Abstract
In embryonic stem cells, Oct-4 concentration is critical in determining the development of endoderm, mesoderm, and trophectoderm. Although Oct-4 expression is essential for mesoderm development, it is unclear whether it has a role in the development of specific mesodermal tissues. In this study, we have examined the importance of Oct-4 in the generation of hematopoietic cells using an inducible Oct-4 ESC line. We demonstrate that Oct-4 has a role in supporting hematopoiesis after specifying brachyury-positive mesoderm. When we suppressed Oct-4 expression before or after mesoderm specification, no hematopoietic cells are detected. However, hematopoiesis can be rescued in the absence of Oct-4 after mesoderm specification if the essential hematopoietic transcription factor stem cell leukemia is expressed. Our results suggest that, for hematopoiesis to occur, Oct-4 is required for the initial specification of mesoderm and subsequently is required for the development of hematopoietic cells from uncommitted mesoderm.
Introduction
Oct-4 is a POU transcription factor that is highly expressed in embryonic stem cells (ESCs), embryonic epiblasts, and primordial germline cells.1-3 The precise level of Oct-4 tightly regulates the differentiation capacity of ESCs. Loss of Oct-4 expression in ESCs results in a loss of pluripotency and the induction of trophectoderm differentiation.4,5 In contrast, doubling Oct-4 levels in ESCs induces differentiation toward the mesodermal and endodermal embryonic lineages.4 Oct-4 is normally expressed in early tissues, including oocyte, blastocysts, and embryonic ectoderm before gastrulation.1,2 Oct-4 is absent in hematopoietic stem cells and is only detected at high levels in germ tissue of adults.2,6 Maintaining expression of Oct-4 during ESC differentiation has been shown to have effects on neurogenesis and hematopoiesis.7,8
In this study, we have used an ESC line (ZHBTc4) in which both endogenous Oct-4 alleles have been deleted and a tetracycline-repressible Oct-4 transgene has been introduced.4 When Oct-4 expression was repressed at the beginning of differentiation (day 0), formation of embryoid bodies (EBs) was severely hindered and there was a decrease in the expression of hematopoietically associated genes. Similar results were observed when we stably transfected the cells with the basic helix-loop-helix transcription factor stem cell leukemia (Scl).9,10 However, if Oct-4 was allowed to express for 48 hours after induction for differentiation, the expression of Scl was able to rescue the ESCs to form functional hematopoietic EBs. Our study demonstrates that Oct-4 is essential for at least 2 different roles in hematopoiesis. It must first specify brachyury-positive (bry+) mesoderm and then facilitate the proper hematopoietic development of uncommitted mesoderm.
Methods
Embryonic stem cells
ZHBTc4 ESCs were generated and cultured as previously described.4 Full-length Scl cDNA was subcloned into the pCAPP chicken β-actin promoter-containing vector. ZHBTc4 cells were electroporated with pCAPP parental or Scl/pCAPP vector using the Bio-Rad Gene Pulser (240 V, 500 μFD; Bio-Rad). Puromycin (2.5 μg/mL; Sigma-Aldrich) was added to culture medium 48 hours after electroporation for clonal selection. All ZHBTc4 and ZHBTc4-derived ESCs (pCAPP control and Scl clones) are Oct-4 knockout cells with tet-regulated Oct-4 transgene.
Reverse-transcribed–polymerase chain reaction analysis
Total RNA was extracted from multiple clones of pCAPP control or Scl ZHBTc4 clones using RNeasy (QIAGEN). First-strand cDNA was synthesized using the Invitrogen cDNA synthesis kit according to the manufacturer's instructions (Invitrogen). Primers used for polymerase chain reaction (PCR) reactions are shown in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).11
Immunoblots
Total protein was extracted from undifferentiated ESCs or differentiating EBs using 1× lysis buffer (50 mM Tris-Cl, pH 7.8, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol, 0.5% NP-40 Alternative; Calbiochem; and 1× protease inhibitor cocktail, Roche Diagnostics). A total of 60 μg of each protein sample was used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently transferred to a polyvinylidene difluoride membrane for immunoblotting. Primary antibodies used were anti–Tal-1 polyclonal (Scl; Calbiochem), anti–Oct-4 monoclonal (Santa Cruz Biotechnology), antihemoglobin-α polyclonal (Santa Cruz Biotechnology), and anti–β-actin monoclonal (Sigma-Aldrich). Secondary antibodies were donkey anti–mouse IgG conjugated with horseradish peroxidase (HRP; GE Healthcare) and sheep anti–rabbit IgG HRP-conjugated (GE Healthcare).
Differentiation of ESCs into embryoid bodies
Embryoid bodies were generated from ESCs either in liquid culture or in semisolid media. For liquid culture, ESCs were cultured in primary differentiation medium according to an established protocol.12 For differentiation in semisolid media, pCAPP control or Scl clones were cultured in conditions as previously described.13
Microscopy
Photomicrographs of EBs formed after 12 to 14 days cultured in methylcellulose medium were taken by Axioskop fluorescence microscopy (Carl Zeiss) via a 10×/0.3 NA air objective. Images taken via AxioCam camera (Carl Zeiss) were analyzed with Slidebook software (Olympus).
Flow cytometry
Day 14 EBs were collected and disaggregated in 0.25% collagenase at 37°C for 1 hour. Single-cell suspensions were collected, washed, and incubated with 100 μL antibody cocktail (CD45-phycoerythrin and CD11b-allophycocyanin, 1:100; BD Biosciences) at ambient temperature for 30 minutes. Cells were washed once and resuspended in phosphate-buffered saline for fluorescence-activated cell sorter analysis (FACSCalibur; BD Biosciences). Data collected were analyzed by FlowJo software (TreeStar).
CD11b staining of macrophages from embryoid bodies
Immunocytochemistry was performed as previously described.14
Results and discussion
Lack of Oct-4 in ESCs hinders embryoid body formation and inhibits hematopoiesis
In pluripotent ESCs, any disruption of Oct-4 natural expression pattern results in an increase or decrease of their mesodermal commitment.4,7 To investigate whether there is a possible role for Oct-4 during hematopoietic differentiation, we systematically switched off Oct-4 at different time points using a tet-regulated, Oct-4 knockin ESC line (ZHBTc4) and examined EB generation. Both EB formation and hematopoietic development were hampered when Oct-4 was turned off at day 0 (supplemental Figure 1A). Reverse-transcribed (RT) PCR analyses showed both mesodermal and hematopoietic markers (Scl, BMP4, Brachyury [bry], flk1, PU.1, EKLF, embryonic globin, and EpoR) were repressed (supplemental Figure 1B).
Because Scl is an early-acting factor required for hematopoietic stem cell development from mesoderm, we generated Scl-expressing ZHBTc4 ESCs (Scl clones) to determine whether Scl clones could bypass the requirement for Oct-4 in hematopoiesis (supplemental Figure 2).10 Scl failed to rescue hematopoiesis when Oct-4 was silenced at day 0 of differentiation (Figure 1A). Major mesodermal and hematopietic markers (bry, PU.1, and EKLF) were down-regulated in both tet-treated control and Scl clones (supplemental Figure 3). However, an increased expression in various globin genes (α-, ζ-, and βmaj-) was noted from the tet-treated Scl ESCs but not the tet-treated control (supplemental Figure 3).
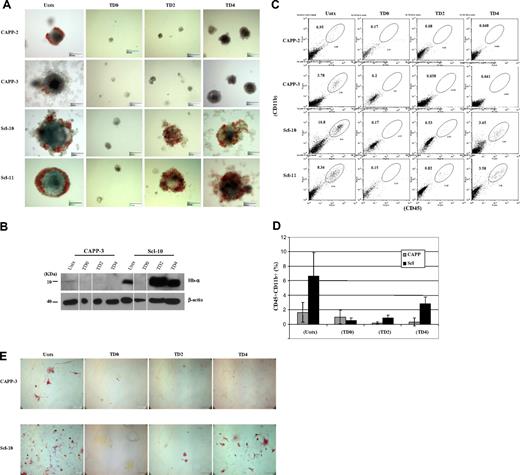
Formation of hematopoietic progenitors from day 14 EBs derived from control and Scl ESCs. (A) Control and Scl ESCs cultured in methylcellulose medium supplemented with hematopoietic cytokines (stem cell factor, interleukin-1, interleukin-3, granulocyte-macrophage colony-stimulating factor, erythropoietin) in the absence of leukemia inhibiting factor. Scale bar represents 150 μm. (B) Immunoblot analysis of total protein extracts prepared from day 14 EBs derived from wild-type and Scl ESC clones. (Top panel) Immunoblotting with probe targeting hemoglobin-α antigen (1:1000). (Bottom panel) Immunoblotting with probe targeting β-actin antigen (1:10 000). Lanes 2 and 6 (TD0) were spliced from another blot because of the extremely low yield of EBs from TD0 ESCs. The results are consistent from separate experiments. (C) Representative histograms of flow cytometric analyses on 2 clones from wild-type ESCs and 2 clones from Scl ESCs. CD45 marker was analyzed by the FL4 channel (y-axis), and CD11b marker was analyzed by the FL2 channel (x-axis). (D) Calculation of the CD45CD11b double-positive cell populations from each clone. All cell preparations were done in duplicates, and each cell population was averaged from 2 separate experiments. Error bars represent 1 SD. (E) CD11b immunostaining on single-cell suspensions prepared from day 11 EBs with or without tet treatment. Single cells prepared from both control (top panel) and Scl EBs (bottom panel) were stained with CD11b antibody, a marker for myeloid progenitors/macrophages. A high number of CD11b+ cells was seen from untreated EBs (wild-type and Scl) and day 4 tet-treated Scl EBs. Scale bar represents 100 μm. CAPP indicates parental vector stably transfected clones (wild-type); Scl, Scl stably transfected clones; Untx, untreated; TD0, tet-treated at day 0; TD2, tet-treated at day 2; TD4, tet-treated at day 4; CAPP-3, CAPP clone 3; Scl-10, Scl clone 10; Hb-α, hemoglobin-α. Both CAPP control and Scl clones are ZHBTc4-derived Oct-4 knockout ESCs with tet-regulated Oct-4 rescue.
Formation of hematopoietic progenitors from day 14 EBs derived from control and Scl ESCs. (A) Control and Scl ESCs cultured in methylcellulose medium supplemented with hematopoietic cytokines (stem cell factor, interleukin-1, interleukin-3, granulocyte-macrophage colony-stimulating factor, erythropoietin) in the absence of leukemia inhibiting factor. Scale bar represents 150 μm. (B) Immunoblot analysis of total protein extracts prepared from day 14 EBs derived from wild-type and Scl ESC clones. (Top panel) Immunoblotting with probe targeting hemoglobin-α antigen (1:1000). (Bottom panel) Immunoblotting with probe targeting β-actin antigen (1:10 000). Lanes 2 and 6 (TD0) were spliced from another blot because of the extremely low yield of EBs from TD0 ESCs. The results are consistent from separate experiments. (C) Representative histograms of flow cytometric analyses on 2 clones from wild-type ESCs and 2 clones from Scl ESCs. CD45 marker was analyzed by the FL4 channel (y-axis), and CD11b marker was analyzed by the FL2 channel (x-axis). (D) Calculation of the CD45CD11b double-positive cell populations from each clone. All cell preparations were done in duplicates, and each cell population was averaged from 2 separate experiments. Error bars represent 1 SD. (E) CD11b immunostaining on single-cell suspensions prepared from day 11 EBs with or without tet treatment. Single cells prepared from both control (top panel) and Scl EBs (bottom panel) were stained with CD11b antibody, a marker for myeloid progenitors/macrophages. A high number of CD11b+ cells was seen from untreated EBs (wild-type and Scl) and day 4 tet-treated Scl EBs. Scale bar represents 100 μm. CAPP indicates parental vector stably transfected clones (wild-type); Scl, Scl stably transfected clones; Untx, untreated; TD0, tet-treated at day 0; TD2, tet-treated at day 2; TD4, tet-treated at day 4; CAPP-3, CAPP clone 3; Scl-10, Scl clone 10; Hb-α, hemoglobin-α. Both CAPP control and Scl clones are ZHBTc4-derived Oct-4 knockout ESCs with tet-regulated Oct-4 rescue.
Scl rescues hematopoietic differentiation when Oct-4 is silenced after bry+ mesoderm specification
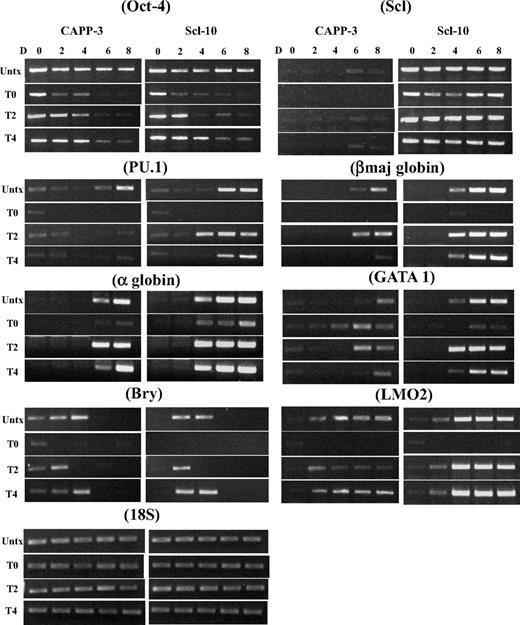
To identify the time point when Oct-4 becomes dispensable for EB and hematopoietic development, we silenced Oct-4 in differentiating ESCs at day 2 or 4 and examined EB development. No hematopoietic EBs could be derived from control ESCs at any time point, although the size of day 4 tet-treated EBs was larger than day 0 and 2 tet-treated EBs (Figure 1A). In Scl ESCs, hemoglobinized red cells were seen in both day 2 and day 4 tet-treated EBs (Figure 1A), and expression of hemoglobin protein in these EBs was confirmed by immunoblot analyses (Figure 1B). The presence of myeloid progenitor population (CD45+CD11b+ cell population) in day 2 and day 4 tet-treated Scl EBs was demonstrated by flow cytometry (Figure 1C-D) and immunostaining (CD11b+; Figure 1E). RT-PCR analysis verified that expression of hematopoietic genes (PU.1, LMO2, Gata1, α-, ζ-, and βmaj-goblins) was restored in Scl EBs when Oct-4 was silenced at day 2 or day 4 of differentiation (Figure 2).
RT-PCR analyses of a time course of mesodermal and hematopoietic gene expression markers in day 0 to day 8 control and Scl EBs. The expression of the indicated genes was analyzed at days 0, 2, 4, 6, and 8 after differentiation induction. EB cultures were either not treated or tet-treated at day 0, day 2, or day 4 after commencement of EB differentiation. Representative PCR data are shown. Each PCR was repeated at least 3 times with cDNA prepared from independent ESC differentiations. Bry indicates Brachyury; Untx, untreated; TD0, tet-treated at day 0; TD2, tet-treated at day 2; TD4, tet-treated at day 4; D, day. Both CAPP control and Scl clones are ZHBTc4-derived Oct-4 knockout ESCs with tet-regulated Oct-4 rescue.
RT-PCR analyses of a time course of mesodermal and hematopoietic gene expression markers in day 0 to day 8 control and Scl EBs. The expression of the indicated genes was analyzed at days 0, 2, 4, 6, and 8 after differentiation induction. EB cultures were either not treated or tet-treated at day 0, day 2, or day 4 after commencement of EB differentiation. Representative PCR data are shown. Each PCR was repeated at least 3 times with cDNA prepared from independent ESC differentiations. Bry indicates Brachyury; Untx, untreated; TD0, tet-treated at day 0; TD2, tet-treated at day 2; TD4, tet-treated at day 4; D, day. Both CAPP control and Scl clones are ZHBTc4-derived Oct-4 knockout ESCs with tet-regulated Oct-4 rescue.
Our data support the notion that, without Oct-4, there is no generation of bry+ mesoderm and thus no mesoderm-derived hematopoietic progenitors. However, if we allow Oct-4 to be expressed for 2 or 4 days after differentiation induction, bry+ progenitors are formed and can be induced to hematopoietic tissue by Scl. In the absence of exogenous Scl, these bry+/Oct-4− progenitors are not induced to become hematopoietic cells. However, silencing Oct-4 at day 6 in control EBs does not inhibit hematopoiesis (not shown). The requirement for Oct-4 correlates with the time (days 2.5-4.0 after differentiation induction) when the early hematopoietic progenitors (hemangioblasts/BL-CFCs) are generated in the EBs.15
In conclusion, we have revealed a novel role for Oct-4 in embryonic development beyond the initial specification of early bry+ mesoderm. By shutting off Oct-4 expression immediately after the formation of early mesoderm, we have shown that Oct-4 is necessary for hematopoietic development. However, this requirement can be bypassed by the expression of Scl in mesoderm.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (Bethesda, MD; CA102283, HL075783; R.H.), the American Cancer Society (Atlanta, GA; RSG-06-170-01; R.D.), the American Society of Hematology (Washington, DC; Junior Faculty Scholar Award; R.D.), and the Leukemia & Lymphoma Society (White Plains, NY; SCOR 7388-06; R.H., R.D.).
National Institutes of Health
Authorship
Contribution: K.Y.K. performed experiments, prepared data for publication, contributed conceptually, and prepared the manuscript; E.A.W., J.H.R., and T.T. performed experiments; and R.H. and R.D. contributed conceptually, supervised experiments, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard Dahl, Department of Internal Medicine and the Cancer Research and Treatment Center, University of New Mexico Health Sciences Center, 900 Camino de Salud, CRF 125A, MSC10 5550, Albuquerque, NM 87131; email: rdahl@salud.unm.edu.