, we still incorporate the use of low-dose aspirin in conjunction with UFH in treating obstetric APS.
A relevant issue that warrants further investigation is the delineation of any potential treatment differences between UFH and LMWH in obstetric APS. In one trial that assessed treatment of APS-related recurrent pregnancy loss, LMWH plus aspirin did not differ compared with aspirin alone.103 This finding is consistent with the results of a study by Laskin et al.104 In this latter study, they assessed a heterogeneous population of women, who all had a history of 2 or more unexplained, consecutive miscarriages (before 32 weeks of gestation), and who were either persistently (8 weeks apart) positive for antiphospholipid antibodies (CL-ELISA IgG and/or IgM or LA) or had a hereditary thrombophilia or who were positive for antinuclear antibodies.104 However, a prospective, controlled, multicenter pilot study has found that LMWH (enoxaparin 40 mg daily subcutaneously) plus low-dose aspirin (81 mg) is equivalent to UFH (5000 units subcutaneously twice daily) plus aspirin (81 mg) for the prevention of recurrent fetal loss in APS patients.105 This is supported by another recent, small, single-center RCT that compared dalteparin plus aspirin to UFH plus aspirin.106
The risk of heparin-induced thrombocytopenia (HIT) is relevant to consider when deciding on using either low-dose UFH plus aspirin or LMWH plus aspirin for obstetric APS. In the general population setting, an increased risk of HIT has been noted with the use of UFH compared with LMWH for VTE prophylaxis after orthopedic surgery.107 This is supported by a meta-analysis, in which most studies included patients being treated for VTE prophylaxis after orthopedic surgery.108 A subsequent meta-analysis that compared the risk of heparin-associated thrombocytopenia (ie, all the studies did not satisfy the stricter diagnostic criteria for HIT) in patients treated with therapeutic doses of either UFH or LMWH for established VTE did not note a difference between the 2 treatments.109 There was insufficient evidence to conclude that HIT rates were different between them.109 Several possibilities were raised to explain the discrepancy with the previous meta-analysis,108 including the extent to which patients were investigated specifically for HIT, different patient populations (postorthopedic surgery vs other medical indications), and different intensity and duration of dosing in the patient populations studied.109
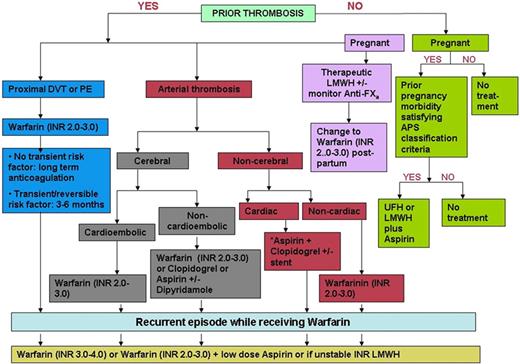
Therapeutic doses of UFH compared with LMWH have been associated with an increased risk of osteopenia when used to treat established VTE in the general patient population who cannot take oral anticoagulants.110 In pregnant women with a history of VTE treated with either adjusted-dose UFH or LMWH, the risk of osteopenia was also greater in those on UFH.111 These clinical finding are supported by work in animal models of heparin-induced osteopenia.112 However, the risk of osteopenia in the specific setting of prophylactic, low doses used for obstetric APS did not differ between UFH and LMWH in a study by Backos et al.113 Another study that compared the effects on bone-mineral density of UFH and LMWH taken during pregnancy for thromboprophylaxis noted that the bone-mineral density was similar with the 2 regimens.114 All the patients received calcium supplementation (500 mg daily). On the basis of the various lines of evidence presented, we deem it reasonable to use either low-dose UFH (twice daily) plus aspirin or low-dose LMWH (once daily) plus aspirin to prevent recurrent miscarriages in obstetric APS pending large, multicenter RCTs directly comparing the 2 treatments (Figure 1).
Treatment algorithm for the thrombotic and obstetric complications associated with persistently positive antiphospholipid antibodies. Readers are referred to current evidence-based consensus guidelines60 for the management of the various acute coronary syndromes, with and without coronary artery stenting, and myocardial infarction.
Treatment algorithm for the thrombotic and obstetric complications associated with persistently positive antiphospholipid antibodies. Readers are referred to current evidence-based consensus guidelines60 for the management of the various acute coronary syndromes, with and without coronary artery stenting, and myocardial infarction.
We commence aspirin before conception, and heparin at the time of confirmation of pregnancy with a pregnancy test. We monitor and investigate for heparin-induced thrombocytopenia according to published guidelines.115 We initially perform weekly platelet counts for 2 consecutive weeks after the initiation of heparin, and then monthly after that. We treat patients at least until 34 weeks of gestation.101 Regular assessment with ultrasound monitoring for intrauterine growth retardation is used from approximately 26 weeks of gestation.
We acknowledge that a firm evidence base to guide optimal duration of thromboprophylaxis in the postpartum period of obstetric APS patients without a history of thrombosis is lacking. In a recent, small, nonrandomized, prospective observational study, Clark et al116 reported that patients with a history of recurrent miscarriages (2 or 3) but no previous thrombosis, who tested repeatedly positive on the CL-ELISA or for LA, did not have an increased risk for thrombotic events in the postpartum period compared with patients with a similar clinical picture but who were negative on autoantibody testing. RCTs are required to establish a standard of care. At the moment, we take a risk-stratified approach to determine who should be considered for postpartum VTE prophylaxis. Factors that we consider include the presence or absence of positivity on multiple assays for antiphospholipid antibodies, in view of retrospective studies suggesting a greater risk of VTE with so-called triple positivity (LA, β2GPI-ELISA, and CL-ELISA).19,117 Other variables that appear to have relevance when considering risk for postpartum VTE are caesarian section versus vaginal delivery, emergency caesarian section versus planned, age older than 35 years, family history to suggest hereditary thrombophilia, obesity, immobility, severe pre-eclampsia, and twin pregnancy.118
Management of the woman with APS who wants to conceive, who has had a previous thrombosis not associated with a transient risk factor, and is on long-term warfarin
Issues regarding management in this area are extrapolated from consensus guidelines pertaining to the management of thrombosis in pregnancy118 due to a lack of RCTs addressing this area. There is strong evidence that warfarin is teratogenic from 6 weeks to the 12th week of gestation (starting from the first day of the last menstrual period), hence our patients are given the choice to switch to LMWH either when trying to conceive or upon confirmation of conception. We acknowledge that UFH can be used instead of LMWH, though at intensities greater than conventional, prophylactic low doses, the risk of osteopenia seems to be greater with UFH.110,111 We emphasize to patients considering starting heparin after conception has been achieved the importance of regular pregnancy testing to ensure that the switch to heparin is made before 6 weeks of gestation.
The optimal LMWH or UFH dosing regimen to use in this setting has yet to be determined, and in the current expert consensus guidelines several options are considered reasonable in conjunction with graduated elastic compression stocking, including (1) weight-adjusted, full-dose LMWH (with or without periodic monitoring; ie, every 1 to 3 months) or UFH (with monitoring), to maintain either the anti-Xa level or the activated partial thromboplastin time, respectively, at a specified level considered therapeutic, (2) 75% of the weight-adjusted dose of LMWH, or (3) intermediate doses of LMWH (eg, dalteparin 5000 U subcutaneously every 12 hours or enoxaparin 40 mg subcutaneously every 12 hours).118 We tend to favor weight-adjusted, full-dose LMWH. We tend to stop treatment with heparin at least 24 hours before elective induction of pregnancy, recommencing as soon as possible thereafter. Oral anticoagulation is subsequently recommenced, while the patient receives bridging therapy with heparin plus graduated elastic compression stockings, and is encouraged to undertake early mobilization in the postpartum period.
Management of the woman with APS who has had a single previous thrombosis that occurred during a previous pregnancy, is now off warfarin, and wants to conceive again
Our approach in this regard is also based on the consensus guidelines.118 Several options are deemed reasonable in the guidelines including treatment during the pregnancy with either prophylactic or intermediate doses of LMWH or UFH, with or without laboratory monitoring of therapy to ensure a prespecified level of anticoagulation.118 We tend to favor the use of intermediate doses of LMWH in conjunction with graduated elastic compression stockings. Therapy is stopped 24 hours before the elective induction of labor. This is followed either by bridging therapy with heparin while commencing oral anticoagulation (INR 2-3) or continuation with subcutaneous injections with LMWH plus the elastic stockings during the high-risk 6-week postpartum period, depending on the patient's preferences.118
Catastrophic APS
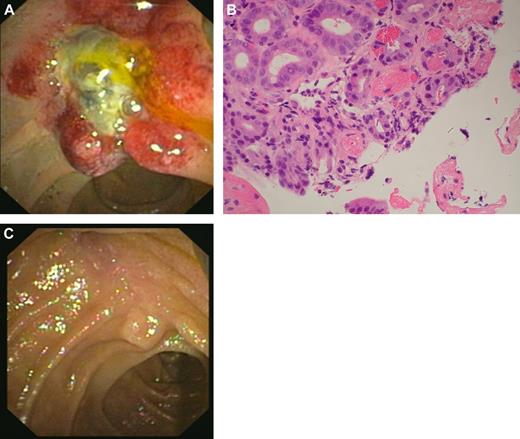
The catastrophic APS is a rare variant of APS characterized by microthrombi in multiple organs119 (Figure 2). It may be triggered by several factors including infection, trauma, surgery, and the withdrawal of oral anticoagulation.120 Due to the rarity or catastrophic APS, RCTs are not available to guide treatment. Investigators have reported several interventions that may be of benefit119-121 and are used by us to guide treatment of these patients. The cornerstone of treatment appears to be the prompt use of anticoagulation (in the absence of concurrent hemorrhage) and the identification and treatment of possible underlying infection. Glucocorticoids, in the absence of infection, also have a role. The use of intravenous immunoglobulin and/or plasma exchange has also been advocated and is something we use. In the context of a lupus flare, cyclophosphamide should be considered. Recurrences tend to be rare, however we tend to leave patients on long-term anticoagulation.
A case of catastrophic APS with involvement of the ampulla of Vater. (A) Morphologic appearance (on endoscopic examination) of ampulla of Vater, which is grossly edematous and unusual in appearance. (B) Ampullary biopsy showing small-vessel thrombosis. (Olympus BX41 microscope, objective lense used was the Olympus UPLANF 40×/0.75NA dry, original magnification ×400, H&E stain; camera: NIKON COOLPIX 995, image acquistion software: Olympus CAMEDIA Master.) (C) Resolution of changes after treatment with intravenous anticoagulation, corticosteroids, antibiotics, plasma exchange, and intravenous immunoglobulin.
A case of catastrophic APS with involvement of the ampulla of Vater. (A) Morphologic appearance (on endoscopic examination) of ampulla of Vater, which is grossly edematous and unusual in appearance. (B) Ampullary biopsy showing small-vessel thrombosis. (Olympus BX41 microscope, objective lense used was the Olympus UPLANF 40×/0.75NA dry, original magnification ×400, H&E stain; camera: NIKON COOLPIX 995, image acquistion software: Olympus CAMEDIA Master.) (C) Resolution of changes after treatment with intravenous anticoagulation, corticosteroids, antibiotics, plasma exchange, and intravenous immunoglobulin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Dr Michael Grimm from the St George Hospital Gastroenterology Department performed the endoscopy and acquired the endoscopic images. Dr Ewan Millar from the St George Hospital Histopathology Department prepared and interpreted the histology slide.
Authorship
Contribution: B.G. and S.A.K. contributed to the writing of this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven A. Krilis, 2 South St, Kogarah, NSW 2217, Australia; e-mail: s.krilis@unsw.edu.au; or Bill Giannakopoulos, 4-10 South St, Research and Education Bldg, Level 3, Kogarah, NSW 2217, Australia; e-mail: billgianna@hotmail.com.