Abstract
Osteopontin (OPN), a multifunctional acidic glycoprotein, expressed by osteoblasts within the endosteal region of the bone marrow (BM) suppresses the proliferation of hemopoietic stem and progenitor cells and also regulates their lodgment within the BM after transplantation. Herein we demonstrate that OPN cleavage fragments are the most abundant forms of this protein within the BM. Studies aimed to determine how hemopoietic stem cells (HSCs) interact with OPN revealed for the first time that murine and human HSCs express α9β1 integrin. The N-terminal thrombin cleavage fragment of OPN through its binding to the α9β1 and α4β1 integrins plays a key role in the attraction, retention, regulation, and release of hemopoietic stem and progenitor cells to, in, and from their BM niche. Thrombin-cleaved OPN (trOPN) acts as a chemoattractant for stem and progenitor cells, mediating their migration in a manner that involves interaction with α9β1 and α4β1 integrins. In addition, in the absence of OPN, there is an increased number of white blood cells and, specifically, stem and progenitor cells in the peripheral circulation.
Introduction
Osteopontin (OPN) is a secreted, multifunctional glycoprotein that exists either as a full-length molecule or as proteolytic fragments. Multiple forms of OPN have been described, including various glycosylated,1 phosphorylated,2 sulfated, and nonsulfated species.3
Many cellular interactions involving OPN are mediated via integrin receptors, which by binding to different domains of OPN can control different cellular functions including differentiation, adhesion, migration, and apoptosis. Specifically, αvβ3, αvβ5, αvβ6, and αvβ1 interact with the Arg-Gly-Asp (RGD) domain of OPN to influence cell adhesion and migration.4-8
A unique N-terminal fragment of OPN produced by thrombin proteolysis 6 amino acids C-terminal of the RGD domain9 is found to contain a “cryptic” integrin binding site.10 Thrombin-cleaved OPN (trOPN) fragments have previously been shown in vivo in plasma and milk and may have physiologically distinct roles.11-13 This cryptic integrin binding site specifically mediates binding to α9β1 and α4β110 and cell migration toward trOPN.14 In contrast to the other integrin OPN receptors, α9β1 recognizes only the trOPN N-terminal fragment and not the full-length form.10
We have previously demonstrated that trOPN acts as a negative regulator of both human and mouse hemopoietic stem and progenitor cell (HSC/HPC) proliferation and differentiation in vitro.15 However, the mechanism of this interaction and whether this OPN fragment has any role in vivo remains unclear. Here we have demonstrated that (1) the cleaved form of OPN is the predominant form present in human and murine bone marrow (BM); (2) murine and human HSCs express α9β1; (3) trOPN interacts with HSC/HPC via α9β1 and α4β1; (4) trOPN interacting via α9β1 and α4β1 is potently chemotactic for human and murine marrow HSC/HPC; (5) the absence of OPN in the hemopoietic microenvironment significantly alters the homing ability of transplanted murine HSC/HPC isolated from the central BM region to the BM; and (6) the absence of OPN in the hemopoietic microenvironment results in a significant endogenous redistribution of BM cells to the peripheral circulation and spleen and augments the mobilization of HSC/HPC in response to granulocyte colony-stimulating factor (G-CSF).
Methods
Umbilical cord blood and human BM
Umbilical cord blood (CB) and human BM was obtained after informed consent, in accordance with the Declaration of Helsinki, from the Mercy Hospital for Women (East Melbourne, Australia) and patients undergoing hip replacement at St Vincent's Public Hospital or St Vincent's Private Hospital (East Melbourne, Australia), respectively, in accordance with procedures approved by each hospital's ethics committees. Samples were collected as previously described.15
Mice
Mice were either bred or purchased from Monash Animal Services (Monash University, Clayton, Australia). Red fluorescence protein (RFP) mice, derived from embryonic stem cells expressing a pβActin-CMV-DsRed T3 transgene, were obtained from the Children's Medical Research Institute (Westmead, Australia), and Opn−/− mutant mice16 from Rutgers University. All strains are maintained on the C57BL/6 background. All animal research was approved by the institutional review boards of all participating institutions.
Cell lines
Chinese hamster ovary (CHO) and 293T cells were maintained in Dulbecco modified Eagle medium: nutrient mixture F-12 (DMEM-F12; Invitrogen), containing 10% fetal bovine serum (FBS; JRH Biosciences) and 2 mM l-glutamine (Invitrogen). CHO cells overexpressing human α9 were made as described previously,17 and overexpressing human α4 were created as described in “Cloning of human α4 into murine stem cell virus retroviral vector.”
Osteopontin
Full-length OPN was isolated from human milk and thrombin cleaved as previously described.15,18 OPN (100 μg) in the same cleavage buffer was also cleaved using 10 μg matrix metalloproteinase-3 (MMP-3) or MMP-7 (both from Chemicon) for 10 minutes at 37°C. For biotinylation, samples were dialyzed against phosphate-buffered saline (PBS) and incubated on ice for 2 hours with 20-fold molar excess of EZ-Link NHS-LC-biotin (Pierce). Excess and unbound biotin was removed by dialysis against PBS and biotinylation confirmed by HABA assay.
Immunohistochemical analysis of human BM
Femoral BM was immersion-fixed, decalcified, embedded in paraffin, cut, and dewaxed as previously described.15,19 Antibody labeling was done using mouse anti–human N-terminal OPN (YGLR epitope, 34E3; ImmunoBiological Laboratories) or mouse immunoglobulin G (IgG; Jackson ImmunoResearch Laboratories) at 5 μg/mL in 0.5% BSA in PBS, before washing and labeling with anti–mouse biotin (1/1000; Jackson ImmunoResearch Laboratories). Sections were washed, amplified with PerkinElmer TSA Biotin System (PerkinElmer) labeled with Streptavidin-Alexa 488 tertiary antibody (1/1000; Molecular Probes), washed and mounted in antifade (Vectashield; Vector Laboratories).
Image acquisition and manipulation
Images were acquired and manipulated as previously described.15
Isolation of enriched populations of murine and human HSC/HPC
Populations of murine HSC/HPC (LSK, Lin−Sca+Kit+) were isolated as previously described,20 except the metaphyseal region of each bone was removed before flushing and added into the endosteal sample to be ground.
Low density mononuclear cells (MNCs) were isolated from CB and human BM as previously described.15 MNCs were incubated with mouse anti–human CD3, CD11b, CD14, CD16, CD20, CD24, and CD235a (BD), washed, and exposed to Dynal sheep anti–mouse IgG beads (Invitrogen, Carlsbad, CA) at 0.5 × 106 beads per 106 MNCs for 5 minutes at 4°C with constant rotation. Rosetted non-CD34+ cells were captured. Rosetting was repeated for 15 minutes and CD34+-enriched MNC kept for antibody staining or immunolabeled with a cocktail of CD34-fluorescein isothiocyanate (FITC) and CD38-phycoerythrin (PE) for subsequent fluorescence-activated cell sorting (FACS).
Flow cytometry
Labeled cells were sorted and reanalyzed for purity on a Cytopeia Influx 516SH cell sorter (Cytopeia) equipped with 5 solid state lasers (355, 405, 488, 561, and 640 nm light emission). Band pass filter settings for the detection of fluorescence pulses for PacificBlue, FITC, PE, allophycocyanin (APC), PE-Cy5.5, and APC-Cy7 conjugates were 460 (± 50), 528 (± 38), 605 (± 40), 660 (± 20), 710 (± 50), and 710 long pass, respectively.
Flow cytometric analysis was performed using a BD LSR II (BD) equipped with 4 solid-state lasers (405-, 488-, 561-, and 640-nm light emission). Filter settings were as described for the Cytopeia in the previous paragraph.
Chemotaxis assay
Stromal cell-derived factor (SDF-1, 90 ng/mL; R&D Systems), full-length OPN, or N-terminal trOPN (200 ng/mL) were loaded into the reservoirs of μ-slide chemotaxis wells (Ibidi). The molecular weight of SDF-1 is approximately 8400 kDa and N-terminal trOPN approximately 24 000 kDa, resulting in concentrations of approximately 0.85 mM and approximately 0.66 mM, respectively. Cells were loaded through the cell inlet in Iscove modified Dulbecco media (IMDM) alone, creating a gradient. Slides were incubated at 37°C for 1 hour, and cell movement was quantitated against control cultures with media alone.
α9β1 expression on murine and human HSC
The expression of α9β1 on enriched murine HSC was tested by sorting murine c-kit-APC+ (10 μg/mL; BD), Sca–Pacific Blue+ (25 μg/mL; Biolegend, San Diego, CA) cells, and labeling with biotinylated rat anti–mouse CD34-FITC (20 μg/mL; BD) and goat anti–mouse α9 (20 μg/mL; R&D Systems), followed by streptavidin PECy5.5 (Caltag, 1:400) and donkey anti–goat AF488 (2.5 μg/mL; Molecular Probes). α9β1 expression by enriched human HSC was tested by sorting human CD34-FITC+ cells (1.25 μg/mL; BD) and labeling with CD38-PeCy7 (1.25 μg/mL; BD), CD90-PE cells (10 μg/mL; BD), and biotinylated mouse anti–human α9β1 (50 μg/mL; Chemicon), followed by Strepavidin-APC (2.5 μg/mL; BD).
The expression of α9β1 by enriched murine HSC was immunohistochemically visualized on sorted murine Sca+c-kit+CD34− cells after labeling with goat anti–mouse α9 and donkey anti–goat AF594 (2.5 μg/mL; Molecular Probes). α9β1 expression by enriched human HSC was visualized after sorting human CD34+CD38−CD90+ cells and labeling with biotinylated mouse anti–human α9β1, followed by streptavidin AF594 (2 μg/mL; Molecular Probes).
α9 gene expression in murine BM and human CB HSC
RNA was extracted from sorted human CB Lin−CD34+CD38−CD90bright HSCs and murine BM Lin−Sca+Kit+CD34− HSCs using TRIzol reagent (Invitrogen). RNA was quantified using a nanospec (NanoDrop ND-1000 Spectrophotometer; BioLab), and contaminating DNA was removed by Turbo DNase treatment (Ambion, Austin, TX). RNA (1 μg) was reverse-transcribed using random primers and Superscript III reverse transcriptase (Invitrogen). cDNA (100 ng) was used in a standard singleplex real-time polymerase chain reaction (PCR) using the Taqman Universal PCR Master mix (Applied Biosystems). Forward and reverse primers (Genesearch) to amplify either human or murine α9 or 18S were used at a final concentration of 300 and 20 nM, respectively. All probes (Applied Biosystems) were used at a final concentration of 100 nM. Real-time PCRs were performed using the 7500 real-time PCR system (Applied Biosystems). Primer and probe sequences used are as follows: human α9: forward: 5′-GCCAGCACCACTTCCTTTA-3′, reverse: 5′-TGCTCGGCTCTGTAGCTCACT-3′, probe: 5′-AGATGAACTCTGAACTTT-FAM-3′; mouse α9: forward: 5′-GGACCTTTTGGGTTGAGCTTATT-3′, reverse: 5′-AAAAAGTCTAACCCCCATATTGGA-3′, probe: 5′-ACAACCCACGAGGTGG-FAM-3′; mouse/human 18s: forward: 5′-TTGGATAACTGTCGTAATTCTAGAGCTAAT-3′, reverse: 5′-CCGGGTTGGTTTTGATCTGA-3′, probe: 5′-ATGCCGACGGGCGCTGACC-VIC-3′.
The resulting PCR products were run on a 2% agarose gel containing ethidium bromide and visualized using a transilluminator (Bio-Rad Laboratories).
Analysis of the interaction between trOPN and α9β1 and/or α4β1 expressed on HSC/HPC
The ability of the cryptic region of OPN to bind to α9β1 and or α4β1 on human and mouse HSC/HPC was tested by incubating human CD34+ cells with 1 μM of the peptide SVVYGLR-FITC or SLAYGLR-FITC for murine LSK cells (Auspep)21 or a scrambled version of the peptides (VRVGLYS-FITC for human and LRAGLRS-FITC for murine cells, respectively). Specific interactions were demonstrated using 10 μg/mL mouse anti–human α9β1 antibody (Millipore) or mouse anti–human α4 antibody (BD) before peptide labeling.
Cloning of human α4 into murine stem cell virus retroviral vector
Human α4 integrin (Itga4) cDNA cloned into pENTR223.1 vector was purchased from Open Biosystems (clone ID, 100015216; National Center for Biotechnology Information [NCBI] GenBank ID, BC146277) and amplified using the following primer pair: forward: 5′-GCGTTACGCGTGCCACCATGGCTTGGGAAGCGAGGCGCG-3′, reverse: 5′-GGGCTCGAGTTAATCATCATTGCTTTTAC-3′.
The PCR product was cloned into pMSCV-IRES-eGFP (kind gift from Stephen Jane, Royal Melbourne Hospital, Melbourne, Australia) to make pMSCV-HUα4-IRES-eGFP. Sequencing was done to validate the construct.
Murine stem cell virus retrovirus generation
pMSCV-huα4-IRES-eGFP DNA (5.7 μg) was mixed with Ampho Vector DNA (3.8 μg; gift from Phil Darcy, Peter MacCallum Cancer Institute, Melbourne, Australia) and added to 19 μL DMEM-F12 medium without serum. In addition, 11 μL Metafectene transfection reagent (Biontex Laboratories) were added to 190 μL DMEM-F12 without serum. Tubes were incubated for 5 minutes, combined, and incubated for 20 minutes, both at room temperature. The mixture was added dropwise to a T25 flask of 293T cells at 50% confluence in 2.5 mL DMEM-F-12 medium, 10% FBS. The cells were incubated at 37°C with 10% CO2 for 24 hours, and the media was replaced with a fresh 5 mL. Virus-containing medium was collected 48 and 72 hours after transfection, and a further 5 mL fresh media was added.
Transduction of CHO cells
Twenty-four hours before retroviral infection, CHO cells were rendered vulnerable using Tunicamycin (Sigma-Aldrich).22 Filtered viral supernatant (2.5 mL) was added to cells with 2.5 mL fresh DMEM-F12, 10% FBS containing 4 μg/mL polybrene (Millipore). Cells were reinfected after 72 hours. A day after the last infection, virus-containing medium was removed, and replaced with fresh medium. After an additional 24 hours, green fluorescent protein–positive (GFP+) CHO cells were selected, further cultured, and reselected. Human α4 integrin expression was confirmed using a mouse anti–human α4 antibody (10 μg/mL; BD).
Analysis of interaction between OPN and α9β1 or α4β1 expressed on CHO cells
A mixture of 50% GFP+α4+ and 50% GFP−α9+ CHO cells were incubated with 30 μg/mL biotinylated N-terminal trOPN at 37°C for 60 minutes, washed, and labeled with Streptavidin-PE (10 μg/mL; BD).
HSC/HPC in Opn−/− and wild-type mice
Peripheral blood (PB) and spleen cells were isolated from Opn−/− and wild-type mice. Cells were counted, and the PB were lysed in 0.83% ammonium chloride. PB cells were labeled with Sca-Pacific Blue and c-kit-APC as described above. Colony-forming cells were assayed by plating 0.6 μL equivalent of PB in a double layer nutrient agar culture system as previously described,23 except that stem cell factor (SCF) was added.
Homing assay
Murine endosteal BM RFP+ (2-4 × 104) and carboxyfluorescein succinimidyl ester positive (CFSE+) central BM HSC/HPC and 2 × 105 unlabeled marrow cells were transplanted into wild-type or Opn−/− recipients by lateral vein injection. After 15 hours, BM was harvested from femurs, tibias, and iliac crests as previously described.20 The proportion of transplanted cells homed to marrow was calculated using the total white blood cell count as the denominator and assuming that cells from one femur, tibia, and iliac crest represent 15% of total BM.
Preparation of CB CD34+ and human bone lysates
Sorted CB CD34+CD38+ and CD34+CD38− cells were washed in protease inhibitors (PI; Roche Diagnostics) then resuspended in lysis buffer; 140 mM NaCl, 50 mM Tris HCl, pH 8.0, 10% glycerol, 2 mM ethylenediaminetetraacetic acid (EDTA), 1% Nonidet P-40 (NP40; Sigma), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium orthovanadate (Na3VO4; Sigma) at 20 × 106 cells/mL for 45 minutes on ice.
Small pieces of human bone were immersed in 3 to 5 mL lysis buffer but containing 5 mM Tris-HCl, then agitated rapidly for 5 minutes. The supernatant was removed, bones finely crushed under liquid nitrogen, and the powder was resuspended at 0.3 mg/mL lysis buffer. The supernatant was combined with the initial collection.
Immunoprecipitation and Western blot analysis
Rat anti–mouse IgG1 beads (Invitrogen) were coupled to mouse anti–human integrin α9β1 (Y9A2; Millipore) or mouse anti–human α4 monoclonal antibodies (BD) and washed in PBS plus 0.1% BSA (buffer). CB cell lysates were precleared with uncoupled beads for 20 minutes at 4°C, incubated for 2 hours at 4°C with appropriately pre-armed rat anti–mouse beads, and then the beads were washed in buffer and resuspended in water. Precipitated proteins were either (1) denatured, deglycosylated with N-glycanase, Sialidase A, and O-glycanase using an enzymatic deglycosylation kit (Prozyme) and resuspended in Western loading buffer containing NuPAGE reducing agent and NuPAGE LDS buffer (Invitrogen; see Figure 3B) or (2) immediately resuspended in Western loading buffer (see Figure 2I). Alternately, samples were boiled in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) reducing buffer (see Figure 1D-H). Samples were run on 4% stacking/10% separating Tris/tricine SDS-PAGE gel (see Figure 1D-H) or a NuPAGE 4% to 12% Bis-Tris precast gel (Invitrogen; see Figures 2I and 3B). Proteins were transferred to an immobilin-FL membrane (Millipore), then incubated in primary antibody for 16 hours at 4°C. Primary antibodies used were 2 μg/mL rabbit anti–human OPN antibody (LF-124; a kind gift from Larry Fisher, National Institute of Dental and Craniofacial Research [NIDCR], Washington, DC), 2 μg/mL mouse anti–human α9 ITGA9 monoclonal antibody, clone 3E4 (Abnova), and 2 μg/mL mouse anti–human integrin β1 (CD29) adhesion blocking monoclonal antibody (mAb) 2253 (Chemicon). Membranes were visualized using 100 ng/mL IRDye 800CW conjugated goat anti–rabbit IgG (LI-COR Biosciences), 200 ng/mL IRDye 680 conjugated goat anti–mouse IgG, or 50 ng/mL IRDye 800CW streptavidin secondary antibodies and the Odyssey infrared imaging system (LI-COR Biosciences).
Mobilization
Mice received subcutaneous injections of G-CSF (Filgrastrim; Amgen) at 250 μg/kg in saline in 100 μL/10g body weight twice daily, 6 to 8 hours apart, for 2, 4, or 6 consecutive days. Marrow, PB, and spleen cells were harvested within 12 hours of the last injection. Control animals received an equivalent volume of saline.
Statistical analysis
Differences between means were evaluated by 1-way analysis of variance (ANOVA), Student t test, or a Mann-Whitney rank sum test where appropriate.
Results
Cleaved OPN is the dominant form present in the BM
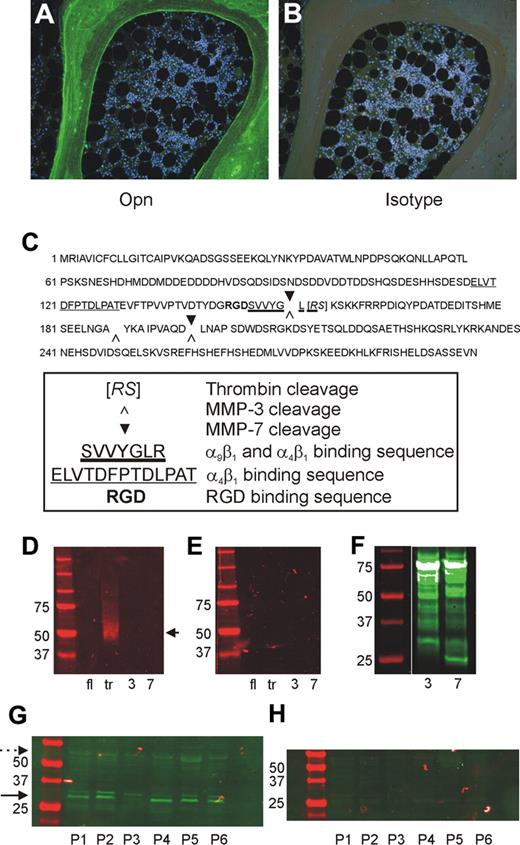
Our previous studies in mice demonstrate a restricted localization of OPN to the endosteal region of BM and that the trOPN exhibits a potent inhibition of human and murine HSC/HPC proliferation and differentiation in vitro.15 However, the exact form of OPN present in the BM niche remains unknown. Immunohistochemical analysis of human BM sections with an antibody specific for the “cryptic” binding site of trOPN, clone 34E3, demonstrated that N-terminal trOPN is abundantly expressed in bone and has a restricted localization to the endosteum (Figure 1A-B). Notably, no OPN was detected within the hemopoietic tissue itself. OPN is cleaved by 2 alternate proteases, MMP-3 (stromelysin-1) and MMP-7 (matrilysin; Figure 1C). However, 34E3 does not recognize the N-terminal fragments of human milk OPN after cleavage with either of these 2 proteases (Figure 1D-E), confirming that the abundant N-terminal OPN detected in human endosteal BM (Figure 1A) is trOPN. Confirmation of MMP-3 and MMP-7 cleavage of full-length OPN was performed through Western blot analysis of biotinylated cleavage fragments (Figure 1F). In addition, Western blot analysis of OPN within lysates of human trabecular BM showed the cleaved N-terminal form of OPN (30-32 kDa) in equivalent or greater amounts compared with the full-length form (approximately 50 kDa; Figure 1G-H). These findings prompted us to examine the mechanism through which this cleaved form of OPN interacts with HSC/HPC.
Presence of full-length and N-terminal trOPN in human BM. (A) Immunohistochemical analysis of human BM sections stained with an antibody specific for the “cryptic” binding site of N-terminal trOPN, demonstrated the restricted presence of N-terminal trOPN in the endosteal region. (B) Isotype control. (C) Identified thrombin, MMP-3, and MMP-7 cleavage sites in human OPN. (D) Western blot analyses using the antibody specific for the “cryptic” binding site of N-terminal trOPN. Data demonstrate recognition of human milk trOPN (lane tr, arrow) but no recognition of full-length human milk OPN (lane fl), or MMP-3 (lane 3), or MMP-7 (lane 7) cleaved human milk OPN. (E) Isotype control. (F) Western blot analysis demonstrating the presence of biotinylated fragments of human milk OPN present postcleavage with MMP-3 (lane 3) or MMP-7 (lane 7) detected using streptavidin. (G) Representative samples of bone lysates from 6 (P1 to P6) of 12 patients run under reduced conditions and immunoblotted with LF-124, an antibody that detects the N-terminal half of OPN. The N-terminal cleaved OPN (→, 30-32 kDa) is at least as dominant as the full-length ( , approximately 55 kDa) form. (H) Neither of these 2 forms were detected in the isotype control.
, approximately 55 kDa) form. (H) Neither of these 2 forms were detected in the isotype control.
Presence of full-length and N-terminal trOPN in human BM. (A) Immunohistochemical analysis of human BM sections stained with an antibody specific for the “cryptic” binding site of N-terminal trOPN, demonstrated the restricted presence of N-terminal trOPN in the endosteal region. (B) Isotype control. (C) Identified thrombin, MMP-3, and MMP-7 cleavage sites in human OPN. (D) Western blot analyses using the antibody specific for the “cryptic” binding site of N-terminal trOPN. Data demonstrate recognition of human milk trOPN (lane tr, arrow) but no recognition of full-length human milk OPN (lane fl), or MMP-3 (lane 3), or MMP-7 (lane 7) cleaved human milk OPN. (E) Isotype control. (F) Western blot analysis demonstrating the presence of biotinylated fragments of human milk OPN present postcleavage with MMP-3 (lane 3) or MMP-7 (lane 7) detected using streptavidin. (G) Representative samples of bone lysates from 6 (P1 to P6) of 12 patients run under reduced conditions and immunoblotted with LF-124, an antibody that detects the N-terminal half of OPN. The N-terminal cleaved OPN (→, 30-32 kDa) is at least as dominant as the full-length ( , approximately 55 kDa) form. (H) Neither of these 2 forms were detected in the isotype control.
, approximately 55 kDa) form. (H) Neither of these 2 forms were detected in the isotype control.
Human and murine HSC express α9β1
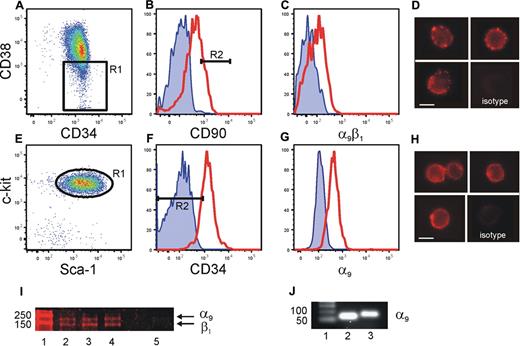
We have previously shown that HSC/HPC interact with OPN via the β1 integrin.15 Thrombin cleavage of OPN reveals a cryptic binding site that specifically binds to α9β1 and α4β1.10,21 Previous studies have demonstrated that α4β1 integrin is expressed by HSC/HPC and plays a role in the homing and mobilization of HSC/HPC to and from the BM.24,25 In contrast, the presence and function of α9β1 integrin on HSC has not been investigated. α9β1 expression by human CB and murine (Figure 2) and human BM (data not shown) HSC was analyzed using flow cytometry. Our data demonstrated robust levels of α9β1 on CB Lin−CD34+CD38−CD90bright HSC26 (Figure 2A-C), which could be clearly visualized by fluorescent microscopy (Figure 2D). Similarly, murine BM Lin−Sca+Kit+CD34− HSC expressed robust levels of α9β1 (Figure 2E-G), which could be clearly visualized by fluorescent microscopy (Figure 2H). The expression of α9 and β1 by CB CD34+CD38− cells was confirmed using Western blot analysis (Figure 2I). In addition, real-time analysis demonstrated the transcript for α9 in murine BM Lin−Sca+Kit+CD34−Flk2+ HSC and human CB Lin−CD34+CD38−CD90bright HSC (Figure 2J). These data are the first demonstration of the expression of α9 by HSC.
Expression of α9β1 on human CB and murine BM cells. Sorted lin−CD34+ CB cells (A) gated for CD38low (R1) and CD90high (B, R2, 20%), then analyzed for α9β1 expression (C). Cell surface expression of α9β1 on Lin−CD34+CD38−CD90bright cells was clearly evident by fluorescence microscopy (D). Endosteal BM sorted for LSK (E, R1) were gated for CD34low (F, R2, < 10%) and analyzed for α9 expression (G). Red line represents specific antibody staining, blue line represents unstained control. Cell surface expression of α9 on Lin−Sca+Kit+CD34− cells was clearly evident by fluorescence microscopy (H). Lysates made from 3 individual sorted human CB Lin−CD34+CD38− cells (lanes 2-4) demonstrate the presence of α9 and β1. Isotype control is in lane 5. (I). Sorted human CB Lin−CD34+CD38−CD90bright HSCs (lane 2) and murine BM Lin−Sca+Kit+CD34− HSCs (lane 3) express α9 gene when analyzed by real-time PCR. (J). Figure shows one representative example of 3 independent biological repeats from each cell population. Scale bar = 5 μm.
Expression of α9β1 on human CB and murine BM cells. Sorted lin−CD34+ CB cells (A) gated for CD38low (R1) and CD90high (B, R2, 20%), then analyzed for α9β1 expression (C). Cell surface expression of α9β1 on Lin−CD34+CD38−CD90bright cells was clearly evident by fluorescence microscopy (D). Endosteal BM sorted for LSK (E, R1) were gated for CD34low (F, R2, < 10%) and analyzed for α9 expression (G). Red line represents specific antibody staining, blue line represents unstained control. Cell surface expression of α9 on Lin−Sca+Kit+CD34− cells was clearly evident by fluorescence microscopy (H). Lysates made from 3 individual sorted human CB Lin−CD34+CD38− cells (lanes 2-4) demonstrate the presence of α9 and β1. Isotype control is in lane 5. (I). Sorted human CB Lin−CD34+CD38−CD90bright HSCs (lane 2) and murine BM Lin−Sca+Kit+CD34− HSCs (lane 3) express α9 gene when analyzed by real-time PCR. (J). Figure shows one representative example of 3 independent biological repeats from each cell population. Scale bar = 5 μm.
trOPN specifically interacts with HSC/HPC via α9β1 and α4β1 and is endogenously bound to CB HSC in vivo
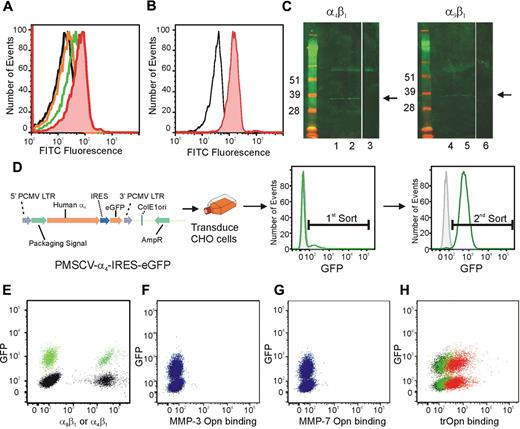
The expression of both α9β1 and α4β1 on HSC led us to analyze the ability of these cells to bind the N-terminal fragment of trOPN. To demonstrate a specific interaction, we synthesized an FITC-conjugated peptide mimicking the cryptic binding site in trOPN (SVVYGLR-FITC for human OPN and SLAYGLR-FITC for murine OPN)21 and assessed its ability to bind with CB and murine HSC/HPC (CD34+ and LSK, respectively) via α9β1 and α4β1 using flow cytometry (Figure 3A-B). In contrast to the scrambled control peptide, SVVYGLR-FITC and SLAYGLR-FITC, specifically bound to CB and murine HSC/HPC, respectively. This interaction was mediated via both α9β1 and α4β1, as preincubating CB cells with blocking antibodies to either of these integrins significantly inhibited the peptide binding (Figure 3A). These data provide further support that both α9β1 and α4β1 present on HSC specifically bind to sequences within N-terminal trOPN. To demonstrate that the N-terminal fragment interacts with HSC via α9β1 and α4β1 in vivo, lysates made from CB HSC/HPC (CD34+CD38− and CD34+CD38+) were immunoprecipitated using antibodies to either α9β1 or α4 before being deglycosylated, reduced, run on a Western blot, and probed for N-terminal OPN (Figure 3C). We demonstrated that a fragment with the appropriate molecular mass of N-terminal trOPN was endogenously bound to α9β1 on CB HSC/HPC. To confirm that this was trOPN, we assessed whether MMP-3– or MMP-7–cleaved OPN binds to α9β1 or α4β1. GFP+ or parental CHO cells were transduced to express equivalent amounts of either human α4β1 or α9β1 (Figure 3D-E). OPN was cleaved with MMP-3 or MMP-7, the cleavage fragments biotinylated, and cleavage confirmed by Western blot analysis (Figure 1F). Binding of biotinylated MMP-3– or MMP-7–cleaved OPN was assessed using a 50:50 mixture of parental α9 expressing and GFP+α4 expressing CHO cells and flow cytometry. Neither MMP-3– nor MMP-7–cleaved OPN bound to CHO cells overexpressing α9 or α4 (Figure 3F-G). In contrast trOPN bound equivalently to CHO cells overexpressing either α9 or α4 in a dose-dependent manner (Figure 3H).
Human CB HSC specifically bind to the cryptic site in N-terminal trOPN via α9β1 and α4β1 and is found endogenously bound to CB HSC. (A) The peptide, SVVYGLR-FITC, representing the cryptic site in human trOPN, was used to demonstrate specific interactions between N-terminal trOPN and human HSC/HPC (CD34+ cells; red line) compared with the scrambled peptide control (black line). Blocking antibodies specific to α9β1 (orange line) and α4 (green line) demonstrated OPN binding via both α9β1 and α4β1. Data are representative of 6 biologic repeats. (B) Similarly, the peptide, SLAYGLR-FITC, representing the cryptic site in murine trOPN, was demonstrated specific interactions between N-terminal trOPN and murine HSC/HPC (LSK cells; red line) compared with the scrambled peptide control (black line). (C) CD34+CD38+ and CD34+CD38− human CB cell lysates were immunoprecipitated using specific α4 and α9β1 antibodies and bound proteins immunoblotted with anti-OPN antibody (LF-124). An N-terminal cleaved form of OPN (approximately 32 kDa) is bound to both α4β1 and α9β1 on CB CD34+CD38+ cells (→, lanes 1 and 4) and CD34+CD38− (→, lanes 2 and 5). No staining is seen with CD34+CD38+ lysates immunoprecipitated using uncoupled beads and immunoblotted with LF-124 (lanes 3 and 6). (D) The GFP+ MSCV vector was used to transduce CHO cells to express human α4. (E) Parental α9+ (black) or GFP+ α4+ (green) CHO cells expressed equivalent levels of integrin when labeled with individual antibodies. (F) CHO cells expressing human α9β1 (GFP−, blue) or human α4β1 (GFP+, blue) were used in a 50:50 mix to determine the binding of OPN cleaved by MMP-3 (F), MMP-7 (G), or thrombin (H). There was no evidence of MMP-3 or MMP-7 cleaved OPN binding, however, trOPN bound equivalently to both integrins and in a dose-dependent manner (red, 30 μg/mL; green, 7.5 μg/mL; and black, secondary alone).
Human CB HSC specifically bind to the cryptic site in N-terminal trOPN via α9β1 and α4β1 and is found endogenously bound to CB HSC. (A) The peptide, SVVYGLR-FITC, representing the cryptic site in human trOPN, was used to demonstrate specific interactions between N-terminal trOPN and human HSC/HPC (CD34+ cells; red line) compared with the scrambled peptide control (black line). Blocking antibodies specific to α9β1 (orange line) and α4 (green line) demonstrated OPN binding via both α9β1 and α4β1. Data are representative of 6 biologic repeats. (B) Similarly, the peptide, SLAYGLR-FITC, representing the cryptic site in murine trOPN, was demonstrated specific interactions between N-terminal trOPN and murine HSC/HPC (LSK cells; red line) compared with the scrambled peptide control (black line). (C) CD34+CD38+ and CD34+CD38− human CB cell lysates were immunoprecipitated using specific α4 and α9β1 antibodies and bound proteins immunoblotted with anti-OPN antibody (LF-124). An N-terminal cleaved form of OPN (approximately 32 kDa) is bound to both α4β1 and α9β1 on CB CD34+CD38+ cells (→, lanes 1 and 4) and CD34+CD38− (→, lanes 2 and 5). No staining is seen with CD34+CD38+ lysates immunoprecipitated using uncoupled beads and immunoblotted with LF-124 (lanes 3 and 6). (D) The GFP+ MSCV vector was used to transduce CHO cells to express human α4. (E) Parental α9+ (black) or GFP+ α4+ (green) CHO cells expressed equivalent levels of integrin when labeled with individual antibodies. (F) CHO cells expressing human α9β1 (GFP−, blue) or human α4β1 (GFP+, blue) were used in a 50:50 mix to determine the binding of OPN cleaved by MMP-3 (F), MMP-7 (G), or thrombin (H). There was no evidence of MMP-3 or MMP-7 cleaved OPN binding, however, trOPN bound equivalently to both integrins and in a dose-dependent manner (red, 30 μg/mL; green, 7.5 μg/mL; and black, secondary alone).
trOPN interacting via α9β1 is potently chemotactic for HSC/HPC, and OPN is critical for the homing of murine HSC/HPC isolated from the central BM, but not those isolated from the endosteal region
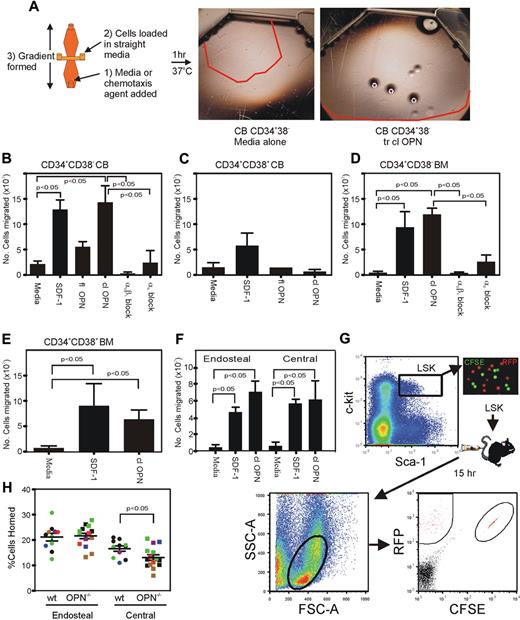
The absence of OPN in vivo results in a significant reduction in the attraction to and retention of HSC/HPC to the endosteal BM niche after transplantation.15 To investigate the reasons for these outcomes, we assessed the potential role of trOPN interacting with α9β1 and/or α4β1 in the chemotactic response of HSC/HPC in vitro (Figure 4A). Our data demonstrated that trOPN also influences the chemotactic activity of HSC/HPC in a hierarchically dependent manner. Analysis of CB and human BM HSC/HPC (CD34+CD38−) demonstrated a significant chemotactic migration to trOPN, equivalent in potency to that of SDF-1 (Figure 4B,D, respectively). The low chemotactic response of CB HSC/HPC to the full-length form of OPN (Figure 4B) could be due either to alternate integrins expressed on HSC/HPC or, more likely, to low levels of contaminating N-terminal OPN in the full-length preparation. Western blot analysis of “full-length” OPN preparations revealed the presence of a contaminating smaller OPN species in addition to the full-length form (data not shown). More committed CB and BM CD34+CD38+ cells also responded to SDF-1, but had a significantly reduced chemotactic response to trOPN or full-length OPN (Figure 4C,E). This chemotactic response of CB and BM HSC/HPC is mediated principally via α9β1. In the presence of an anti-α9β1 antibody, HSC/HPC did not migrate toward trOPN, whereas some migration toward trOPN remained in the presence of anti-α4 antibody (Figure 4B,D). Specificity of the anti-α9β1 antibody was confirmed by both flow cytometric labeling of CB cells with the appropriate isotype control and the use of the isotype control in the chemotaxis assay. No binding or effect of the isotype control was evident in either of these 2 assays (data not shown). These findings were mirrored in the murine system, where HSC/HPC isolated from the endosteal and central BM regions showed significant chemotactic response to trOPN, which was similar to that evident in response to SDF-1 (Figure 4F).
trOPN interacting via α9β1 is potently chemotactic for HSC/HPC, and OPN is critical for the homing of murine HSC/HPC isolated from the central BM, but not those isolated from the endosteal region. (A) FACS isolated CB HSC/HPC were tested in a chemotactic assay for their response to SDF-1 or OPN and compared with medium alone. The red lines indicate the distance migrated by cells stimulated under each condition. (B) A chemotactic response to full-length OPN and trOPN was compared with cell migration in medium alone and SDF-1 for CB HSC (CD34+CD38−), (C) CB progenitor cells (CD34+CD38+), (D) human BM HSC (CD34+CD38−), (E) human BM progenitor cells (CD34+CD38+), or (F) murine endosteal and central BM HSC/HPC (LSK). Human CD34+CD38− and murine LSK cells demonstrated equivalent chemotaxis to SDF-1 compared with trOPN (P < .05). To determine the mechanism of the observed migration of HSC to trOPN, CD34+CD38− cells were preincubated in blocking antibodies to either α9β1 or α4. Values are the mean plus the standard error of the mean (SEM) of the number of migrated cells. (G) LSK harvested from the endosteal (RFP+ mice) or central (CFSE+ labeled C57/B6 mice) BM regions of wild-type mice were transplanted into nonablated wild-type or Opn−/− recipients. There was a significant decrease in the homing efficiency of LSK isolated from the central BM region homing into an Opn−/− microenvironment (P < .05). (H) Data are the mean plus SEM from 10 wild-type and 13 Opn−/− recipients in 3 independent replicates as expressed by different colors.
trOPN interacting via α9β1 is potently chemotactic for HSC/HPC, and OPN is critical for the homing of murine HSC/HPC isolated from the central BM, but not those isolated from the endosteal region. (A) FACS isolated CB HSC/HPC were tested in a chemotactic assay for their response to SDF-1 or OPN and compared with medium alone. The red lines indicate the distance migrated by cells stimulated under each condition. (B) A chemotactic response to full-length OPN and trOPN was compared with cell migration in medium alone and SDF-1 for CB HSC (CD34+CD38−), (C) CB progenitor cells (CD34+CD38+), (D) human BM HSC (CD34+CD38−), (E) human BM progenitor cells (CD34+CD38+), or (F) murine endosteal and central BM HSC/HPC (LSK). Human CD34+CD38− and murine LSK cells demonstrated equivalent chemotaxis to SDF-1 compared with trOPN (P < .05). To determine the mechanism of the observed migration of HSC to trOPN, CD34+CD38− cells were preincubated in blocking antibodies to either α9β1 or α4. Values are the mean plus the standard error of the mean (SEM) of the number of migrated cells. (G) LSK harvested from the endosteal (RFP+ mice) or central (CFSE+ labeled C57/B6 mice) BM regions of wild-type mice were transplanted into nonablated wild-type or Opn−/− recipients. There was a significant decrease in the homing efficiency of LSK isolated from the central BM region homing into an Opn−/− microenvironment (P < .05). (H) Data are the mean plus SEM from 10 wild-type and 13 Opn−/− recipients in 3 independent replicates as expressed by different colors.
In addition to the impaired ability of transplanted wild-type HSC/HPC to lodge within the endosteal region of a Opn−/− recipient,15 wild-type HSC/HPC isolated from the central marrow core also showed reduced ability to home to the BM of wild-type recipients compared with those isolated from the endosteal BM region.20 We have now tested whether there is impaired homing of transplanted wild-type HSC/HPC isolated from the endosteal and central BM regions into Opn−/− mice (Figure 4G). Indeed, we demonstrated a significant impairment (∼ 2-fold) in the ability of wild-type HSC/HPC isolated from the central BM region to home into an Opn−/− microenvironment compared with a wild-type microenvironment (P = .002, Figure 4H). In contrast, HSC/HPC isolated from the wild-type endosteal marrow region showed no impairment in homing to an Opn−/− microenvironment. At 15 hours posttransplantation, greater than 20% of HSC/HPC isolated from the endosteal region had homed to the BM of either wild-type or Opn−/− recipients (Figure 4H). The mechanism for this phenomenon of decreased homing of central HSC/HPC remains unclear. One of the potential mechanisms for this involves a differential expression of α9β1 or α4β1 on these cells, but no difference in expression of either integrin was detected between LSK isolated from the endosteal or central regions (data not shown).
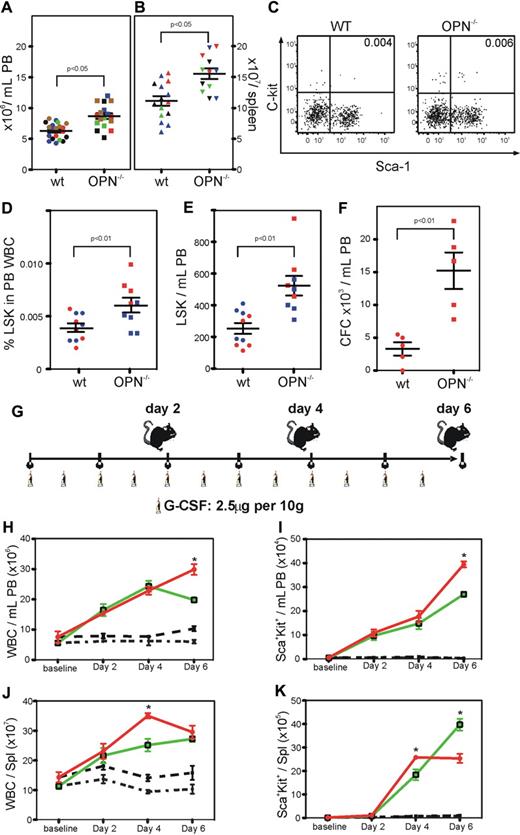
Opn−/− mice show significant levels of endogenous mobilization and exhibit enhanced mobilization in response to G-CSF
Compared with wild-type mice, Opn−/− mice had a significant increase in white blood cells in the PB and spleen (P < .05; Figure 5A-B). In addition, there was a significant increase in the incidence of HSC and progenitors in the PB (P < .01; Figure 5C-D), resulting in a significant increase in total circulating stem and progenitor cells (P < .01; Figure 5E). There was also a significant increase in the number of high-proliferative potential colony-forming cells in the PB of Opn−/− mice compared with their wild-type controls (P < .01; Figure 5F).
Opn−/− mice show significant levels of endogenous mobilization and exhibit enhanced mobilization in response to G-CSF. (A) Opn−/− mice exhibited significantly increased numbers of white blood cells (WBC) in the PB (B) and spleen compared with wild-type controls (P < .05). (C-D). This increase in WBC correlated with a significant increase in the percent, (E) number of HSC/HPC, (F) and frequency of CFC in the PB (P < .01). (G) Groups of 5 mice were mobilized with G-CSF for up to 6 days, before the analysis of the PB and spleen. (H) Opn−/− mice exhibited significantly increased numbers of WBC, (I) and percent HSC/HPC in the PB 6 days after G-CSF. In addition, Opn−/− mice exhibited significantly increased numbers of WBC (J) and percent HSC/HPC (K) in the spleen after 4 but not 6 days after G-CSF. *P < .05.
Opn−/− mice show significant levels of endogenous mobilization and exhibit enhanced mobilization in response to G-CSF. (A) Opn−/− mice exhibited significantly increased numbers of white blood cells (WBC) in the PB (B) and spleen compared with wild-type controls (P < .05). (C-D). This increase in WBC correlated with a significant increase in the percent, (E) number of HSC/HPC, (F) and frequency of CFC in the PB (P < .01). (G) Groups of 5 mice were mobilized with G-CSF for up to 6 days, before the analysis of the PB and spleen. (H) Opn−/− mice exhibited significantly increased numbers of WBC, (I) and percent HSC/HPC in the PB 6 days after G-CSF. In addition, Opn−/− mice exhibited significantly increased numbers of WBC (J) and percent HSC/HPC (K) in the spleen after 4 but not 6 days after G-CSF. *P < .05.
Due to the significant endogenous mobilization, we explored the differences in response to G-CSF for 2, 4, or 6 days in the Opn−/− mice compared with their wild-type controls (Figure 5G). After 6 days of mobilization with G-CSF, there was a significant increase in white blood cells, including stem and progenitor cells, in the PB of Opn−/− mice compared with their wild-type controls (Figure 5H-I). In contrast, while there was a significant increase in white blood cells and stem and progenitor cells in the spleen of Opn−/− mice compared with their wild-type controls 4 days after G-CSF, there were no further increases over the next 2 days, resulting in an equivalent number of white blood cells and a significant decrease in stem and progenitor cells in the spleen of Opn−/− mice compared with their wild-type controls 6 days after G-CSF (Figure 5J-K).
Discussion
HSCs have previously been described to express a plethora of receptors for cytokines and adhesion molecules, including at least 6 (αvβ3, α4β1, α2β1, α5β1, α6β1, αLβ2)27-33 of the known 25 different integrin heterodimers.34 Herein we have described, for the first time, expression of α9β1 on human and murine HSCs. The integrin α9 subunit forms a single known heterodimer, α9β1, that has previously been described to be expressed on epithelial and muscle cells and neutrophils.17,35 Structurally, the α9 subunit is closely related to the α4 subunit, and based on sequence homology, these 2 α subunits form a separate subfamily of integrin subunits.35
The expression of these 2 integrins has previously been described to be distinct on different marrow cell types. α9β1 has only been shown to be abundantly expressed on neutrophils, where it has been described to be involved in adhesion and migration.36,37 In contrast, α4 has been shown to be expressed by several mature BM cell types, as well as BM progenitors,38 and its expression is normally lost during neutrophil maturation.39 In the present study, we have demonstrated α9β1 expression on human and murine HSC/HPC. This raises the question of the role of α9β1 in HSC biology.
Many studies have analyzed the distribution of OPN in various tissues and, more recently, have specifically investigated the distribution and function of different forms of OPN. For example, trOPN is elevated in synovial fluid of rheumatoid arthritis patients where its interaction with both α9β1 and α4β1 integrins on monocytes is critical in inflammatory cell responses.40 However, 3 previous studies describing the presence and location of OPN mRNA and protein, respectively, within the BM, while demonstrating its restriction to the endosteal region,15,41,42 did not determine whether OPN was full-length or cleaved. Our study demonstrates that N-terminal trOPN is the predominant species in human BM. The use of C-terminal fragment-specific OPN antibodies did not detect endogenous C-terminal OPN in human BM (data not shown). In addition, the specific labeling of trOPN in the endosteal BM region as well as endogenously bound to α9β1 and α4β1 suggests that N-terminal trOPN may be primarily responsible for the negative regulatory effects of OPN on HSCs in the endosteal stem cell niche.15 A recent study43 demonstrated that cultured murine osteoblasts produce full-length and C-terminal OPN. However, as 2A1, which recognizes an epitope in the C-terminal fragment of OPN, was used to purify OPN from these cultures, the N-terminal fragment of OPN would not have been separated, so it is impossible to determine whether both species were present.
In accord with previous studies,44 we have demonstrated that MMP-3 and MMP-7 can cleave full-length OPN at multiple sites (Figure 1C), but found no evidence that any of the cleavage products bound to CHOα4+ or CHOα9+ cells (Figure 3F,G). In addition, due to the lack of antibodies specific for the MMP-3– and/or MMP-7–cleaved OPN, it is not possible to analyze their presence in the BM microenvironment. However, our finding that fragments of OPN generated by MMP-3 or MMP-7 did not bind to either α9β1 or α4β1 integrins on HSC/HPC suggests that even though these 2 enzymes are present within BM,45,46 there is not a functional role for MMP-3– or MMP-7–cleaved OPN fragments in regulating HSC/HPC. As is the case in vitro with cell lines,44 the presence of MMP-3– and/or MMP-7–cleaved OPN fragments in BM may have alternate unique functional roles in hemopoiesis, attributed to binding via the RGD sequence of OPN and not the cryptic sequence that binds to α9β1 or α4β1 integrins.
In the present study, we report that N-terminal trOPN mediates chemotaxis of HSC/HPC. Previous studies have shown that both full-length and trOPN induce chemotactic migration of monocytes, macrophages, neutrophils, and lymphocytes.17,40,47-50 To date, the most potent known chemoattractant for HSC/HPC is the α-chemokine SDF-1.51,52 In the present study, we demonstrated here that the N-terminal fragment of OPN is equally effective as SDF-1 in eliciting chemoattraction of HSC/HPC, and this response appears to be mediated via an interaction with α9β1 integrin. This finding provides a mechanism for the in vivo spatial distribution studies demonstrating that in addition to membrane-bound SCF,53 hyaluronic acid (HA),54 and CD44 (unpublished observations), OPN15 promoted the directed migration and/or retention of HSC/HPC to the endosteal region where OPN is expressed at high levels.15 Our data now suggest that these affects can be attributed to N-terminal trOPN.
Previous studies have identified a role for multiple cell adhesion molecules including the integrin α4β1 in the homing of transplanted HSC/HPC to the BM posttransplant.55 We have now identified a role for OPN in the recipient microenvironment for the homing of HSC/HPC isolated from the central marrow, but not the endosteal region. The mechanism underlying this difference remains unclear and is the subject of further investigation. However, these experiments highlight another distinct difference between cells with the Lin−Sca+Kit+ phenotype isolated from the endosteal and central marrow regions. LSK cells isolated from the endosteal region have a significantly increased ability to home to the BM and engraft within the endosteal BM region and have a significantly higher hemopoietic reconstitutive potential posttransplant compared with those isolated from the central BM region.20 The current study is the first description of the different effects of OPN on the homing of these 2 HSC/HPC populations.
Previous studies analyzing the conditional α4−/− mouse demonstrated endogenous HSC/HPC mobilization, with a significant increase in circulating colony-forming cells,55,56 and one-third of α4−/− PB transplant recipients surviving long-term compared with no wild-type PB transplant recipients.57 Surprisingly, there were no alterations in the biodistribution of transplanted HPC from the conditional MxCre+β1f/f mouse,58 which led to the speculation that β1 integrins expressed in nonhemopoietic niche cells are more important.29,59 In the current study, we describe that the absence of 1 α4β1 ligand, OPN, leads to an increase in white blood cells in the PB and spleen, as well as an increase in the number of circulating colony-forming progenitors. These data support the earlier hypothesis of OPN “anchoring” HSC/HPC within the BM niche.15,42 In light of the endogenous mobilization of HSC/HPC evident in Opn−/− mice, it is not surprising that our study demonstrates that compared with wild-type controls, Opn−/− mice exhibit increased mobilization after G-CSF. Our data also support a role for α4 integrin and its ligands in HSC/HPC mobilization. This is in accord with previous studies demonstrating that a function-blocking α4 integrin antibody also significantly augments the effects of G-CSF mobilization.33,60
In conclusion, we have shown that in the BM OPN exists predominantly as thrombin-cleaved forms. Notably, the N-terminal cleavage fragment binds to both α9β1 and α4β1 integrins, which are expressed on HSC/HPC. That N-terminal trOPN induces chemotaxis of HSC/HPC strongly suggests that in vivo the N-terminal form of this protein plays a key role in the homing and lodgment of HSC/HPC. A potential application of this knowledge is that OPN synthesis and cleavage may be modulated as a means to promote endogenous reseeding of residual HSC/HPC and marrow recovery during BM conditioning therapy and microenvironmental damage. In the case of HSC transplants, cleaved OPN might synergize with SDF-1 to promote HSC homing, lodgment, and engraftment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge help from Kate Rutherford with animal work, Andrew Fryga and Darren Ellemor with flow cytometry, the Mercy Hospital for Women, in particular Gabrielle Fleming and Anne Beeston, for coordinating CB collections, and St Vincent's Public and Private Hospitals, in particular Michelle Dowsey, for coordinating human marrow collections.
This work was supported in part by a grant to S.N. from the National Health and Medical Research Council (NHMRC) of Australia and a grant from the German Cancer Aid to J.G.
Authorship
Contribution: J.G. and M.S. designed and performed experiments, analyzed data, and contributed to writing the manuscript; G.O.H., B.W., G.A.W., A.V., C.L.B., and S.L. performed experiments; P.F.C. was responsible for collection of clinical material; E.S.S., P.P.L.T., D.T.D., and D.S. contributed vital new reagents and contributed to data analysis and critical appraisal of the manuscript; and D.H. and S.N. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan K. Nilsson, PO Box 8002, Monash University LPO, VIC 3168, Australia; e-mail: Susie.nilsson@stemcellcentre.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal