We analyzed 338 adult patients with acute myeloid leukemia (AML) with t(8;21) and inv(16) undergoing stem cell transplantation (SCT) who were registered in the Japan Society for Hematopoietic Cell Transplantation database. At 3 years, overall survival (OS) of patients with t(8;21) and inv(16) was 50% and 72%, respectively (P = .002). Although no difference was observed when restricted to allogeneic SCT in first complete remission (CR; 84% and 74%), OS of patients with t(8;21) and inv(16) undergoing allogeneic SCT in second or third CR (45% and 86% at 3 years; P = .008) was different. OS was not different between patients in first CR who received allogeneic SCT and those who received autologous SCT for both t(8;21) AML (84% vs 77%; P = .49) and inv(16) AML (74% vs 59%; P = .86). Patients with inv(16) not in CR did better after allogeneic SCT than those with t(8;21) (70% and 18%; P = .03). Patients with t(8;21) and inv(16) should be managed differently as to the application of SCT. SCT in first CR is not necessarily recommended for inv(16). For t(8;21) patients in first CR, a prospective trial is needed to clarify the significance of autologous SCT and allogeneic SCT over chemotherapy.
Introduction
Core binding factor (CBF) acute myeloid leukemia (AML) including t(8;21)(q22;q22) and inv(16)(p13q22)/t(16;16)(p13;q22) [t(8;21) and inv(16)] is considered to be a favorable cytogenetic subgroup in clinical studies.1,,–4 Patients with t(8;21) and inv(16) have shown a markedly improved outcome with repetitive use of high-dose cytarabine.5,,,,,,,–13 However, the major treatment failure is disease recurrence.14,–16 These patients frequently become stem cell transplantation (SCT) candidates
Both t(8;21) and inv(16) AMLs are associated with disruption of genes encoding subunits of the CBF, a heterodimeric transcriptional factor involved in the regulation of hematopoiesis.17 18 Although these 2 different cytogenetics also share common clinical characteristics, they are associated with different clinical features such as morphologic presentation and immunophenotypic marker expression.19
Several reports demonstrated inferior outcome of t(8;21) compared with inv(16), but the number of patients who underwent transplantation was limited.14,15 20 A recent study from the Dana-Farber Cancer Institute reported that both patients with t(8;21) and inv(16) de novo AML who underwent allogeneic transplantation performed favorably compared with other karyotypes.21 To identify the survival data and prognostic factors among the CBF leukemia population who received SCT, we conducted a retrospective analysis using a Japanese multi-institution database with a large number of patients.
Methods
Study population
A total of 2802 adult patients who underwent autologous or allogeneic SCT from 1996 and 2004 for AML were registered in the Japan Society for Hematopoietic Cell Transplantation (JSHCT) database. Patients who underwent SCT from unrelated donors were registered in the different registry in the study period, but not all of the patients undergoing unrelated SCT were registered in the JSHCT database. Demographic, diagnostic, clinical, cytogenetics, induction, and outcome information were collected for each patient, and were sent to a central registration center. Cytogenetic studies were performed in each center, but a central review of cytogenetic analysis was not performed.
Patients with de novo AML aged 16 to 70 years who received hematopoietic SCT as the first transplant were included in the study. No patients with prior history of autologous or allogeneic SCT were included in the study. Of the remaining 2164 patients, 178 patients with t(15;17) or PML/RARα were excluded from the analysis below (Table 1). Finally, of the 1986 patients included in the analysis, 255 were reported to have t(8;21) abnormality, and 83 to have inv(16). A total of 194 patients had no available cytogenetic data. The remaining 1454 patients with normal karyotype and other cytogenetic abnormalities were further coded and analyzed according to published Southwest Oncology Group (SWOG) criteria.3 The intermediate risk category included patients characterized by +8, −Y, +6, del(12p), or normal karyotype. The unfavorable risk category was defined by the presence of one or more of −5/del(5q), −7/del(7q), abn 3q, 11q, 20q, or 21q, del(9q), t(6;9), t(9;22), abn 17p, and complex karyotypes defined as 3 or more abnormalities. Patients with other cytogenetic aberrations were considered an unknown risk group, and were analyzed together with 194 patients with no cytogenetic data.
Cytogenetic risk groups of patients with AML who received autologous SCT and allogeneic SCT
| Cytogenetic risk groups . | No. patients . | ||
|---|---|---|---|
| Auto-SCT . | Allo-SCT . | Total . | |
| t(8;21) | 61 | 194 | 255 |
| inv(16) | 17 | 66 | 83 |
| t(15;17)* | 65 | 113 | 178 |
| Intermediate | 140 | 749 | 889 |
| Unfavorable | 35 | 325 | 360 |
| Unknown | |||
| Unknown cytogenetic risk | 27 | 178 | 205 |
| No available cytogenetic data | 44 | 150 | 194 |
| Total | 389 | 1775 | 2164 |
| Cytogenetic risk groups . | No. patients . | ||
|---|---|---|---|
| Auto-SCT . | Allo-SCT . | Total . | |
| t(8;21) | 61 | 194 | 255 |
| inv(16) | 17 | 66 | 83 |
| t(15;17)* | 65 | 113 | 178 |
| Intermediate | 140 | 749 | 889 |
| Unfavorable | 35 | 325 | 360 |
| Unknown | |||
| Unknown cytogenetic risk | 27 | 178 | 205 |
| No available cytogenetic data | 44 | 150 | 194 |
| Total | 389 | 1775 | 2164 |
Auto-SCT indicates autologous stem cell transplantation; Allo-SCT, allogeneic stem cell transplantation.
Patients with t(15;17) were excluded from the analysis.
This study was approved by the Committee for Nationwide Survey Data Management of the JSHCT. Informed consent was obtained in accordance with the Declaration of Helsinki.
Transplantation
A total of 1662 patients underwent allogeneic SCT, and 324 underwent autologous SCT. Patients were treated with various conditioning regimens, but most of those who underwent autologous transplantation received non–total body irradiation (TBI) regimens (97%), including busulfan (BU), cytarabine (CA), and etoposide. The most frequently used conditioning regimens before allogeneic SCT were cyclophosphamide (Cy) plus TBI (n = 327 patients), and BU plus Cy (n = 267). Conditioning regimens before allogeneic SCT also included more intensified regimens such as CA plus Cy plus TBI (n = 262) and BU plus Cy plus TBI (n = 146), or reduced-intensity conditioning regimens with fludarabine (n = 241) or cladribine (n = 19).
Stem cell sources for allogeneic SCT were bone marrow in 871 patients, peripheral blood stem cell in 570 patients, bone marrow plus peripheral blood stem cell in 23 patients, and cord blood in 190 patients. A total of 1242 patients underwent allogeneic SCT from a related donor, and 404 patients underwent SCT from an unrelated donor.
Of the 1637 patients who had available data, 74% received transplants from human leukocyte antigen (HLA)–matched donors. Among patients who received unrelated bone marrow transplants, 156 patients were HLA genotypically matched and 51 were HLA mismatched. HLA data for 39 mismatched unrelated bone marrow transplantation patients were available. A total of 32 patients were one locus mismatched, and 7 patients were 2 loci mismatched. Among patients receiving unrelated cord blood transplants, 19 patients were serologically HLA matched and 170 patients were mismatched. HLA incompatibility was 5 of 6 HLA matched in 57 patients, 4 of 6 HLA matched in 99 patients, 3 of 6 HLA matched in 7 patients, and 1 of 6 HLA matched in 1 patient.
Graft-versus-host disease (GVHD) prophylaxis mostly consisted of methotrexate and a calcineurin inhibitor, either cyclosporin A or tacrolimus. Several other prophylaxes include mycophenolate mofetil, antithymocyte globulin, and CD34+ selection. The incidence of acute GVHD was evaluated in 1488 patients who survived more than 28 days, and chronic GVHD was evaluated in 1302 patients who survived more than 100 days after allogeneic SCT. GVHD was evaluated in each center.
Statistical analysis
Correlation between the 2 groups was examined with the chi-square test, Fisher exact test, and the Mann-Whitney U test. Disease-free survival (DFS) was calculated from the date of transplantation until the date of relapse or the date of death in CR. Patient survival data were analyzed with the method of Kaplan and Meier and compared by the log-rank test.
Univariate and multivariate analyses for OS were performed with the aid of the Cox proportional hazard regression model, and variables were selected with the stepwise method. The following variables were evaluated: age, sex, and disease status at transplantation; CR versus not in CR; the number of induction courses to achieve CR; one course versus more than one course and failure; type of transplantation (allogeneic SCT vs autologous SCT); conditioning regimen (reduced intensity vs myeloablative); TBI regimen or not; and the existence of additional karyotype abnormalities or not. For those who received allogeneic SCT, in addition to these variables, the following were also evaluated: type of GVHD prophylaxis; short-course methotrexate plus cyclosporin A or short methotrexate plus FK506; acute GVHD, grade II to IV or grade III to IV; chronic GVHD; HLA mismatch; donor; and donor source. The doses of methotrexate were not surveyed. Each factor was considered to be prognostic if the P value was less than .05. Data were analyzed with the Stata 9.2 statistical software (College Station, TX).
Results
Initial characteristics of patients
The median age of all patients with AML in total was 41 years old (range, 16-70 years old). Median follow-up period of living patients was 37.3 months (range, 0.4-108 months). Patients were categorized into 5 cytogenetic subgroups: with t(8;21), with inv(16), intermediate risk cytogenetics, unfavorable cytogenetics, and an unknown risk group. Table 1 shows the number of patients in each cytogenetic subgroup and patients with t(15;17), who were excluded from the analysis.
Characteristics of the patients with CBF who underwent allogeneic SCT or autologous SCT are shown in Table 2. No significant difference was observed between characteristic of 2 groups of patients with CBF who received autologous SCT, except for the initial white blood cell count.
Characteristics of patients with CBF AML
| . | Auto-SCT . | Allo-SCT . | ||||
|---|---|---|---|---|---|---|
| t(8;21)(n = 61), no. . | inv(16)(n = 17), no. . | P . | t(8;21)(n = 194), no. . | inv(16)(n = 66), no. . | P . | |
| Median age, y (range) | 44 (17-68) | 37 (19-61) | .59 | 39 (16-70) | 34 (16-64) | .054 |
| Median WBC, g/L (range) | 8.8 (0.2-94) | 33 (2.1-199) | .02 | 11 (.6-366) | 53 (1.8-284) | < .001 |
| Sex | ||||||
| Male | 41 | 12 | .79 | 117 | 40 | .93 |
| Female | 20 | 5 | 74 | 26 | ||
| No. of induction chemotherapy at diagnosis of AML | ||||||
| 1 course | 48 | 15 | .72 | 125 | 55 | .002 |
| > 1 or failure* | 11 | 2 | 56 | 7 | ||
| Additional cytogenetic abnormalities | ||||||
| None | 53 | 15 | > .999 | 153 | 54 | .61 |
| Positive | 8 | 2 | 41 | 12 | ||
| Disease status at SCT | ||||||
| CR | 55 | 16 | > .999 | 108 | 52 | < .001 |
| Not in CR | 6 | 1 | 85 | 11 | ||
| CR1 | 43 | 13 | .98 | 49 | 21 | .29 |
| CR2 | 7 | 1 | 45 | 26 | ||
| CR3 | 0 | 1 | 5 | 4 | ||
| Conditioning regimen | ||||||
| TBI | 0 | 1 | .22 | 118 | 47 | .078 |
| Not TBI | 61 | 16 | 71 | 16 | ||
| . | Auto-SCT . | Allo-SCT . | ||||
|---|---|---|---|---|---|---|
| t(8;21)(n = 61), no. . | inv(16)(n = 17), no. . | P . | t(8;21)(n = 194), no. . | inv(16)(n = 66), no. . | P . | |
| Median age, y (range) | 44 (17-68) | 37 (19-61) | .59 | 39 (16-70) | 34 (16-64) | .054 |
| Median WBC, g/L (range) | 8.8 (0.2-94) | 33 (2.1-199) | .02 | 11 (.6-366) | 53 (1.8-284) | < .001 |
| Sex | ||||||
| Male | 41 | 12 | .79 | 117 | 40 | .93 |
| Female | 20 | 5 | 74 | 26 | ||
| No. of induction chemotherapy at diagnosis of AML | ||||||
| 1 course | 48 | 15 | .72 | 125 | 55 | .002 |
| > 1 or failure* | 11 | 2 | 56 | 7 | ||
| Additional cytogenetic abnormalities | ||||||
| None | 53 | 15 | > .999 | 153 | 54 | .61 |
| Positive | 8 | 2 | 41 | 12 | ||
| Disease status at SCT | ||||||
| CR | 55 | 16 | > .999 | 108 | 52 | < .001 |
| Not in CR | 6 | 1 | 85 | 11 | ||
| CR1 | 43 | 13 | .98 | 49 | 21 | .29 |
| CR2 | 7 | 1 | 45 | 26 | ||
| CR3 | 0 | 1 | 5 | 4 | ||
| Conditioning regimen | ||||||
| TBI | 0 | 1 | .22 | 118 | 47 | .078 |
| Not TBI | 61 | 16 | 71 | 16 | ||
Correlation between the two groups was examined.
WBC indicates white blood cell count; g/L, 109/L; CR1, first complete remission; and CR2 or 3, second or third CR.
More than 1 or failure includes patients who did not achieve complete remission after first course of induction chemotherapy, and those who were resistant to induction chemotherapy.
Of the 259 patients with CBF who received allogeneic SCT, significantly more patients with t(8;21) had failed to achieve CR with a single course of induction chemotherapy at diagnosis (P = .002), and were not in CR at the time of transplantation (P < .001). Among patients in CR at transplantation, the ratio of those in first, second, or third CR was not different between t(8;21) and inv(16) subgroups. Significantly more patients with inv(16) received transplants from an unrelated donor (P = .004). Table 3 and Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) summarize the transplantation data of those undergoing allogeneic SCT. More patients with inv(16) received unrelated transplants compared with t(8;21) patients (P = .004).
Summary of allogeneic SCT
| . | t(8;21) (n = 194), no. . | inv(16), (n = 66), no. . | P . |
|---|---|---|---|
| Conditioning regimen | |||
| RIST | 31 | 9 | .66 |
| Myeloablative | 161 | 56 | |
| GVHD prophylaxis* | |||
| sMTX+CyA | 136 | 48 | .78 |
| sMTX+FK | 20 | 8 | |
| HLA | |||
| Match | 146 | 47 | .5 |
| Mismatch | 45 | 18 | |
| Donor | |||
| Related | 161 | 44 | .004 |
| Unrelated | 32 | 22 | |
| Stem cell source | |||
| BM | 101 | 40 | .27 |
| PB | 72 | 17 | |
| CB | 18 | 7 | |
| aGVHD grade | |||
| 0-I | 117 | 37 | .54 |
| II-IV | 60 | 22 | |
| cGVHD type | |||
| None | 64 | 28 | .28 |
| Lmt/Ext | 67 | 20 |
| . | t(8;21) (n = 194), no. . | inv(16), (n = 66), no. . | P . |
|---|---|---|---|
| Conditioning regimen | |||
| RIST | 31 | 9 | .66 |
| Myeloablative | 161 | 56 | |
| GVHD prophylaxis* | |||
| sMTX+CyA | 136 | 48 | .78 |
| sMTX+FK | 20 | 8 | |
| HLA | |||
| Match | 146 | 47 | .5 |
| Mismatch | 45 | 18 | |
| Donor | |||
| Related | 161 | 44 | .004 |
| Unrelated | 32 | 22 | |
| Stem cell source | |||
| BM | 101 | 40 | .27 |
| PB | 72 | 17 | |
| CB | 18 | 7 | |
| aGVHD grade | |||
| 0-I | 117 | 37 | .54 |
| II-IV | 60 | 22 | |
| cGVHD type | |||
| None | 64 | 28 | .28 |
| Lmt/Ext | 67 | 20 |
Correlation between the two groups was examined. Some of the missing data was not available, and total numbers do not add up to the number of the patients in each group.
RIST indicates reduced intensity stem cell transplantation; sMTX, short-course methotrexate; CyA, cyclosporin A; FK, tacrolimus; BM, bone marrow; PB, peripheral blood; CB, cord blood; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; Lmt, limited; and Ext, extensive.
Dose of methotrexate was not surveyed in the study. Detail of other GVHD prophylaxis regimens are in Table S1.
Overall survival
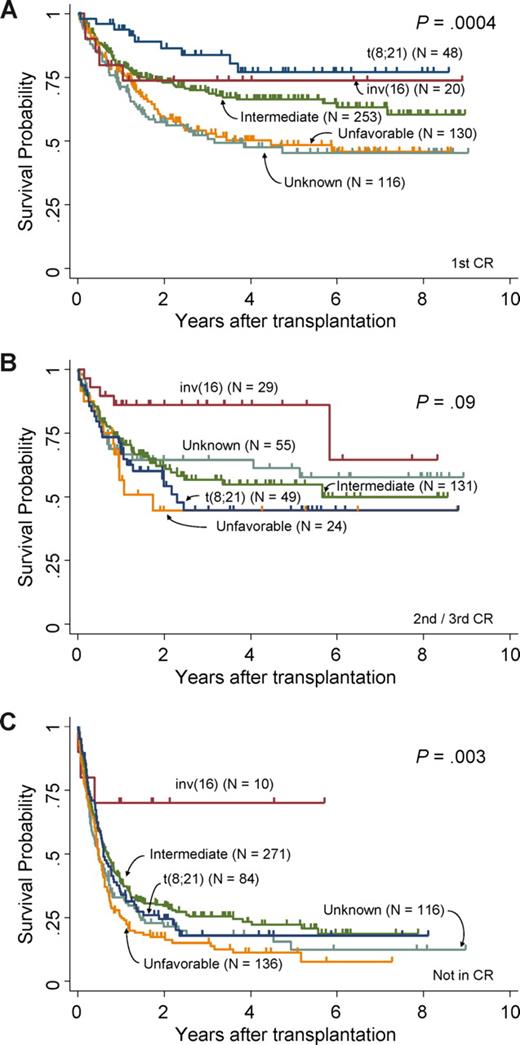
The OS of 1986 patients with AML at 3 years was 48%, and those with t(8;21), inv(16), intermediate, unfavorable, and unknown cytogenetic risks showed OS of 50%, 72%, 52%, 35%, and 45%, respectively (P < .001). Figure 1 shows survival curves of patients with AML patients who underwent allogeneic SCT in first CR (Figure 1A), in second or third CR (Figure 1B), or not in CR (Figure 1C), categorized by the cytogenetic abnormalities. Survival data are listed in Table 4. The OS of patients with t(8;21), inv(16), and intermediate, unfavorable, and unknown risk undergoing allogeneic SCT in first CR was 84%, 74%, 69%, 53%, and 52%, respectively (P < .001), and that of patients undergoing allogeneic-SCT in second or third CR was 45%, 86%, 57%, 44%, and 64%, respectively (P = .09). OS of patients undergoing allogeneic SCT not in CR was 18%, 70%, 25%, 15%, and 18%, respectively (P = .003).
OS difference of patients undergoing allogeneic SCT between cytogenetic subgroups. (A) Survival curves of patients undergoing allogeneic SCT in first CR. (B) Survival curve of patients undergoing allogeneic SCT in second or third CR. (C) Survival curves of patients undergoing allogeneic SCT not in CR. Each are categorized by cytogenetic risk groups, respectively.
OS difference of patients undergoing allogeneic SCT between cytogenetic subgroups. (A) Survival curves of patients undergoing allogeneic SCT in first CR. (B) Survival curve of patients undergoing allogeneic SCT in second or third CR. (C) Survival curves of patients undergoing allogeneic SCT not in CR. Each are categorized by cytogenetic risk groups, respectively.
Outcome of the AML patient population by cytogenetic risk groups
| . | t(8;21) . | inv(16) . | Intermediate . | Unfavorable . | Unknown . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | ||
| OS | |||||||||||
| Allogeneic SCT | |||||||||||
| CR1 | 84 | 48 | 74 | 20 | 69 | 253 | 53 | 130 | 52 | 116 | < .001 |
| CR2/CR3 | 45 | 49 | 86 | 29 | 57 | 131 | 44 | 24 | 64 | 55 | .09 |
| Non-CR | 18 | 84 | 70 | 10 | 25 | 271 | 15 | 136 | 18 | 116 | .003 |
| Autologous SCT | |||||||||||
| CR1 | 77 | 42 | 59 | 13 | 74 | 89 | 38 | 15 | 71 | 39 | .05 |
| CR2/CR3 | 43 | 7 | 50 | 2 | 59 | 15 | 44 | 6 | 42 | 18 | .8 |
| Non-CR | 17 | 6 | 100 | 1 | 25 | 16 | 0 | 10 | 13 | 8 | .35 |
| DFS | |||||||||||
| Allogeneic SCT | |||||||||||
| CR1 | 78 | 48 | 73 | 19 | 63 | 249 | 47 | 129 | 48 | 113 | < .001 |
| CR2/CR3 | 43 | 48 | 71 | 27 | 47 | 129 | 42 | 22 | 57 | 54 | .32 |
| Non-CR | 18 | 81 | 75 | 8 | 22 | 255 | 10 | 128 | 16 | 107 | .005 |
| Autologous SCT | |||||||||||
| CR1 | 73 | 41 | 62 | 13 | 64 | 81 | 33 | 15 | 61 | 36 | .09 |
| CR2/CR3 | 43 | 7 | 50 | 2 | 36 | 14 | 50 | 6 | 39 | 18 | .89 |
| Non-CR | 17 | 6 | 100 | 1 | 25 | 16 | 0 | 10 | 17 | 6 | .45 |
| . | t(8;21) . | inv(16) . | Intermediate . | Unfavorable . | Unknown . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | ||
| OS | |||||||||||
| Allogeneic SCT | |||||||||||
| CR1 | 84 | 48 | 74 | 20 | 69 | 253 | 53 | 130 | 52 | 116 | < .001 |
| CR2/CR3 | 45 | 49 | 86 | 29 | 57 | 131 | 44 | 24 | 64 | 55 | .09 |
| Non-CR | 18 | 84 | 70 | 10 | 25 | 271 | 15 | 136 | 18 | 116 | .003 |
| Autologous SCT | |||||||||||
| CR1 | 77 | 42 | 59 | 13 | 74 | 89 | 38 | 15 | 71 | 39 | .05 |
| CR2/CR3 | 43 | 7 | 50 | 2 | 59 | 15 | 44 | 6 | 42 | 18 | .8 |
| Non-CR | 17 | 6 | 100 | 1 | 25 | 16 | 0 | 10 | 13 | 8 | .35 |
| DFS | |||||||||||
| Allogeneic SCT | |||||||||||
| CR1 | 78 | 48 | 73 | 19 | 63 | 249 | 47 | 129 | 48 | 113 | < .001 |
| CR2/CR3 | 43 | 48 | 71 | 27 | 47 | 129 | 42 | 22 | 57 | 54 | .32 |
| Non-CR | 18 | 81 | 75 | 8 | 22 | 255 | 10 | 128 | 16 | 107 | .005 |
| Autologous SCT | |||||||||||
| CR1 | 73 | 41 | 62 | 13 | 64 | 81 | 33 | 15 | 61 | 36 | .09 |
| CR2/CR3 | 43 | 7 | 50 | 2 | 36 | 14 | 50 | 6 | 39 | 18 | .89 |
| Non-CR | 17 | 6 | 100 | 1 | 25 | 16 | 0 | 10 | 17 | 6 | .45 |
When patients undergoing allogeneic SCT in first CR were analyzed, 3-year OS was not significantly different between patients with t(8;21) and inv(16) (84% and 74%, respectively; P = .28), between inv(16) and intermediate risk groups (74% and 69%, respectively; P = .84), or between t(8;21) and intermediate risk groups (84% and 69%, respectively; P = .06). However, when patients undergoing allogeneic SCT in second or third CR were analyzed, the 3-year OS of patients with inv(16) was significantly better than patients with t(8;21) (86% and 45%, respectively; P = .008), and better than intermediate risk patients (86% and 57%, respectively; P = .03). Difference was not significant between patients in the intermediate risk group and t(8;21) undergoing allogeneic SCT in second or third CR (P = .36). The OS of inv(16) patients undergoing allogeneic SCT not in CR was 70% at 3 years, which was also significantly better than that of t(8;21) (18%; P = .03) and the intermediate risk group (25%; P = .045).
In addition, the OS of t(8;21) undergoing allogeneic SCT in first CR was significantly better than that of the unfavorable risk group (84% and 53%, respectively; P < .001), but the difference between the 2 groups was not significant among patients undergoing allogeneic SCT in second or third CR. In contrast, OS was not different between inv(16) and unfavorable groups undergoing allogeneic SCT in first CR, but it was significantly different when they underwent allogeneic SCT in second or third CR (86% and 44%, for inv(16) and unfavorable groups, respectively; P = .01) or allogeneic SCT in non-CR (70% and 15%, respectively; P = .006).
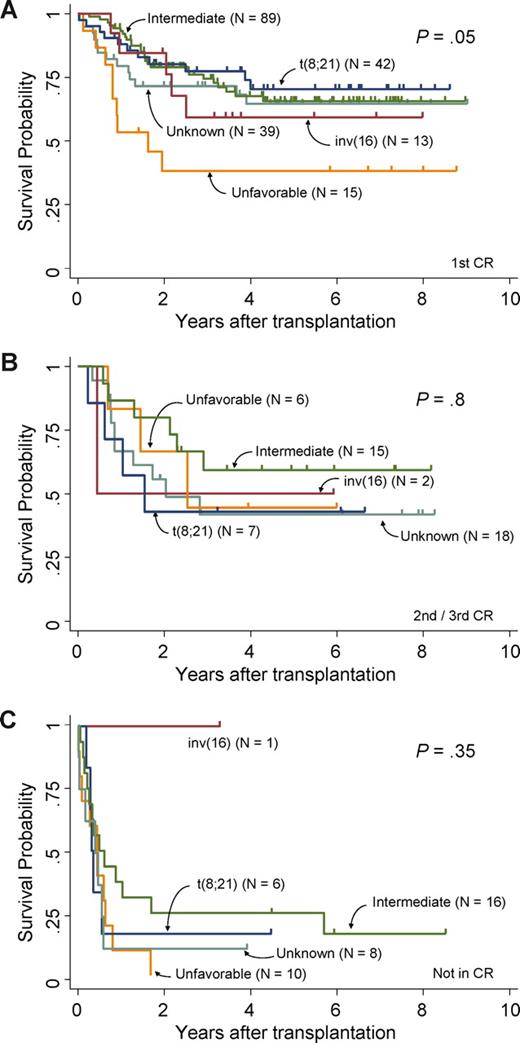
Survival curves of patients who underwent autologous SCT in first CR, second or third CR, and not in CR are shown in Figure 2A, 2B, and 2C, respectively. The overall survival of patients with t(8;21), inv(16), and intermediate, unfavorable, and unknown cytogenetic risks in first CR was 77%, 59%, 74%, 38%, and 71%, respectively (P = .049), while that of patients undergoing autologous SCT in second or third CR was 43%, 50%, 59%, 44%, and 42%, respectively (P = .8). The OS of patients undergoing autologous SCT not in CR with t(8;21), inv(16), intermediate, and unknown risks was 17%, 100%, 25%, and 13%, respectively, and the survival curve of patients in the unfavorable risk group did not reach 3 years (P = .35).
OS difference of patients undergoing autologous SCT between cytogenetic subgroups. (A) Survival curves of patients undergoing autologous SCT in first CR. (B) Survival curves of patients undergoing autologous SCT in second or third CR. (C) Survival curves of patients undergoing autologous SCT not in CR. Each are categorized by cytogenetic risk groups, respectively.
OS difference of patients undergoing autologous SCT between cytogenetic subgroups. (A) Survival curves of patients undergoing autologous SCT in first CR. (B) Survival curves of patients undergoing autologous SCT in second or third CR. (C) Survival curves of patients undergoing autologous SCT not in CR. Each are categorized by cytogenetic risk groups, respectively.
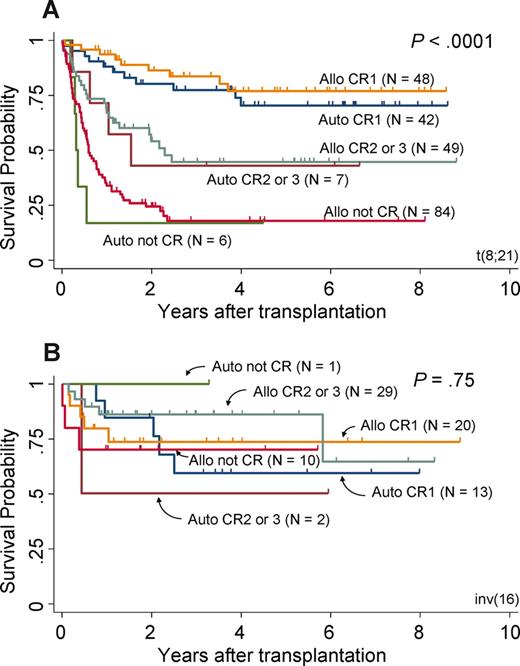
Figure 3A and B focus on t(8;21) and inv(16) patients, stratified according to the type of (allogeneic or autologous) and disease status at the time of transplantation (first CR, second or third CR, and not in CR). The 3-year overall survival of t(8;21) patients in first CR was not different between allogeneic and autologous transplantation (84% and 77%, respectively), as well as that of patients in second or third CR (45% and 43%, respectively) and patients not in CR (18% and 17%, respectively). Similarly, the 3-year OS of inv(16) patients was not different between allogeneic and autologous transplantation when they underwent transplantation in first CR (74% and 59%). A significant difference was observed among the 3 disease status groups of t(8;21) patients (P < .001; Figure 3A), but not inv(16) patients (P = .75; Figure 3B).
OS of patients with CBF. Survival curves of patients with t(8;21) (A) and with inv(16) (B). Both are stratified according to the type of transplantation (allogeneic or autologous) and disease status at the time of transplantation (first CR, second or third CR, and not in CR).
OS of patients with CBF. Survival curves of patients with t(8;21) (A) and with inv(16) (B). Both are stratified according to the type of transplantation (allogeneic or autologous) and disease status at the time of transplantation (first CR, second or third CR, and not in CR).
The OS of allogeneic SCT, excluding cord blood transplantation, was not different from the analysis presented here, including bone marrow, peripheral blood, and cord blood transplantation (Table S2; Figures S1,S2).
DFS after SCT was also different among cytogenetic risk groups (P < .001). DFS of patients with inv(16) (69% at 3 years) was better compared with t(8;21) (49%), intermediate (46%), unfavorable (31%), and unknown (41%) risk groups. Among patients undergoing allogeneic SCT in first CR, DFS was also different among cytogenetic subgroups (P < .001). When t(8;21), inv(16), and intermediate cytogenetic subgroups undergoing allogeneic SCT in first CR were compared, the difference was not statistically significant between t(8;21) and inv(16) (78% and 73% at 3 years; P = .58), between t(8;21) and intermediate risk group (78% and 63%; P = .1), nor between inv(16) and intermediate risk group (73% and 63%; P = .65). DFS of patients with t(8;21) undergoing allogeneic SCT in first CR was better than that of the unfavorable risk group (78% and 47%, respectively; P < .001), but the difference was not significant between inv(16) and unfavorable risk groups (73% and 47%, respectively; P = .16).
DFS was not significantly different when 5 cytogenetic subgroups among patients undergoing allogeneic SCT in second or third CR were compared (P = .32). The DFS of patients undergoing allogeneic SCT in second or third CR was not significantly different between t(8;21) and inv(16) (43% and 71% at 3 years; P = .053), t(8;21) and the intermediate group (43% and 47%; P = .76), or inv(16) and the intermediate group (71% and 47%; P = .06). The difference was also not significant between t(8;21) and unfavorable risk groups (43% and 42%; P = .7), nor between inv(16) and unfavorable risk groups (71% and 42%; P = .06). The DFS of patients undergoing allogeneic SCT who were not in CR was significantly different among the 5 cytogenetic subgroups (P = .005), and that of inv(16) (75% at 3 years) was significantly better than t(8;21) (18%; P = .02), the intermediate risk group (22%; P = .03) and the unfavorable risk group (10%; P = .003).
Relapse and TRM
The relapse rate (RR) after SCT also differed among cytogenetic subgroups (P < .001). The RR of patients with inv(16) (18% at 3 years) was lower than t(8;21) (38%), intermediate (38%), and unfavorable (56%) risk groups. The RR of t(8;21) and inv(16) after allogeneic SCT was not statistically different in either first CR (16% and 6%; P = .45) or second or third CR (34% and 16%, respectively; P = .09).
Transplantation-related mortality (TRM) of all patients with AML was 22% at 3 years. The TRM of t(8;21) (18%), inv(16) (11%), and intermediate (21%), unfavorable (24%), and unknown risk groups (27%) was significantly different among cytogenetic risk groups (P = .02).
Evaluation of prognostic variables in CBF
Univariate analyses of t(8;21) showed that age (P = .004), not in CR at transplantation (P < .001), allogeneic SCT (P = .01), and TBI regimen (P = .006) were significant prognostic factors indicating poor OS (Table 5). Multivariate analysis for OS revealed older age (P = .01) and not in CR at transplantation (P < .001) as the independent prognostic variables. Univariate analyses of t(8;21) patients who received allogeneic SCT in CR showed that age (P = .02), TBI regimen (P = .01), and second and third CR at transplantation (P < .001) were also significantly prognostic for poor OS. These variables remained significant after multivariate analysis. Univariate analyses for inv(16) patients showed only age (P = .009) to be a significant prognostic factor (Table 5). The univariate analysis of inv(16) patients who underwent allogeneic SCT in CR showed only additional karyotype abnormalities to be an unfavorable prognostic variable (P = .009).
Prognostic factors affecting overall survival of patients with t(8;21)
| Variables . | Unfavorable factors . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| t(8;21) | ||||
| Age | 1.02 | 1.01-1.04 | .004 | |
| Disease status at SCT | Not in CR | 4.4 | 3.1-6.5 | < .001 |
| Transplantation | Allo-SCT | 1.9 | 1.2-3.0 | .01 |
| Conditioning regimen | TBI | 1.7 | 1.2-2.5 | .005 |
| inv(16) | ||||
| Age | 1.1 | 1.0-1.1 | .009 |
| Variables . | Unfavorable factors . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| t(8;21) | ||||
| Age | 1.02 | 1.01-1.04 | .004 | |
| Disease status at SCT | Not in CR | 4.4 | 3.1-6.5 | < .001 |
| Transplantation | Allo-SCT | 1.9 | 1.2-3.0 | .01 |
| Conditioning regimen | TBI | 1.7 | 1.2-2.5 | .005 |
| inv(16) | ||||
| Age | 1.1 | 1.0-1.1 | .009 |
CI indicates confidence interval.
Additional cytogenetic abnormalities to CBF
A total of 49 patients with t(8;21) and 14 with inv(16) had additional cytogenetic abnormalities. Data for additional cytogenetic abnormalities were obtained in 42 patients with t(8;21) and 13 patients with inv(16) (Table 6). Additional abnormalities were selected that have been reported to be prognostic by others, including loss of sex chromosome (X or Y), trisomy 8, trisomy 4, del(7q), and del(9q) for the t(8;21) group, and trisomy 22, trisomy 8, trisomy 21, del(7q), and del(9q) for the inv(16) group.14,15,20,22 23 There were no patients with trisomy 21 in the data of patients with CBF. Patients with t(8;21) and patients with inv(16) were analyzed separately. Among t(8;21) patients undergoing allogeneic SCT, survival was not different between patients with and without additional karyotype abnormalities. When patients with inv(16) were analyzed, the survival was not different between patients with (n = 13) and without (n = 67) additional abnormalities (61% and 74%, respectively; P = .07). The survival of patients undergoing allogeneic SCT without additional abnormality (n = 52) was significantly better than that with additional abnormality (n = 11), (85% and 53%, respectively; P = .004). When analysis was restricted to patients in CR with inv(16) undergoing allogeneic SCT, a similar difference was observed (86% without additional abnormality [n = 42], and 60% with additional abnormality [n = 8], respectively; P = .03). Difference in OS was observed among non-CR patients with (n = 9) and without (n = 1) additional abnormality, but this difference may not be relevant with too few patients in the analysis. We further analyzed subgroups of additional abnormalities of the patients with inv(16). Although the number of patients were limited, significant difference was found among 3 groups of patients; trisomy 8 or trisomy 22 as a sole abnormality (n = 4), without additional abnormality (n = 69), and other additional abnormality to inv(16) (n = 10). The OS at 3 years were 100%, 74%, and 42%, respectively (P = .002). The OS of patients undergoing allogeneic SCT was also different among these 3 groups (100%, n = 3; 85%, n = 52; and 33%, respectively; P < .001).
Additional cytogenetic abnormalities among patients with CBF
| Additional cytogenetic abnormalities . | t(8;21), no. . | inv(16), no. . |
|---|---|---|
| None | 206 | 69 |
| With additional abnormalities | 49 | 14* |
| −Y | 10 | 0 |
| −X | 5 | 0 |
| Trisomy 22 | 0 | 3† |
| Trisomy 8 | 0 | 2† |
| Trisomy 4 | 2* | 0 |
| Complex | 7 | 4 |
| del(7q) | 1† | 2 |
| del(9q) | 6 | 0 |
| Other abnormalities | 27 | 9‡ |
| Unknown | 7 | 1 |
| Additional cytogenetic abnormalities . | t(8;21), no. . | inv(16), no. . |
|---|---|---|
| None | 206 | 69 |
| With additional abnormalities | 49 | 14* |
| −Y | 10 | 0 |
| −X | 5 | 0 |
| Trisomy 22 | 0 | 3† |
| Trisomy 8 | 0 | 2† |
| Trisomy 4 | 2* | 0 |
| Complex | 7 | 4 |
| del(7q) | 1† | 2 |
| del(9q) | 6 | 0 |
| Other abnormalities | 27 | 9‡ |
| Unknown | 7 | 1 |
Patients with additional change to inv(16) and trisomy 4 with t(8;21) tended to show poor survival tendency, with P < .1.
All patients with trisomy 22, trisomy 8 with inv(16), and del(7q) with t(8;21) were alive and censored at survival analysis.
Other abnormalities with inv(16) was poorly prognostic, with P < .001.
Discussion
We analyzed the outcome of a large group of patients with adult CBF AML in Japan who were treated with SCT. The current study focused on the different outcome of the 2 different cytogenetic subgroups of patients with CBF AML undergoing SCT. Our study demonstrated a comparable outcome between patients with t(8;21) and inv(16) undergoing SCT in first CR, but the prognosis between these 2 cytogenetic subgroups was different beyond first CR.
In the literature, there have been several reports showing inferior survival of patients with t(8;21) compared with inv(16) patients undergoing induction chemotherapy and SCT.14,15 20 Other studies categorized both patients with t(8;21) and inv(16) undergoing allogeneic SCT together as good-risk CBF AML,1 21 with a relatively comparable prognosis. In our study, OS of patients with t(8;21) undergoing allogeneic SCT in first CR was not statistically different from intermediate cytogenetic subgroup (84% and 79% at 3 years, respectively; P = .058). Moreover, the survival of inv(16) (74% at 3 years) and intermediate cytogenetic subgroups showed no statistically significant difference.
In contrast, we have here demonstrated that the prognosis of patients with t(8;21) undergoing allogeneic SCT with second or third CR disease was significantly poor compared with those with inv(16). This finding is consistent with those of other studies reporting differences between the 2 types of CBF AML.14 15 In the present study, non-CR disease with t(8;21) was also significantly poor compared with patients with inv(16). The Acute Leukemia French Association reported that allogeneic donor availability among patients with CBF AML who were in second CR was a prognostic factor for better survival.16 We believe that different treatment strategies should be applied for patients with t(8;21) and those with inv(16) other than first CR.
Patients with t(8;21) undergoing allogeneic SCT and autologous SCT had a similar survival rate when they underwent transplantation in first CR, and in further CR. No survival difference between allogeneic SCT and autologous SCT was also observed among inv(16) patients receiving SCT in first CR (74% and 59%, respectively). The University of California, San Francisco (UCSF) group described the good results of patients with advanced AML undergoing autologous SCT in second or third remission, including patients with CBF.24 As in our study, the European Group for Blood and Marrow Transplantation (EBMT) reported that the survival rate of t(8;21) patients who received allogeneic bone marrow transplantation was not significantly different from that of patients who received autologous SCT.1 Results by others showed that allogeneic SCT in first CR did not benefit good-risk cytogenetic subgroups.3,25 26 Schlenk et al also demonstrated that t(8;21) patients receiving allogeneic SCT or chemotherapy showed no difference in outcome.23 These results suggest that autologous SCT can be considered as postremission therapy for patients with CBF AML, but it remains unclear whether SCT is more beneficial for patients with CBF than high-dose cytarabine. Survival of patients with inv(16) was favorable beyond first CR. Patients with inv(16) in second or third CR, or even non-CR patients, are good candidates for allogeneic SCT. There are long-term survivors after allogeneic SCT in non-CR disease, so t(8;21) patients with no other choice of treatment, such as those in further CR or non-CR, can proceed to allogeneic SCT. In order to confirm the appropriate treatment for t(8;21) patients in first CR, a prospective trial is needed to compare the results of autologous SCT for t(8;21) in first CR with standard chemotherapy. t(8,21) patients with suitable related or well-matched donors should be recommended to participate in a risk-adopted prospective trial when they receive allogeneic SCT in first CR.
There were differences between the 2 types of CBF AML with respect to prognostic valuables. Age was a significant and independent prognostic variable in both t(8;21) and inv(16) patients, a finding in agreement with reports from some,14 27 but not all, investigators.28 Transplantation in CR was a significant and independent prognostic factor for patients with t(8;21), but not for those with inv(16). The Cancer and Leukemia Group B (CALGB) also reported differences between t(8;21) and inv(16) in prognostic factors, in terms of race, sex, and secondary cytogenetic abnormalities.14 Among patients with CBF AML, t(8;21) and inv(16) patients undergoing SCT should be considered 2 separate clinical entities in future clinical studies.
Several specific additional karyotype abnormalities have been reported to be prognostic in patients with CBF AML. Among t(8;21) patients, no specific additional karyotype abnormality was prognostic for overall survival. The poor prognosis of t(8;21) patients with trisomy 4 has been reported by others,22 but the survival difference was not statistically significant (P = .085) in our case series. Since there were limited numbers of patients with additional abnormalities, the real significance of each additional abnormality should be investigated in large numbers of patients.
The reason for the different survival results between patients with t(8;21) and inv(16) undergoing allogeneic SCT in our study remains unclear. The impact of additional mutational events such as c-Kit, FLT3, RAS, and gene-expression profiles was reported to be associated with the clinical outcome of patients with CBF AML.29,,,,–34 The effects of these additional mutational events and gene-expression profiles on the clinical outcome of autologous and allogeneic SCT have not yet been studied. Which proportion of the patients with CBF AML benefited from earlier SCT remains to be identified in future clinical studies. Recent studies by others also suggested that prognosis of CBF AML could differ among different ethnic groups or races.14 35–37 The background molecular basis among the Japanese population must also be taken into account in future studies.
In conclusion, the survival outcome of patients with CBF AML was similar when they received allogeneic or autologous SCT in first CR. However, the outcomes were significantly different between t(8;21) and inv(16) when they received allogeneic SCT beyond first CR. Therefore, these 2 kinds of CBF AML should be managed differently when applying SCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of the staff of the participating institutions of the Japan Society for Hematopoietic Cell Transplantation Registry. We thank Dr Y. Inamoto for thoughtful discussion.
Authorship
Contribution: Y. Kuwatsuka, K.M., and R.S. contributed to data collection, designed and performed the study, analyzed the data, and wrote the manuscript; M.K., A.M., H.O., R.T., S.T., K.K., K.Y., Y.A., T.Y., and H.S. contributed to data collection and analysis and writing of the paper; and Y. Kodera contributed to data collection and writing of the paper, conceived the study, and provided intellectual input.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yachiyo Kuwatsuka, Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, Aichi 466-8550, Japan; e-mail: kuwatsuka-ngy@umin.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal