Hematopoietic stem cells (HSCs) reside in association with bone marrow (BM) sinusoidal vessels in vivo, but the function of BM endothelial cells (ECs) in regulating hematopoiesis is unclear. We hypothesized that hematopoietic regeneration following injury is regulated by BM ECs. BALB/c mice were treated with total body irradiation (TBI) and then infused with C57Bl6-derived endothelial progenitor cells (EPCs) to augment endogenous BM EC activity. TBI caused pronounced disruption of the BM vasculature, BM hypocellularity, ablation of HSCs, and pancytopenia in control mice, whereas irradiated, EPC-treated mice displayed accelerated recovery of BM sinusoidal vessels, BM cellularity, peripheral blood white blood cells (WBCs), neutrophils, and platelets, and a 4.4-fold increase in BM HSCs. Systemic administration of anti–VE-cadherin antibody significantly delayed hematologic recovery in both EPC-treated mice and irradiated, non–EPC-treated mice compared with irradiated controls. These data demonstrate that allogeneic EPC infusions can augment hematopoiesis and suggest a relationship between BM microvascular recovery and hematopoietic reconstitution in vivo.
Introduction
Cellular or pharmacologic therapies capable of augmenting hematopoietic stem cell (HSC) reconstitution in vivo could have utility in the treatment of patients undergoing chemo- or radiotherapy.1 An obvious resource to identify cells and soluble factors that support HSC regeneration is the bone marrow (BM) microenvironment, wherein HSCs reside.2,,,–6 HSCs reside in close association with BM osteoblasts, which support the maintenance of the HSC pool via Jagged-Notch interactions and Tie2-angiopoietin signaling.2,–4 HSCs also reside adjacent to BM sinusoidal vessels, raising the possibility that BM endothelial cells (ECs) or supportive reticular cells also modulate HSC fate in vivo.6,–8 Since ECs critically regulate the onset of hematopoiesis during embryogenesis9,10 and since adult sources of ECs support the expansion and repair of BM stem/progenitor cells in vitro,11,–13 we hypothesized that BM ECs regulate hematopoietic reconstitution in vivo. Since BM ECs are profoundly sensitive to radiation and chemotherapy-induced toxicity,14,15 we propose that systemic administration of endothelial progenitor cells (EPCs) could replenish endogenous EC activity and augment hematopoietic regeneration following myelotoxic conditioning. Herein, we demonstrate that infusion of EPCs into irradiated mice causes the accelerated reconstitution of BM HSCs, peripheral blood (PB) counts, and BM sinusoidal vessels. These results suggest a relationship between BM microvascular recovery and hematopoietic reconstitution following myelotoxicity
Methods
EPC infusions and measurements of hematopoietic recovery
BALB/c mice aged 12 weeks (The Jackson Laboratory, Bar Harbor, ME) received 550 cGy total body irradiation (TBI) and, 4 hours later, were given intravenous infusions of 106 C57Bl6 fetal blood EPCs, which were generated in culture (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). On days 1 to 4, mice received 2.5 × 106 EPCs/day intraperitoneally. Between days 5 and 30, subsets of mice that received EPC infusions or phosphate-buffered saline (PBS) injections were killed, and bilateral femurs were harvested and stained with hematoxylin and eosin (H&E) and anti–MECA-32 antibody (BD Biosciences, San Jose, CA). BM HSC content was measured via competitive repopulating assays,2,5 BM cells were incubated with antileukocyte antibodies as previously described11,14,16 to measure c-kit+sca-1+lin− (KSL) subsets, and PB white blood cells (WBCs), neutrophils, and platelet counts were also measured as previously described.14 All animal studies were approved by the Duke University Animal Care and Use Committee.
Microscopic image acquisition was performed using a Zeiss Axiovert 200 inverted microscope (Carl Zeiss Microimaging, Thornwood, NY) with an LD-plan NEOFLUAR 40×/0.6 objective lens at 25°C in Cytoseal XYL imaging medium (Richard Allen Scientific). Image acquisition and analysis was conducted using an AxioCam MRc camera and Zeiss Axiovision 4.5 software (Carl Zeiss Microimaging).
Results and discussion
EPCs were generated from C57Bl6-derived fetal blood cells17 following previously described culture methods.18 These cells expressed EC antigens, lacked monocyte markers, and possessed features of endothelial colony-forming cells (ECFCs; Figure S1; Table S1).18
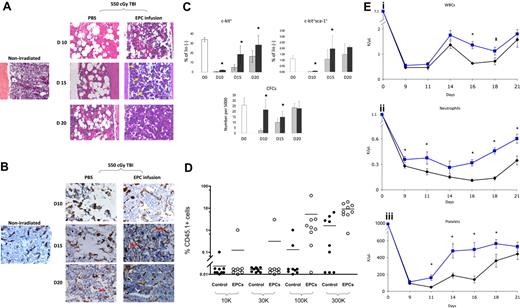
We first compared the hematologic recovery of BALB/c mice that were irradiated with 550 cGy TBI, which is sublethal but myelotoxic in BALB/c mice and, beginning 4 hours after exposure, received either EPC infusions alone or PBS for 5 days. Control mice demonstrated pronounced BM hypocellularity and hemorrhage at day 10, whereas EPC-treated mice displayed largely preserved BM cellularity and less vascular disruption at all time points (Figure 1A). Following TBI, control mice displayed marked disruption of BM sinusoidal vessels at days 10 and 15 compared with nonirradiated controls (Figure 1B). In contrast, EPC-treated mice maintained more intact BM sinusoidal vessels at day 10, and displayed neovasculogenesis at day 15 and essentially normal BM sinusoidal vessel architecture by day 20, ahead of irradiated controls (Figure 1B). These data indicated that the early restoration of hematopoiesis in mice following TBI was temporally associated with the re-establishment of BM sinusoidal vessels in mice that had received transplants of EPCs.
EPC infusions accelerate the recovery of BM sinusoidal vessels, BM cellularity, HSC regeneration, and mature blood counts. (A) EPC infusion for 5 days accelerates recovery of BM cellularity following total body irradiation. BM cross-sections (× 400) are shown from a normal BALB/c mouse and from BALB/c mice following 550 cGy TBI and treatment with PBS or EPCs for 5 days (“Methods”). BM hypocellularity and BM vasculature disruption were more pronounced in the PBS treatment group at all time points. Recovery of megakaryocytes (yellow arrows) occurred earlier in mice that received EPCs (day 15) compared with controls. (B) EPC infusions accelerate the recovery of BM sinusoidal vessels following TBI. Expression of MECA-32 was examined in BM sections from normal and irradiated BALB/c mice. BM from a normal mouse demonstrated narrow sinusoidal vessels (brown). Overt disruption of BM vasculature was evident in irradiated, PBS-treated mice at days 10 and 15 as compared with normal mice. Preservation of sinusoidal vessels (day 10) and assembly of nascent vessels (day 15; red arrows) was evident earlier in the EPC-treated mice compared with PBS-treated mice (day 20). The BM vasculature in EPC-treated mice approached normal appearance by day 20. (C) EPC infusions augment the recovery of phenotypic BM hematopoietic progenitor cells in irradiated mice. EPC-treated mice (■) demonstrated significantly increased percentages of BM c-kit+lin− progenitors (top left) at days 10, 15, and 20 following 550 cGy irradiation (P = .004, P = .004, and P = .04, respectively) and BM KSL cells at days 10 and 15 after irradiation (right; P = .02 and P = .04) compared with controls (▩). The numbers of BM CFCs were also significantly increased at days 10 and 15 in EPC-treated mice compared with controls (bottom; P = .001 and P = .003). Data represent the mean plus SD of n = 6 experiments. *P < .05 for difference between EPC-treated mice and controls. (D) The scatterplot shows the percentage of CD45.1+ donor cell engraftment in the PB of CD45.2+ mice that received transplants of limiting doses of CD45.1+ BM cells collected at day 20 from mice irradiated with 700 cGy TBI followed by PBS treatments (control) or mice irradiated identically and then infused with EPCs. Mice that received transplants of BM cells from EPC-treated donors (□) demonstrated significantly higher donor CD45.1+ cell repopulation at 12 weeks after transplantation than mice that received transplants of BM from PBS-treated mice (■). Each dot represents a mouse that underwent transplantation (n = 76 mice). Donor BM cell doses transplanted per mouse are shown at the bottom of the figure. All mice received transplants of 105 host BM cells for competition. Lines represent the mean levels of CD45.1+ cell engraftment in each group. (Ei) EPC-treated mice (blue line) demonstrated earlier recovery of WBCs compared with controls (black line). *P = .006 and P = .04 for difference in WBCs between EPC-treated mice and controls at days 16 and 18. (Eii) EPC-treated mice also demonstrated increased neutrophil counts compared with controls. *P = .04, P = .04, P < .001, P < .001, and P = .002 for differences between EPC-treated mice and controls at days 9, 11, 16, 18, and 21. (Eiii) Platelet recovery was also significantly accelerated in EPC-treated mice compared with controls. *P = .007, P = .006, P = .002, and P = .03 for differences between EPC-treated mice and controls at days 11, 14, 16, and 18. Data represent the means plus SEM of n = 10 to 12 mice PB samples per time point.
EPC infusions accelerate the recovery of BM sinusoidal vessels, BM cellularity, HSC regeneration, and mature blood counts. (A) EPC infusion for 5 days accelerates recovery of BM cellularity following total body irradiation. BM cross-sections (× 400) are shown from a normal BALB/c mouse and from BALB/c mice following 550 cGy TBI and treatment with PBS or EPCs for 5 days (“Methods”). BM hypocellularity and BM vasculature disruption were more pronounced in the PBS treatment group at all time points. Recovery of megakaryocytes (yellow arrows) occurred earlier in mice that received EPCs (day 15) compared with controls. (B) EPC infusions accelerate the recovery of BM sinusoidal vessels following TBI. Expression of MECA-32 was examined in BM sections from normal and irradiated BALB/c mice. BM from a normal mouse demonstrated narrow sinusoidal vessels (brown). Overt disruption of BM vasculature was evident in irradiated, PBS-treated mice at days 10 and 15 as compared with normal mice. Preservation of sinusoidal vessels (day 10) and assembly of nascent vessels (day 15; red arrows) was evident earlier in the EPC-treated mice compared with PBS-treated mice (day 20). The BM vasculature in EPC-treated mice approached normal appearance by day 20. (C) EPC infusions augment the recovery of phenotypic BM hematopoietic progenitor cells in irradiated mice. EPC-treated mice (■) demonstrated significantly increased percentages of BM c-kit+lin− progenitors (top left) at days 10, 15, and 20 following 550 cGy irradiation (P = .004, P = .004, and P = .04, respectively) and BM KSL cells at days 10 and 15 after irradiation (right; P = .02 and P = .04) compared with controls (▩). The numbers of BM CFCs were also significantly increased at days 10 and 15 in EPC-treated mice compared with controls (bottom; P = .001 and P = .003). Data represent the mean plus SD of n = 6 experiments. *P < .05 for difference between EPC-treated mice and controls. (D) The scatterplot shows the percentage of CD45.1+ donor cell engraftment in the PB of CD45.2+ mice that received transplants of limiting doses of CD45.1+ BM cells collected at day 20 from mice irradiated with 700 cGy TBI followed by PBS treatments (control) or mice irradiated identically and then infused with EPCs. Mice that received transplants of BM cells from EPC-treated donors (□) demonstrated significantly higher donor CD45.1+ cell repopulation at 12 weeks after transplantation than mice that received transplants of BM from PBS-treated mice (■). Each dot represents a mouse that underwent transplantation (n = 76 mice). Donor BM cell doses transplanted per mouse are shown at the bottom of the figure. All mice received transplants of 105 host BM cells for competition. Lines represent the mean levels of CD45.1+ cell engraftment in each group. (Ei) EPC-treated mice (blue line) demonstrated earlier recovery of WBCs compared with controls (black line). *P = .006 and P = .04 for difference in WBCs between EPC-treated mice and controls at days 16 and 18. (Eii) EPC-treated mice also demonstrated increased neutrophil counts compared with controls. *P = .04, P = .04, P < .001, P < .001, and P = .002 for differences between EPC-treated mice and controls at days 9, 11, 16, 18, and 21. (Eiii) Platelet recovery was also significantly accelerated in EPC-treated mice compared with controls. *P = .007, P = .006, P = .002, and P = .03 for differences between EPC-treated mice and controls at days 11, 14, 16, and 18. Data represent the means plus SEM of n = 10 to 12 mice PB samples per time point.
Irradiated mice in both conditions showed a marked decline in BM c-kit+lin− progenitors by day 10, but EPC-treated mice showed significantly earlier recovery of BM c-kit+lin− progenitors compared with controls (Figure 1C). Similarly, EPC-treated mice contained significantly increased numbers of BM KSL stem/progenitor cells14 at days 10 and 15 compared with controls (Figure 1C). A corresponding increase in BM colony-forming cell (CFC) numbers was also demonstrated at days 10 and 15 in the EPC-treated mice compared with controls (Figure 1C).
To determine whether EPC infusions augmented BM HSC reconstitution following TBI, competitive repopulating assays were performed in which lethally irradiated (950 cGy single-fraction) C57Bl6 (CD45.2+) mice were given transplants of limiting dilutions of BM cells collected from EPC-treated or PBS-treated Bl6.SJL (CD45.1+) mice at day 20 following sublethal TBI (700 cGy). At 12 weeks after treatment, a significantly higher percentage of mice (15 of 37; 41%) that underwent transplantion with BM cells from EPC-treated mice demonstrated donor CD45.1+ cell engraftment in the PB compared with mice that received transplants of BM cells from PBS-treated controls (5 of 39; 13%; Figure 1D; Table S2). Poisson statistical analysis demonstrated that the frequency of competitive repopulating units (CRUs) within EPC-treated mice was 1 in 164 565 BM cells (95% confidence interval [CI]: 1 in 95 243-284 338), whereas the CRU frequency within PBS-treated mice was 1 in 719 452 (95% CI: 1 in 301 009-1 719 586). Therefore, EPC infusions induced a 4.4-fold increase in BM HSC content following TBI compared with control, irradiated mice. Multilineage engraftment of myeloid cells, B lymphoid cells, T cells, and erythroid progenitors was also significantly increased in mice that received transplants of BM from EPC-treated mice as compared with mice that received transplants of BM from controls (Figure S2).
In addition to augmenting BM HSC reconstitution, EPC-treated mice displayed significantly increased PB absolute neutrophil counts and platelet counts over time following TBI (Figure 1Eii,iii). These data indicated that EPC infusions not only accelerated BM HSC reconstitution, but also the production of mature blood elements, which affect clinical outcomes in chemotherapy- and radiotherapy-treated patients.19
To determine whether infused EPCs engrafted within the BM vascular niche following irradiation, we irradiated BALB/c mice with 550 cGy TBI, injected them intravenously with 106 GFP+ EPCs, and measured their engraftment in multiple tissues. GFP+ cells were detected within the lungs of EPC-treated mice at 24 hours, day 4, and day 7 after infusion, but were not detected in the BM, spleen, or liver in any mice (Figure S3). Therefore, EPC treatment did not augment hematopoiesis via direct reconstitution of the BM vascular niche. To determine whether the effects of EPC infusions could be secondary to nonspecific effects of an allogeneic cell infusion, we also measured hematologic recovery in irradiated BALB/c mice that received 5 days of infusions of irradiated C57Bl6 fibroblasts (Figure S4). Infusions of irradiated fibroblasts failed to reproduce the effects of EPC infusions on hematologic recovery following irradiation. In vitro studies demonstrated that noncontact culture with EPCs supported the expansion of normal BM KSL cells and the recovery of BM progenitor cells following irradiation (Figure S5). Taken together, these data suggested that the observed effects of the EPC infusions were not nonspecific, and were possibly mediated by factors secreted by EPCs.
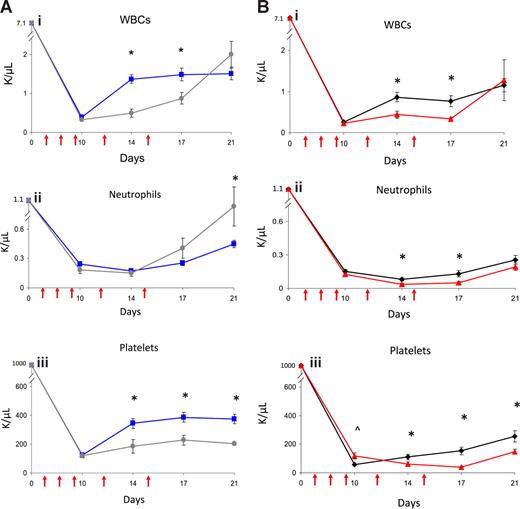
Since EPC-treated mice displayed concordant early recovery of BM sinusoidal vessels and hematopoiesis, this suggested the possibility of a causal relationship between BM vascular recovery and hematopoietic reconstitution in mice that received transplants of EPCs. To test this, we irradiated BALB/c mice sublethally (550 cGy TBI) and subsequently treated them with anti–VE-cadherin, which binds VE-cadherin, a BM EC adhesion molecule that mediates vessel assembly in vivo.20,–22 Avecilla et al previously showed that treatment of mice with anti–VE-cadherin was associated with inhibition of BM sinusoidal vessel recovery and BM megakaryocytopoiesis following 5-FU chemotherapy.22 We compared multilineage hematologic recovery in irradiated mice that were subsequently treated with EPCs alone, EPCs plus VE-cadherin antibody, VE-cadherin antibody alone, or PBS injections. No mice received hematopoietic cell infusions in these experiments. Interestingly, irradiated mice treated with VE-cadherin antibody plus EPCs showed significantly delayed recovery of WBCs and platelets compared with mice treated with EPCs alone (Figure 2A). Irradiated mice that were treated with VE-cadherin antibody alone also demonstrated significantly delayed recovery of WBCs, neutrophils, and platelets compared with irradiated, control mice (Figure 2B). These data suggested a relationship between BM microvascular recovery and hematopoietic reconstitution following irradiation.
Administration of VE-cadherin antibody abrogates the effect of EPC infusions on hematopoietic recovery. PB WBCs, neutrophil counts, and platelet counts were measured over time in BALB/c mice that were irradiated with 550 cGy TBI and then treated systemically with either EPCs alone, EPCs plus VE-cadherin antibody, VE-cadherin antibody alone, or PBS as described in “Methods.” VE-cadherin treatments are represented by red arrows. (A) Comparison in WBC (Ai), neutrophils (Aii), and platelet count (Aiii) recovery in mice treated with EPCs alone versus mice treated with EPCs plus VE-cadherin treatments. Mice that were treated with EPCs plus VE-cadherin antibody (gray line) demonstrated significantly delayed recovery of WBCs and platelets compared with mice treated with EPCs alone (blue line). *P < .001 and P = .006 for differences in WBCs at days 14 and 17 (Ai). Platelet recovery was significantly delayed in mice treated with EPCs plus VE-cadherin versus mice treated with EPCs alone (Aiii). *P = .01, P = .002, and P < .001 for differences in platelet counts at days 14, 17, and 21. (B) Treatment with VE-cadherin antibody alone (red line) delayed WBC, neutrophil, and platelet recovery in irradiated mice compared with irradiated control mice (black line). *P = .003 and P = .002 for differences in WBCs at days 14 and 17 (Bi WBCs). *P = .002 and P = .007 for differences in neutrophil counts between VE-cadherin antibody–treated mice and controls at days 14 and 17 (Bii Neutrophils). *P = .03, P < .001, and P = .01 for differences in platelet counts between VE-cadherin antibody–treated mice and controls at days 14, 17, and 21. ∧P = .01 for increase in platelet count in VE-cadherin antibody–treated mice versus controls at day 10 (Biii Platelets). Data points represent the means plus or minus SEM of n = 10 to 15 mice per time point.
Administration of VE-cadherin antibody abrogates the effect of EPC infusions on hematopoietic recovery. PB WBCs, neutrophil counts, and platelet counts were measured over time in BALB/c mice that were irradiated with 550 cGy TBI and then treated systemically with either EPCs alone, EPCs plus VE-cadherin antibody, VE-cadherin antibody alone, or PBS as described in “Methods.” VE-cadherin treatments are represented by red arrows. (A) Comparison in WBC (Ai), neutrophils (Aii), and platelet count (Aiii) recovery in mice treated with EPCs alone versus mice treated with EPCs plus VE-cadherin treatments. Mice that were treated with EPCs plus VE-cadherin antibody (gray line) demonstrated significantly delayed recovery of WBCs and platelets compared with mice treated with EPCs alone (blue line). *P < .001 and P = .006 for differences in WBCs at days 14 and 17 (Ai). Platelet recovery was significantly delayed in mice treated with EPCs plus VE-cadherin versus mice treated with EPCs alone (Aiii). *P = .01, P = .002, and P < .001 for differences in platelet counts at days 14, 17, and 21. (B) Treatment with VE-cadherin antibody alone (red line) delayed WBC, neutrophil, and platelet recovery in irradiated mice compared with irradiated control mice (black line). *P = .003 and P = .002 for differences in WBCs at days 14 and 17 (Bi WBCs). *P = .002 and P = .007 for differences in neutrophil counts between VE-cadherin antibody–treated mice and controls at days 14 and 17 (Bii Neutrophils). *P = .03, P < .001, and P = .01 for differences in platelet counts between VE-cadherin antibody–treated mice and controls at days 14, 17, and 21. ∧P = .01 for increase in platelet count in VE-cadherin antibody–treated mice versus controls at day 10 (Biii Platelets). Data points represent the means plus or minus SEM of n = 10 to 15 mice per time point.
In summary, these results demonstrate that infusion of EPCs alone mediates BM HSC reconstitution and hematologic recovery in vivo and is a candidate cellular therapy to augment hematopoiesis in patients. It remains to be determined whether these effects are specific to EPCs or whether infusion of other supportive cells (eg, mesenchymal stem cells) can reproduce such effects. Systemic administration of anti–VE-cadherin delayed hematologic recovery following irradiation in both EPC-treated and nontreated mice, suggesting a relationship between microvascular recovery and hematopoietic recovery following injury.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Chad May (ImClone Systems, New York, NY) for provision of the anti–VE-cadherin antibody.
This work was supported in part by a grant from the National Institutes of Health (Bethesda, MD; National Institute of Allergy and Infectious Diseases grant no. AI067798 to J.P.C.). Support was also provided by the Koskinen Regenerative Medicine Endowment (Durham, NC).
National Institutes of Health
Authorship
Contribution: A.B.S. performed research, analyzed data and wrote the paper; S.K.M., G.G.M., H.H., P. Doan, P. Daher, and L.R. performed research and analyzed data; B.C. contributed a vital reagent and performed research; N.J.C. analyzed data and wrote the paper; and J.P.C. designed research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Corresponding author: John P. Chute, Associate Professor of Medicine, Division of Cellular Therapy, Department of Medicine, Duke University Medical Center, 2400 Pratt Street, Durham, NC 27710; e-mail: john.chute@duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal