Abstract
By screening 720 natural compounds in a standard 2-way allogeneic mixed leukocyte reaction assay, we identified a potent immunosuppressive capacity of crassin acetate (CRA), a coral-derived cembrane diterpenoid. CRA efficiently inhibited allogeneic mixed leukocyte reaction as well as antigen-specific activation of CD4 T cells by bone marrow–derived dendritic cells (DCs). With regard to cellular targets, CRA suppressed not only mitogen-triggered T-cell activation, but also lipopolysaccharide-induced DC maturation, indicating dual functionality. Treatment with CRA at nontoxic doses induced heme oxygenase-1 (HO-1) mRNA/protein expression and HO-1 enzymatic activity in DCs, suggesting a unique mechanism of action. In fact, lipopolysaccharide-induced DC maturation was also inhibited by structurally unrelated compounds known to induce HO-1 expression or carbon monoxide (CO) release. Allergic contact hypersensitivity response to oxazolone and oxazolone-induced Langerhans cell migration from epidermis were both prevented almost completely by systemic administration of CRA. Not only do our results support the recent concept that HO-1/CO system negatively regulates immune responses, they also form both conceptual and technical frameworks for a more systematic, large-scale drug discovery effort to identify HO-1/CO-targeted immunosuppressants with dual target specificity.
Introduction
Activation of immune responses is required for protection against invading pathogens. At the same time, induction of excessive, prolonged, or dysregulated immune responses against innocuous environmental antigens, self-antigens, and allo-antigens causes various forms of immunologic disorders. Only a few immunosuppressive drugs, including glucocorticoids, cyclosporine A, tacrolimus, and rapamycin, are currently available for patients with these diseases, and their clinical utility is limited further by deleterious adverse effects.1-3 Interestingly, although these drugs were originally thought to primarily suppress the function of effector lymphocytes, more recent studies have revealed their pharmacologic activities to inhibit the function of dendritic cells (DCs).4-6
In the present study, we launched a simple, small-scale drug-screening program as an initial step toward the development of a newer generation of immunosuppressants. We chose to use a standard 2-way allogeneic mixed leukocyte reaction (allo-MLR) assay as a screening system because it would enable us to identify the compounds inhibiting the function of DCs, T cells, or both populations. In this study, we describe in vitro and in vivo activities of one of the hit compounds discovered in this fashion. This compound is a bifunctional immunosuppressant blocking the function of both DCs and T cells. With regard to mechanisms of action, our results suggest the involvement of a rate-limiting enzyme in heme catabolism.
Heme oxygenease (HO) catalyzes the first step in the oxidative degradation of heme (ferriprotoporphyrin IX) into biliverdin-IXα, which is further metabolized to bilirubin-IX by nicotinamide adenine dinucleotide phosphate:biliverdin reductase. The cleavage of the heme ring releases free iron and carbon monoxide (CO).7 HO-1 is a stress-responsive isoform known to be induced in various cell types by diverse stimuli, including oxidative stress, ischemia, heavy metals, lipid metabolites, and cytokines.7 HO-1 knockout mice have been shown to exhibit prenatal death, developmental failure, iron deficiency, anemia, and chronic inflammatory disorders, indicating its diverse physiologic functions.8,9 Moreover, similar phenotypes have been observed in human HO-1 deficiency as well.10 Conversely, overexpression of HO-1 achieved by pharmacologic agents or gene transfer exerts beneficial effects in various pathologic conditions and disease models, including sepsis, asthma, lung injury, cardiovascular injury, ischemia/perfusion, and organ transplantation.11 These protective effects of HO-1 induction can be mimicked, in most cases, by administration of CO, biliverdin, or bilirubin, suggesting that these by-products play functional roles.11,12 Thus, it is now a well accepted view that HO-1 is involved in broad spectra of biologic processes. In this regard, our new finding provides important knowledge to the rapidly growing field of HO-1/CO system in immune regulation.
Methods
Animals and cells
C57BL/6 and BALB/c mice (6- to 10-week-old females) were bred and maintained in the Animal Research Center facilities at the University of Texas Southwestern Medical Center or purchased from The Jackson Laboratory. CD3+ T cells were purified from C57BL/6 spleen cells by a mouse T-cell enrichment column system (R&D Systems), followed by depletion of major histocompatibility complex (MHC) class II+ cells with anti-MHC class II microbeads (Miltenyi Biotec). Splenic CD4+ T cells were purified from DO11.10 T-cell receptor (TCR) transgenic mice (BALB/c background) using a CD4-negative isolation kit (Dynal Biotech), as described before.13 DC cultures were generated from BALB/c bone marrow (BM) cells in complete RPMI 1640 supplemented with 10 ng/mL murine granulocyte-macrophage colony-stimulating factor (R&D Systems).13 All animal experiments were approved by the Institutional Review Boards at University of Texas Southwestern Medical Center and at University of Toledo College of Medicine and conducted according to guidelines of National Institutes of Health.
Drug screening in allo-MLR
The Natural Product Library was purchased from MicroSource Discovery Systems, and individual compounds were tested at 2 μM with the final dimethyl sulfoxide (DMSO) concentration of 0.1% in the first screening. For 2-way allo-MLR screening, unfractionated spleen cells freshly isolated from C57BL/6 mice and from BALB/c mice were cultured together at equal ratio in 96-well plates (2 × 105 cells in total/well) in the presence of each test compound or vehicle alone and pulsed for 16 hours with 3H-thymidine (1 μCi/well; ICN Biomedicals) on day 3. The cells were harvested onto glass-fiber filters (Packard Instrument), and radioactivity was counted in a TopCount NXT (PerkinElmer).
DC-dependent T-cell activation assays
To assess the impact of crassin acetate (CRA) on one-way allo-MLR, CD3+ T cells purified from C57BL/6 mice (2 × 105 cells/well) were cultured with BM-DCs (1.2 × 104 cells/well) propagated from BALB/c mice in the presence of CRA or vehicle alone, followed by measurement of 3H-thymidine uptake. Human 2-way allo-MLR assays were similarly performed by coculturing mononuclear cell fractions (2 × 105 cells/donor/well) isolated from unrelated donors using HISTOPAQUE-1077 and -1119 (Sigma-Aldrich). To test the impact on antigen-specific T-cell response, CD4+ T cells (5 × 104 cells/well) from DO11.10 TCR transgenic mice were cocultured with BM-DCs (3 × 102 cells/well) and 2 μg/mL ovalbumin (OVA)323-339 peptide in the presence of CRA or vehicle alone.
Measurement of in vitro impacts of CRA on mitogen-induced T-cell activation
CD3+ T cells (2 × 105 cells/well) were stimulated with 3 nM phorbol myristate acetate (PMA) plus 300 nM ionomycin or with 5 μg/mL concanavalin A (Con A; Sigma-Aldrich) in the presence of CRA or vehicle alone. T-cell proliferation was measured by 3H-thymidine uptake on day 2 (PMA plus ionomycin) or day 4 (Con A). To test interleukin 2 (IL-2) dependent proliferation of T-cell blasts, CD3+ T cells were treated with PMA plus ionomycin for 24 hours, washed extensively, and then examined for their proliferative responses to 10 U/mL murine recombinant IL-2 (R&D Systems) in the presence of CRA or vehicle alone. To examine the impact on cytokine production, CD3+ T cells were stimulated with PMA plus ionomycin for 24 hours in the presence of CRA or vehicle alone, and the culture supernatants were tested for interferon (IFN)-γ, IL-2, and IL-13 using the enzyme-linked immunosorbent assay kits (R&D Systems). The same CD3+ T cells were also assessed for surface expression of CD25, CD44, and CD69 and cell viability by propidium iodide (PI) uptake with the FACSCalibur (BD Biosciences).
Gene array analysis
BM-DCs were incubated for 6 hours with CRA (10 μM) or PBS in the presence or absence of lipopolysaccharide (LPS; 10 ng/mL). Total RNA samples extracted by the TRIzol reagent (Invitrogen) and an RNeasy plus mini kit (Qiagen) were examined for expression profiles of 47 000 genes with the Sentrix Mouse-6 Array (Illumina) in the Microarray Core at University of Texas Southwestern Medical Center using the standard protocol (http://microarray.swmed.edu).
Measurement of in vitro impacts of CRA on LPS-induced DC maturation
BM-DCs (106 cells/mL) were stimulated with LPS (Escherichia coli 026:B6; Sigma-Aldrich) in the presence of CRA, cobalt protoporphyrin IX (CoPPIX; Frontier Scientific), tricarbonyldichlororuthenium (II) dimmer (CORM-2; Sigma-Aldrich), or vehicle alone for 24 hours, and culture supernatants were tested for cytokines, including IL-1β, IL-6, IL-10, IL-12 p40, IL-12 p70, tumor necrosis factor-α, and macrophage-inflammatory protein-1α. The same BM-DCs were evaluated for surface expression of MHC class II, CD40, CD80, CD86, and CC-chemokine receptor 7 (CCR7). To examine HO-1 mRNA expression, total RNA was converted into cDNA using a Superscript III first strand system kit (Invitrogen). Real-time polymerase chain reaction (PCR) was performed using ABI PRISM 7300 Sequence Detection System (Applied Biosystems) with SYBR Green PCR master mix and the specific primers for HO-1 (SuperArray Bioscience). HO-1 mRNA expression was determined relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA using the comparative cycle threshold method. For Western blotting, BM-DCs were washed 3 times with ice-cold phosphate-buffered saline (PBS) and suspended with 20 μL of cell lysis buffer (Pierce). Protein concentration was determined by a bicinchoninic acid assay kit (Pierce). The samples (20 μg/lane) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes (Bio-Rad). To prevent nonspecific binding, the membrane was blocked with PBS/0.1% Tween 20 containing 2% bovine serum albumin overnight at 4°C, followed by incubation with rabbit anti-rat HO-1 polyclonal antibody (SPA895; StressGen Biotechnologies) for 2 hours and then with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Bio-Rad) for an additional 1 hour. Membranes were developed by chemiluminescent method using the SuperSignal West Pico Chemiluminescent Substrate (Pierce), followed by detection on x-ray film. To measure HO-1 enzymatic activity, BM-DCs were suspended in potassium phosphate buffer, freeze thawed 3 times with liquid nitrogen. The samples (0.85 mg/mL) were added to a reaction mixture containing 0.25 mM nicotinamide adenine dinucleotide phosphate (Sigma-Aldrich), 1 mg/mL rat liver cytosol as a source of bilirubin reductase, and 15 μM hemin (Sigma-Aldrich). The reaction was conducted at 37°C in the dark for 2 hours, followed by extraction of bilirubin with chloroform. The bilirubin concentration was calculated by the difference in absorbance between 460 and 530 nm (ϵ = 40 mM−1cm−1).
In vivo testing of pharmacologic activities of CRA
BALB/c mice received intraperitoneal injection of CRA (10 μg/mouse) or vehicle alone (0.5% DMSO), followed immediately by topical application of 1.25% oxazolone (OX; Sigma-Aldrich) or vehicle alone (acetone/olive oil) on the ear. On day 1, the ear skin samples were harvested to examine the number of MHC class II-positive Langerhans cells (LCs) in epidermal sheet preparations using an Olympus BX60 microscope equipped with an Olympus DPIO digital camera and MetaMorph software (Universal Imaging).13 To test allergic contact hypersensitivity responses, BALB/c mice were sensitized by topical application of 1.25% OX on the shaved abdominal skin (day −4) and challenged with 0.5% OX on the right ear or vehicle alone on left ear (day 0). The swelling responses (the right ear thickness minus the left ear thickness) were examined on days 1 and 2 using an engineer's micrometer.6 CRA (10 μg/mouse) freshly prepared in 0.5% DMSO in PBS or vehicle alone was intraperitoneally injected into these mice. Primary irritant contact hypersensitivity responses were induced by topical application of 10% benzalkonium chloride (BAC) or 30% ethyl phenylpropiolate (EPP) on the right ear.14
Statistical analyses
Data are expressed as means plus or minus SD. Differences in measured variables between the experimental and control groups were assessed with 2-tailed Student t test, except for the analysis of dose response, which was done using an analysis of variance and Dunnett test. Comparisons among more than 3 groups were assessed by Student-Newman-Keuls multiple comparison test. All experiments were repeated more than twice to assess reproducibility.
Results
Identification of immunosuppressive activities of CRA
We screened 720 natural compounds in the standard 2-way allo-MLR assay, in which unfractionated spleen cells from C57BL/6 mice and from BALB/c mice were cocultured in the absence of γ-irradiation. This simple assay produces robust T-cell proliferation, which represents highly complex, bidirectional (C57BL/6 →BALB/c and BALB/c → C57BL/6) alloresponses triggered by DCs and other antigen-presenting cells via direct and indirect presentation mechanisms. In the first screening, each test compound was added to the MLR cocultures at 2 μM. Based on the extent of variations observed in control cocultures receiving vehicle alone, we defined the cut-off level as the mean −2 SD (Figure 1A left panel). A total of 20 compounds was found to inhibit T-cell proliferation below this level (Figure 1A right panel). The “hit” compounds were subsequently tested at different concentrations in a more refined 1-way MLR assay in which T cells purified from C57BL/6 mice were cocultured with BM-DCs propagated from BALB/c mice. From the second screening, CRA was chosen for further analyses because of its potent pharmacologic activity. CRA is a diterpenoid with a 14-carbone cembrane ring structure (Figure 1B). This compound was originally isolated from whole dried gorgonians in 196015 and later found to exhibit modest in vitro cytotoxicity against fibroblasts, human leukemic cells, and HeLa cells in the anti–cancer drug discovery program at the National Cancer Institute.16-18
Identification of CRA as an immunosuppressive compound. (A) Two-way allo-MLR microcultures were tested in the presence of vehicle alone (0.1% DMSO; left panel) or test compounds (2 μM; right panel). Each dot represents the actual level of 3H-thymidine uptake observed in each coculture, and the mean value observed in the vehicle treatment group is shown with a bold line. Twenty compounds reducing T-cell proliferation below the cut-off level (the mean − 2 SD in the vehicle-treated group) were considered as positive in the first screening. The arrow indicates the impact of CRA. (B) The chemical structure of CRA. (C) T cells purified from C57BL/6 mice were cultured with CRA at the indicated concentrations in the presence (●) or absence of allogeneic BM-DCs (○). (D) CD4+ T cells purified from the DO11.10 TCR transgenic mice and BM-DCs were cocultured with the indicated concentrations of CRA in the presence of OVA323-339 peptide (●) or vehicle alone (○). Data shown are 3H-thymidine uptake on day 4 (mean ± SD, n = 3). (E) Mononuclear cell fractions isolated from unrelated donors were cocultured (circles) or cultured independently (triangles) in the presence of CRA in the indicated concentrations. Data shown are the means ± SD (n = 3) of 3H-thymidine uptake on day 5 (open symbols) and day 7 (closed symbols). Asterisks indicate statistically significant (**P < .01) differences compared with the vehicle-treated control samples.
Identification of CRA as an immunosuppressive compound. (A) Two-way allo-MLR microcultures were tested in the presence of vehicle alone (0.1% DMSO; left panel) or test compounds (2 μM; right panel). Each dot represents the actual level of 3H-thymidine uptake observed in each coculture, and the mean value observed in the vehicle treatment group is shown with a bold line. Twenty compounds reducing T-cell proliferation below the cut-off level (the mean − 2 SD in the vehicle-treated group) were considered as positive in the first screening. The arrow indicates the impact of CRA. (B) The chemical structure of CRA. (C) T cells purified from C57BL/6 mice were cultured with CRA at the indicated concentrations in the presence (●) or absence of allogeneic BM-DCs (○). (D) CD4+ T cells purified from the DO11.10 TCR transgenic mice and BM-DCs were cocultured with the indicated concentrations of CRA in the presence of OVA323-339 peptide (●) or vehicle alone (○). Data shown are 3H-thymidine uptake on day 4 (mean ± SD, n = 3). (E) Mononuclear cell fractions isolated from unrelated donors were cocultured (circles) or cultured independently (triangles) in the presence of CRA in the indicated concentrations. Data shown are the means ± SD (n = 3) of 3H-thymidine uptake on day 5 (open symbols) and day 7 (closed symbols). Asterisks indicate statistically significant (**P < .01) differences compared with the vehicle-treated control samples.
CRA dose dependently inhibited the proliferation of C57BL/6-derived T cells triggered by BALB/c-derived DCs, with a significant effect (P < .01) observed at 2 μM and almost complete (> 95%) inhibition achieved at 5 μM (Figure 1C). We also tested the impact of CRA on OVA-specific CD4+ T cells freshly isolated from the DO11.10 TCR transgenic mice (BALB/c background). Robust proliferation of these T cells was observed only in the presence of BM-DCs (propagated from BALB/c mice), indicating DC dependency. CRA inhibited the DC-dependent, antigen-specific T-cell proliferation even more efficiently with more than 90% inhibition observed at 3 μM (Figure 1D). Although beyond the scope of this study, we also tested the impact of CRA in human 2-way allo-MLR assays. Once again, CRA at 1 to 3 μM induced significant (P < .01) inhibition of human T-cell proliferation triggered by allogeneic antigen-presenting cells (Figure 1E).
Dual target specificity of CRA-induced immunosuppression
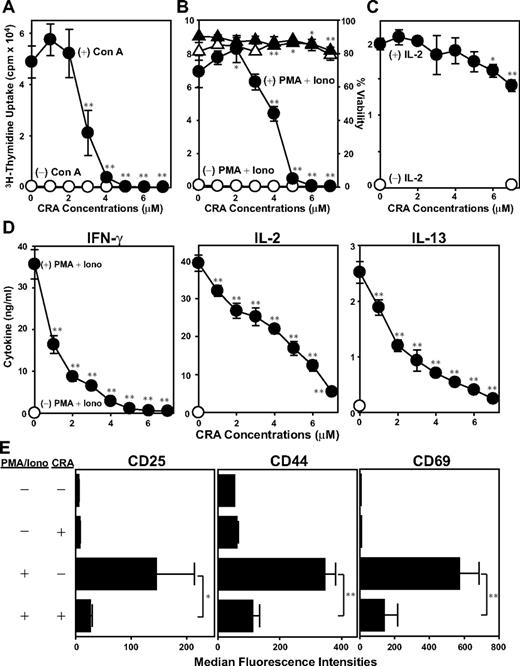
A key question concerned relevant cellular target(s) of CRA. We first determined whether CRA suppresses mitogen-induced activation of T cells in the absence of DCs. Con A-induced T-cell proliferation was inhibited efficiently by CRA at similar concentrations (3-5 μM; Figure 2A). Likewise, CRA (4-6 μM) potently inhibited T-cell proliferation triggered by PMA plus ionomycin (Figure 2B). Importantly, T-cell viability remained almost unaffected after CRA treatment at the tested range (1-7 μM). By contrast, IL-2–dependent proliferation of T-cell blasts preactivated by PMA plus ionomycin was suppressed only marginally (< 30%) by CRA even at the highest concentration tested (7 μM; Figure 2C). PMA plus ionomycin stimulation induced significant release of IL-2, IFN-γ, and IL-13 by T cells. CRA inhibited the production of each of these cytokines in dose-dependent fashions with 50% to 90% inhibition achieved at 4 μM (Figure 2D). Furthermore, PMA plus ionomycin elevated surface expression of CD25, CD44, and CD69 on T cells, and CRA inhibited the up-regulation of all 3 markers uniformly (Figure 2E). Thus, CRA is capable of efficiently blocking all tested changes that accompany mitogen-induced T-cell activation (ie, proliferation, cytokine release, and surface expression of activation markers), with the implication that T cells serve as a relevant target of CRA.
In vitro impacts of CRA on T cells. (A) T cells were cultured with CRA at the graded concentrations in the presence of Con A (●) or vehicle alone (○), and then examined 3H-thymidine uptake on day 4. (B) T cells were cultured in the presence of CRA at the graded concentrations with PMA plus ionomycin (●) or vehicle alone (○), followed by measuring 3H-thymidine uptake on day 2. The viability of these cells was evaluated by PI uptake in the presence of PMA plus ionomycin (▴) or vehicle alone (▵). (C) T cells were incubated with PMA plus ionomycin for 24 hours. After extensive washing, the T cells were cultured again with CRA at indicated concentrations with (●) or without IL-2 (○), and then examined 3H-thymidine uptake on day 2. (D) Cytokine concentrations were measured in the culture supernatants from T cells treated with (●) or without PMA plus ionomycin (○) in the presence of CRA at the indicated doses for 24 hours. (E) Surface expression of CD25, CD44, and CD69 on T cells was tested by flow cytometry. Data shown in this figure are the mean ± SD (n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the vehicle-treated control samples.
In vitro impacts of CRA on T cells. (A) T cells were cultured with CRA at the graded concentrations in the presence of Con A (●) or vehicle alone (○), and then examined 3H-thymidine uptake on day 4. (B) T cells were cultured in the presence of CRA at the graded concentrations with PMA plus ionomycin (●) or vehicle alone (○), followed by measuring 3H-thymidine uptake on day 2. The viability of these cells was evaluated by PI uptake in the presence of PMA plus ionomycin (▴) or vehicle alone (▵). (C) T cells were incubated with PMA plus ionomycin for 24 hours. After extensive washing, the T cells were cultured again with CRA at indicated concentrations with (●) or without IL-2 (○), and then examined 3H-thymidine uptake on day 2. (D) Cytokine concentrations were measured in the culture supernatants from T cells treated with (●) or without PMA plus ionomycin (○) in the presence of CRA at the indicated doses for 24 hours. (E) Surface expression of CD25, CD44, and CD69 on T cells was tested by flow cytometry. Data shown in this figure are the mean ± SD (n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the vehicle-treated control samples.
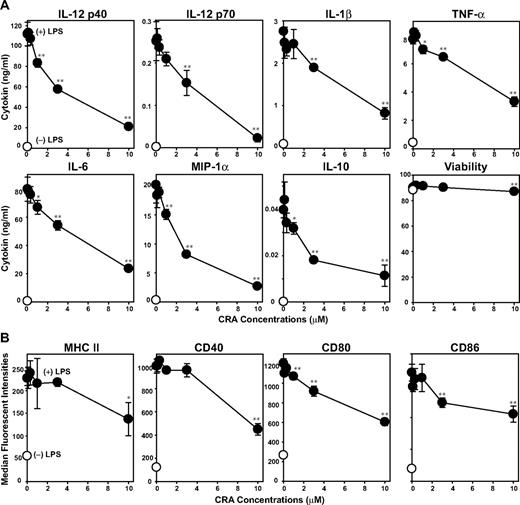
We next examined direct effects of CRA on LPS-induced DC maturation. Upon stimulation with LPS, BM-DCs elaborated IL-12 p40, IL-12 p70, IL-1β, tumor necrosis factor-α, IL-6, IL-10, and macrophage-inflammatory protein-1α. CRA efficiently inhibited the production of all these cytokines in dose-dependent manners with 20%–60% inhibition achieved at 3 μM (Figure 3A). Consistent with our findings with T cells, DCs remained mostly viable after CRA treatment at the tested doses (0.3-10 μM). After LPS stimulation, BM-DCs exhibited markedly elevated surface expression of MHC class II, CD40, CD80, and CD86. This phenotypic maturation was uniformly blocked by CRA at 3-10 μM (Figure 3B). Our observations imply that DCs also serve as a relevant target of CRA.
In vitro impacts of CRA on DCs. (A) BM-DCs were cultured in the presence of CRA at the indicated concentrations with LPS (●) or vehicle alone (○) for 24 hours. Culture supernatants of the BM-DC cultures were examined for the release of indicated cytokines (mean ± SD, n = 3). The cell viability was determined by PI uptake. (B) Surface expression of MHC class II, CD40, CD80, and CD86 was examined on BM-DCs cultured with CRA at different concentrations in the presence of LPS (●) or vehicle alone (○; mean ± SD, n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the vehicle-treated control samples.
In vitro impacts of CRA on DCs. (A) BM-DCs were cultured in the presence of CRA at the indicated concentrations with LPS (●) or vehicle alone (○) for 24 hours. Culture supernatants of the BM-DC cultures were examined for the release of indicated cytokines (mean ± SD, n = 3). The cell viability was determined by PI uptake. (B) Surface expression of MHC class II, CD40, CD80, and CD86 was examined on BM-DCs cultured with CRA at different concentrations in the presence of LPS (●) or vehicle alone (○; mean ± SD, n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the vehicle-treated control samples.
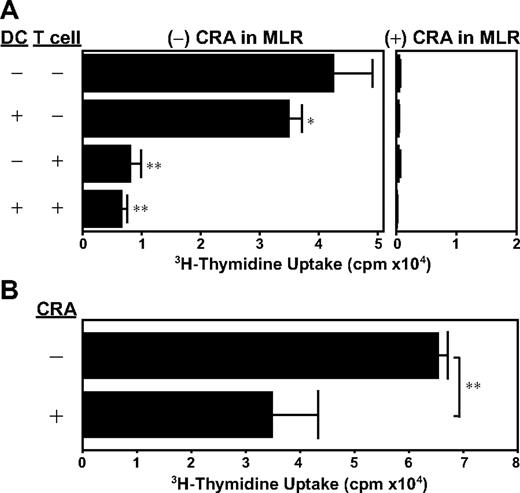
We also performed CRA pretreatment experiments, in which C57BL/6-derived CD3+ T cells (or BALB/c-derived BM-DCs) were incubated for 24 hours with 5 μM CRA or vehicle alone, washed extensively, and then cocultured with allogeneic DCs (or allogeneic T cells). We observed that CRA pretreatment of DCs was sufficient to produce statistically significant (P < .05), albeit modest, inhibition of allo-MLR, whereas CRA pretreatment of T cells resulted in almost complete inhibition of their subsequent proliferation (Figure 4A). Not only do DCs deliver activation signals to T cells, they also receive maturation signals from responding T cells. Thus, we next pretreated DCs with CRA together with a DC-activation signal. CRA pretreatment in the presence of LPS resulted in almost 50% inhibition of T-cell–stimulatory capacity of DCs (Figure 4B). These observations support our conclusion that CRA interferes with DC-dependent T-cell activation efficiently by acting on both populations.
Dual target specificity of CRA. (A) BM-DCs (from BALB/c mice) and/or CD3+ T cells (from C57BL/6 mice) were preincubated for 24 hours with CRA (10 μM) or vehicle alone. After extensive wash, the DCs and T cells were then cocultured in the presence or absence of CRA (10 μM). Data shown are 3H-thymidine uptake on day 4 (mean ± SD, n = 3). (B) BM-DCs (from BALB/c mice) were preincubated for 24 hours with CRA (10 μM) or vehicle alone, washed extensively, and then cocultured with T cells (from C57BL/6 mice). Data shown are 3H-thymidine uptake on day 4 (mean ± SD, n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the control panel in which DCs and T cells were both pretreated with vehicle alone.
Dual target specificity of CRA. (A) BM-DCs (from BALB/c mice) and/or CD3+ T cells (from C57BL/6 mice) were preincubated for 24 hours with CRA (10 μM) or vehicle alone. After extensive wash, the DCs and T cells were then cocultured in the presence or absence of CRA (10 μM). Data shown are 3H-thymidine uptake on day 4 (mean ± SD, n = 3). (B) BM-DCs (from BALB/c mice) were preincubated for 24 hours with CRA (10 μM) or vehicle alone, washed extensively, and then cocultured with T cells (from C57BL/6 mice). Data shown are 3H-thymidine uptake on day 4 (mean ± SD, n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the control panel in which DCs and T cells were both pretreated with vehicle alone.
CRA treatment triggers HO-1 expression in DCs
Although many diterpenoids with cembrane rings have been shown to antagonize the neuronal nicotinic acetylcholine and γ-aminobutyric acidA receptor,19-21 no information is available with regard to mechanisms of action for CRA. As an initial step to answer this question, we exposed BM-DCs to CRA or vehicle alone for 6 hours and then compared mRNA expression profiles using the Sentrix Mouse-6 Array. CRA treatment was found to induce a 12-fold increase in HO-1 mRNA (NM_010442) compared with vehicle treatment (see the whole-gene-array datasets deposited in Gene Expression Omnibus with accession number GSE15360). In addition, CRA also induced greater than 3-fold up-regulation of several genes involved in redox reactions, including biliverdin reductase B (AY340485); glutamate-cysteine ligase catalytic subunit and modifier subunit (NM_010295 and NM_008129); carboxyl reductase 3 (NM_173047); sulfiredoxin 1 homolog (NM_029688); solute carrier family 7, 11, and 40 (NM_011990, NM_013612, and NM_016917); oxidative stress-induced growth inhibitor 1 (NM_027950); and nicotinamide adenine dinucleotide phosphate+-dependent malic enzyme 1 (AK006387).
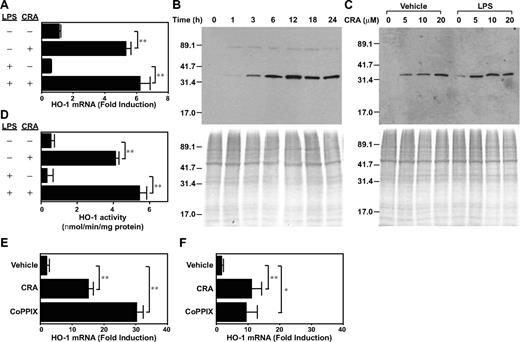
Although the above gene array results remain to be validated by other methods, we have confirmed HO-1 mRNA up-regulation by real-time PCR (Figure 5A). CRA treatment (10 μM) elevated HO-1 mRNA expression in BM-DCs significantly (P < .01, 4.8-fold). To determine whether maturation signals might override the observed response, BM-DCs were exposed to CRA or vehicle alone in the continuous presence of LPS. Under these conditions, CRA treatment caused similar (P < .01, 10.6-fold) HO-1 mRNA induction over vehicle treatment (Figure 5A bottom panels). Western blot experiments revealed rapid (within 3 hours) and continuous (up to 24 hours) HO-1 protein expression in BM-DCs after CRA treatment (Figure 5B). CRA as low as 5 μM was sufficient to induce HO-1 protein expression, and, once again, the presence of LPS did not hamper this response (Figure 5C). Finally, we measured HO-1 enzymatic activities in BM-DCs after exposure to CRA or vehicle alone. As shown in Figure 5D, CRA treatment caused 7.6-fold or 16.3-fold increase in HO-1 activity in the absence or presence of LPS, respectively. Thus, CRA at pharmacologic doses is capable of inducing HO-1 expression in DCs at mRNA, protein, and functional levels.
CRA-induced HO-1 expression in DCs. (A) BM-DCs were treated with CRA (10 μM) in the presence of vehicle alone or LPS (10 ng/mL) for 6 hours. Total RNA was isolated from these cells, and subjected to real-time PCR analysis for mRNA expression for HO-1 and GAPDH (mean ± SD, n = 3). Data are normalized to GAPDH mRNA expression as mentioned in “Measurement of in vitro impacts of CRA on LPS-induced DC maturation.” (B-C) BM-DCs were cultured with CRA in the presence of vehicle alone or LPS to examine time kinetics (10 μM; B) and dose dependency (6 hours) for HO-1 protein expression (C). The whole-cell extracts were assessed by Western blotting for HO-1 protein expression. The whole-cell extracts were also subjected to Coomassie blue staining. (D) HO-1 enzymatic activity was examined in whole-cell extracts from BM-DCs treated with CRA or vehicle alone in the presence or absence of LPS for 6 hours. Data are shown as nmol of bilirubin/minute per mg protein (mean ± SD, n = 3). (E-F) CD3+ T cells freshly purified from C57BL/6 mice were treated with CRA (10 μM) or CoPPIX (50 μM) in the presence (F) or absence (E) of PMA plus ionomycin for 6 hours and then examined for HO-1 mRNA expression by real-time PCR. Data are normalized to GAPDH mRNA expression (mean ± SD, n = 3). Asterisks indicate statistically significant (**P < .01) differences compared with the nontreated control samples.
CRA-induced HO-1 expression in DCs. (A) BM-DCs were treated with CRA (10 μM) in the presence of vehicle alone or LPS (10 ng/mL) for 6 hours. Total RNA was isolated from these cells, and subjected to real-time PCR analysis for mRNA expression for HO-1 and GAPDH (mean ± SD, n = 3). Data are normalized to GAPDH mRNA expression as mentioned in “Measurement of in vitro impacts of CRA on LPS-induced DC maturation.” (B-C) BM-DCs were cultured with CRA in the presence of vehicle alone or LPS to examine time kinetics (10 μM; B) and dose dependency (6 hours) for HO-1 protein expression (C). The whole-cell extracts were assessed by Western blotting for HO-1 protein expression. The whole-cell extracts were also subjected to Coomassie blue staining. (D) HO-1 enzymatic activity was examined in whole-cell extracts from BM-DCs treated with CRA or vehicle alone in the presence or absence of LPS for 6 hours. Data are shown as nmol of bilirubin/minute per mg protein (mean ± SD, n = 3). (E-F) CD3+ T cells freshly purified from C57BL/6 mice were treated with CRA (10 μM) or CoPPIX (50 μM) in the presence (F) or absence (E) of PMA plus ionomycin for 6 hours and then examined for HO-1 mRNA expression by real-time PCR. Data are normalized to GAPDH mRNA expression (mean ± SD, n = 3). Asterisks indicate statistically significant (**P < .01) differences compared with the nontreated control samples.
As shown in Figure 5E, CRA treatment significantly (P < .05) up-regulated HO-1 mRNA expression in CD3+ T cells freshly purified from C57BL/6 mice. A similar up-regulation was also observed even when T cells were treated with CRA in the presence of PMA plus ionomycin, indicating that T-cell activation signals failed to override the effect of CRA (Figure 5F). The level of HO-1 mRNA expression inducible by CRA was substantial compared with that observed after treatment with CoPPIX, a standard compound used widely to trigger HO-1 expression.22-25 Thus, we interpreted our data to suggest a relatively potent capacity of CRA to induce HO-1 expression in T cells. On the other hand, HO-1 has been reported in the literature to suppress IL-2 production and proliferation by T cells,26 augment activation-induced T-cell death,27 and play crucial roles in regulatory T-cell function.28,29 Thus, we chose to further investigate immune-regulatory properties of CRA by focusing on its HO-1–inducing activity in DCs.
Mechanisms of action for CRA
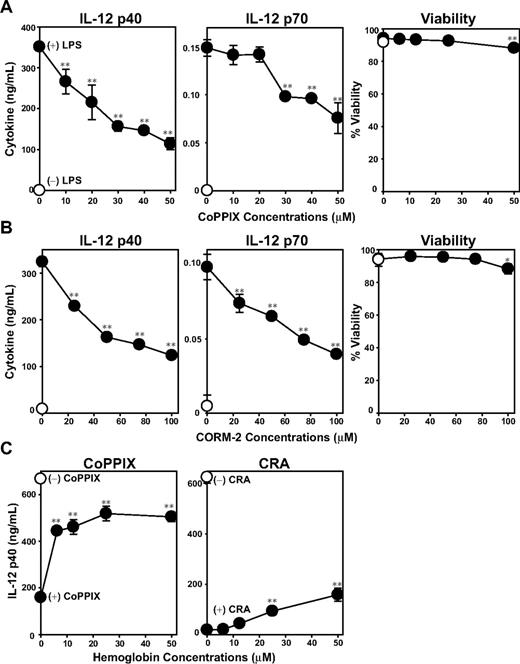
Our experiments described to date unveiled 2 effects of CRA on DCs, that is, inhibition of LPS-induced maturation and induction of HO-1 expression. To assess whether the 2 effects might be causally related to each other, we stimulated BM-DCs with LPS in the presence of CoPPIX. This compound, indeed, inhibited LPS-induced IL-12 p40 and IL-12 p70 production by DCs significantly at subcytotoxic doses, thus duplicating the effect of CRA (Figure 6A). CO, a byproduct of HO-1–mediated heme catalysis, has been shown to modulate a variety of cellular functions by binding not only to hemoglobin, but also to cytochrome c oxidase, nitric oxide synthase, and guanylate cyclase.12,30 Tricarbonydichlororuthenium (II) dimmer is a small chemical compound, also known as the CO-releasing molecule-2 (CORM-2), which has been used to trigger CO release.31 CORM-2 also mimicked CRA by inhibiting LPS-induced IL-12 p40 and IL-12 p70 production by DCs (Figure 6B). We interpreted these data to indicate that induction of HO-1 or release of CO is sufficient to inhibit DC function. We then reasoned that one should be able to abrogate the immunosuppressive effects of CRA by scavenging newly released CO molecules, if CO acts as the main mediator. Once again, CoPPIX and CRA both inhibited LPS-induced IL-12 p40 production by BM-DCs (Figure 6C). When hemoglobin was added as a CO scavenger to this experimental system, it dose dependently blocked the effect of CoPPIX, with greater than 50% inhibition achieved at 6 μM. Hemoglobin also blocked the effect of CRA, although much higher concentrations (25-50 μM) were required for significant (P < .01) and rather partial (< 30%) inhibition. These observations suggest that HO-1–mediated release of CO molecules serves as one, but not the only, mechanism by which CRA negatively regulate DC function.
Role of HO-1 and CO on DC activation. BM-DCs were cultured for 24 hours with the graded concentrations of CoPPIX (A) or CORM-2 (B) in the presence of LPS (●) or vehicle alone (○). The culture supernatants were examined for the indicated cytokines. The cell viability was examined by PI uptake (mean ± SD, n = 3). (C) BM-DCs were pretreated for 1 hour with hemoglobin at the indicated concentrations, and then cultured for 24 hours with LPS in the presence of CoPPIX (left panel) or CRA (right panel; mean ± SD, n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the nontreated control samples.
Role of HO-1 and CO on DC activation. BM-DCs were cultured for 24 hours with the graded concentrations of CoPPIX (A) or CORM-2 (B) in the presence of LPS (●) or vehicle alone (○). The culture supernatants were examined for the indicated cytokines. The cell viability was examined by PI uptake (mean ± SD, n = 3). (C) BM-DCs were pretreated for 1 hour with hemoglobin at the indicated concentrations, and then cultured for 24 hours with LPS in the presence of CoPPIX (left panel) or CRA (right panel; mean ± SD, n = 3). Asterisks indicate statistically significant (*P < .05; **P < .01) differences compared with the nontreated control samples.
Preclinical efficacy and safety of CRA
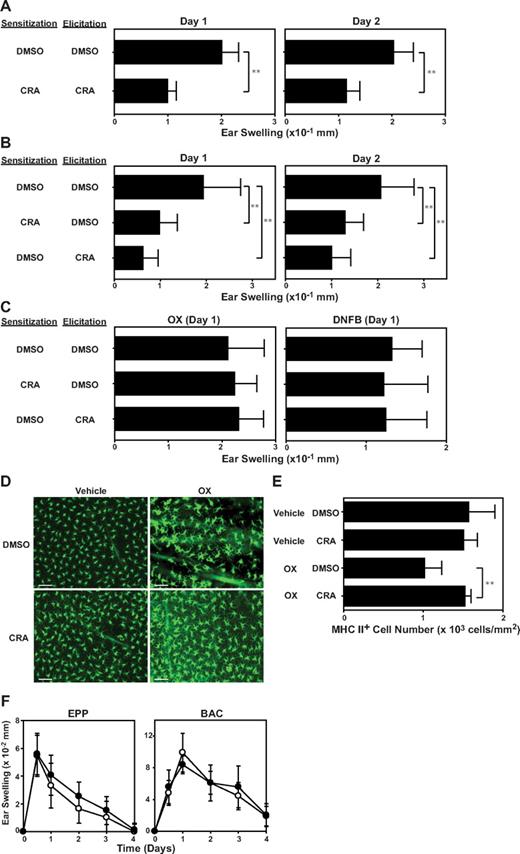
To assess preclinical efficacy of CRA for the treatment of T-cell–mediated immunologic disease, we used a standard mouse model of allergic contact dermatitis. BALB/c mice were sensitized on day −4 by topical application of OX on the abdomen, challenged on day 0 by applying the same hapten on the ear skin, and measured for ear-swelling responses on days 1 and 2. In the first series of experiments, we administered CRA or vehicle alone (DMSO in PBS) repeatedly during both the sensitization phase and the elicitation phase. When administered according to this protocol, CRA significantly (P < .01) diminished the extent of ear-swelling responses compared with DMSO treatment (Figure 7A). In the next series, CRA was administered during the sensitization or elicitation phase (Figure 7B). The 2 protocols both produced significant (P < .01) and equally efficacious outcomes, suggesting that CRA inhibits both primary and secondary immune responses. When these mice receiving CRA during the sensitization or elicitation phase were resensitized with the same hapten (OX) or an irrelevant hapten (2,4-dinitro-1-fluorobenzene [DNFB]) 1 month later, they exhibited robust ear-swelling responses upon challenge with OX or DNFB, and the magnitudes of such responses were comparable with those observed in control mice pretreated with DMSO. Thus, CRA treatment does not appear to induce long-lasting immunologic tolerance or cause long-term impairment of immune responsiveness to unrelated antigens.
In vivo immnosuppressive effects of CRA. (A-B) BALB/c mice were sensitized on day −4 with OX and challenged on day 0 with the same hapten on the ears. CRA (10 μg/animal) or DMSO alone was intraperitonially injected during the sensitization phase (days −4 and −3) and/or the elicitation phase (days 0 and 1). Data shown are the ear-swelling responses on days 1 and 2 (mean ± SD, n = 10). (C) The mice examined in panel B were resensitized and rechallenged 1 month later with OX or DNFB. Data shown are the ear swelling responses on day 1 (mean ± SD, n = 5). Asterisks indicate statistically significant (**P < .01) differences compared with vehicle-treated mice. (D-E) BALB/c mice received intraperitoneal injection of CRA (10 μg/animal) or DMSO alone, followed immediately by topical application of 1.25% OX or vehicle (acetone/olive oil). The number of MHC class II-positive epidermal cells was counted 24 hours after OX application (mean ± SD, n = 10). (F) BALB/c mice were painted with 10% BAC or 30% EPP on the ears on day 0 and examined for ear-swelling responses. CRA (●) or DMSO alone (○) was injected intraperitonially on days 0 and 1 (mean ± SD, n = 5). All animal experimental data shown in this figure are representative of at least 3 independent experiments producing similar results.
In vivo immnosuppressive effects of CRA. (A-B) BALB/c mice were sensitized on day −4 with OX and challenged on day 0 with the same hapten on the ears. CRA (10 μg/animal) or DMSO alone was intraperitonially injected during the sensitization phase (days −4 and −3) and/or the elicitation phase (days 0 and 1). Data shown are the ear-swelling responses on days 1 and 2 (mean ± SD, n = 10). (C) The mice examined in panel B were resensitized and rechallenged 1 month later with OX or DNFB. Data shown are the ear swelling responses on day 1 (mean ± SD, n = 5). Asterisks indicate statistically significant (**P < .01) differences compared with vehicle-treated mice. (D-E) BALB/c mice received intraperitoneal injection of CRA (10 μg/animal) or DMSO alone, followed immediately by topical application of 1.25% OX or vehicle (acetone/olive oil). The number of MHC class II-positive epidermal cells was counted 24 hours after OX application (mean ± SD, n = 10). (F) BALB/c mice were painted with 10% BAC or 30% EPP on the ears on day 0 and examined for ear-swelling responses. CRA (●) or DMSO alone (○) was injected intraperitonially on days 0 and 1 (mean ± SD, n = 5). All animal experimental data shown in this figure are representative of at least 3 independent experiments producing similar results.
LCs and/or dermal DCs are considered to serve as primary antigen-presenting cells in the induction of allergic contact sensitivity responses.32 Skin exposure to reactive haptens triggers migration of LCs from the epidermis to draining lymph nodes.33 Thus, we next sought to study the impact of CRA treatment on LC migration. In control mice receiving DMSO injection, topical application of OX resulted in significant (P < .01, 35%) reduction in the number of MHC class II-positive epidermal cells (ie, epidermal LCs). Importantly, systemic administration of CRA completely inhibited OX-induced LC migration (Figures 6E and 7D). Because CCR7 has been shown to mediate chemotactic migration of mature LCs from epidermis to lymph nodes,34 it was of interest to examine the effect of CRA on CCR7 expression by DCs. Mouse BM-DCs were found to up-regulate CCR7 expression significantly (P < .01) after treatment with CRA (10 μM; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Primary irritant contact dermatitis represents acute responses of the skin to chemically induced tissue injury.14 We found that CRA administration showed no detectable effects on irritant contact hypersensitivity responses induced by topical application of skin-irritating chemicals, EPP and BAC (Figure 7F). Thus, it appears reasonable to conclude that CRA efficiently inhibits T-cell–mediated immune reactions without dampening general host responsiveness to tissue injury.
Discussion
The present study has unveiled a previously unrecognized capacity of CRA to suppress various immune functions. To recapitulate the essence of our findings, CRA inhibits (1) DC-dependent activation of allogeneic T cells; (2) DC-dependent activation of OVA-specific CD4+ T cells; (3) mitogen-triggered T-cell activation; (4) LPS-induced DC maturation; (5) allergic, but not irritant, contact hypersensitivity responses in mice; and (6) hapten-induced LC migration in mice. With regard to mechanisms, CRA was found to trigger marked expression of HO-1 mRNA, protein, and activity in DCs. Other structurally unrelated compounds known to induce HO-1 expression or CO release also inhibited LPS-induced IL-12 production by DCs, whereas the observed inhibitory effects of CRA on DCs were abrogated significantly (although partially) by a CO scavenger. Taken together, these observations illustrate the functional involvement of HO-1/CO system in CRA-induced immune suppression.
Some of our results are consistent with recent reports. Chauveau et al demonstrated that CoPPIX (HO-1 inducer) inhibits LPS-induced phenotypic maturation and cytokine production by DCs and abolishes their ability to activate allogeneic T cells.22 The only discordance relates to the impacts on IL-10 production, which was inhibited by CRA, but preserved after CoPPIX treatment. Using experimental autoimmune encephalomyelitis as a model, Chora et al showed that HO-1–deficient mice exhibit more exacerbated histologic and immunologic features than do wild-type mice, where experimental autoimmune encephalomyelitis progression in wild-type mice can be reversed by systemic administration of CoPPIX.23 Moreover, progression of type I diabetes in nonobese diabetic mice has been delayed or abrogated by systemic administration of CoPPIX or virus-mediated delivery of HO-1 gene.24,25,35 Likewise, CoPPIX treatment has been shown to diminish allo-immune responses and prolong survival of allografts in mice.27,36,37 Taken together, these observations now form the conceptual basis for the future development of a new generation of immunosuppressants that are designed to trigger HO-1 induction in immune cells.
A series of synthetic triterpenoid analogues of oleanolic acid have been developed as anti-inflammatory agents blocking IFN-γ–dependent induction of nitric oxide synthase and cyclooxygenase 2.38 Dinkova-Kostova et al demonstrated that anti-inflammatory properties of these triterpenoid correlate with their abilities to induce HO-1, and proposed that currently available anti-inflammatory agents should be examined for their potentials to trigger HO-1 expression.39 CRA is a naturally occurring diterpenoid with a cembrane ring structure originally purified from gorgonian Pseudoplexaura porosa. To the best of our knowledge, this is the first report documenting immunosuppressive and HO-1–inducing properties of cembrane diterpenoids. Interestingly, rapamycin, which potently inhibits proliferation of T cells and endothelial cells by inactivating mammalian targets of rapamycin, has been reported to induce HO-1 in vascular endothelial cells, albeit in mammalian targets of rapamycin-independent mechanisms.40 Although our limited in vivo experimental data do not allow one to predict the potential efficacy of CRA for any clinical use, it is important to describe our preliminary results with regard to its relative safety. None of the mice receiving CRA treatment died during the experimental procedures. Four repeated intraperitoneal injections of CRA (10 μg/injection/animal) did not affect the body weight (data not shown) or caused apparent changes in any tested serum parameters of hepatic and renal function, except for a significant (P < .01, 50%) increase in alkaline phosphatase (Table S1). Further studies will be required to assess whether CRA and its derivatives may serve as promising candidates of a newer generation of immunosuppressants.
CoPPIX is a prototypic HO-1 inducer that has been tested in a variety of in vitro and animal experimental models. Indeed, we observed CRA and CoPPIX both inhibited LPS-induced cytokine production by DCs. It should be mentioned in this study, however, that CoPPIX required approximately 10-fold higher concentration (30 μM) than did CRA (3 μM) to produce 50% inhibition of IL-12 production (compare Figure 3A with Figure 5A). Systemic administration of CoPPIX reportedly induces many side effects, including weight loss,41 whereas no major adverse effects were observed after repeated CRA injections at a therapeutic dose. CRA is a relatively small compound with a molecular weight of 376.5 compared with CoPPIX with a molecular weight of 654.6. Thus, it appears that CRA may be used as a potent and relatively safe inducer of HO-1 for studying functional roles played by the HO-1/CO system at cellular and animal levels. One may interpret the above observations to suggest apparent advantages of CRA (and its derivatives) as a drug candidate.
In summary, we have discovered a novel immunosuppressive compound with dual target specificity triggering HO-1 induction in DCs. Not only do our findings support the recent concept that the HO-1/CO system negatively regulates inflammatory and immunologic responses, they may also lead to a more systematic, large-scale drug discovery program for the identification of HO-1/CO–targeted immunosuppressants.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (Bethesda, MD) grants RO1-AI46755, RO1-AR35068, RO1-AR43777, and RO1-AI43232 (to A.T.).
National Institutes of Health
Authorship
Contribution: H.M. performed experiments, analyzed data, and drafted the manuscript; H.T. assisted in vitro and in vivo experiments; N.M. helped in vitro drug-screening experiments; and A.T. designed the overall study, interpreted data, and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akira Takashima, Department of Medical Microbiology and Immunology, University of Toledo College of Medicine, 3000 Arlington Ave, Toledo, OH 43614-5806; e-mail: Akira.Takashima@UToledo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal