Abstract
Iron overload is common in patients undergoing allogeneic hematopoietic cell transplantation (HCT), but the mechanisms leading to overload are unknown. Here, we determined iron levels and the expression of iron regulatory proteins in the liver and gut of nonobese diabetic–severe combined immunodeficient (NOD/SCID) mice that underwent transplantation with syngeneic (histocompatible) or allogeneic (histoincompatible) T lymphocytes. Infusion of histoincompatible T cells resulted in a significant rise in serum iron levels and liver iron content. Iron deposition was accompanied by hepatocyte injury and intestinal villous damage. Feeding of low- or high-iron diet was associated with appropriate ferroportin 1 and hepcidin responses in mice given histocompatible T cells, whereas mice given histoincompatible T cells showed inappropriate up-regulation of duodenal ferroportin 1 and a loss of expression of hepatic hepcidin. These findings suggest that alloreactive T cell–dependent signals induced dysregulation of intestinal iron absorption, which contributed to liver iron overload after HCT.
Introduction
Iron overload is common in patients undergoing hematopoietic cell transplantation (HCT).1-3 Many of these patients develop iron overload even without heavy red cell transfusion support; the mechanisms are largely unknown. High-dose conditioning preceding HCT may result in hyperferremia and increased non–transferrin-bound iron, in association with the cessation of erythropoietic activity.4,5 In many patients hyperferremia persists after HCT,1,2 suggesting aberrant iron release into the circulation.
The major regulator of iron release into the circulation is hepcidin (Hamp), secreted mainly by the liver.6 Hepcidin binds to the iron transporter ferroportin 1 (Fpn), expressed on enterocytes and macrophages, which delivers iron from inside the cell to the circulation.7 Hepcidin binding results in internalization and cytoplasmic degradation of ferroportin. As both the intestinal tract and liver are targets of graft-versus-host disease (GVHD),2,8,9 we hypothesized that one effect of allogeneic transplantation may be direct or indirect interference by T lymphocytes with the expression or function of iron regulatory proteins in liver and gut, thereby contributing to iron overload. Here we characterized Hamp and Fpn expression and dietary iron uptake in a murine model of histoincompatible allogeneic T-lymphocyte transplantation.
Methods
Female NOD/LtSz-scid/scid (NOD/SCID [H-2d]), C57BL/6J (H-2b), and BALB/cJ mice (H-2d) were purchased from The Jackson Laboratory (Bar Harbor, ME). NOD/SCID mice were maintained in a pathogen-free environment and kept on normal chow (iron content ∼35 ppm) or chow with low (≤ 1 ppm) or high (30 000 ppm) iron content (Test Diets, Richmond, IN), for 14 to 28 days before transplantation. Histocompatible (syngeneic; H-2d) and histoincompatible (allogeneic; H-2b) T lymphocytes were prepared from spleens obtained from BALB/c (H-2d) and C57BL/6J (H-2b) mice, respectively. Single-cell suspensions of nonadherent cells were adjusted to a concentration of 5 × 106 cells/mL. Contamination by CD3-negative cells was 8% to 12%. Recipient mice received 107 or 3.0 × 107 histocompatible or incompatible T cells intravenously via the lateral tail vein (3-8 recipient mice per experiment). The use of mice in this study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Fred Hutchinson Cancer Research Center.
Mice were euthanized at 7 or 14 days after transplantation (3-6 weeks after initiating a particular diet) by CO2 inhalation. Blood was collected via cardiac puncture and total serum iron was measured. Hepatic iron content was determined using a colorimetric assay as described.10 Tissue for histology was fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin and with Perl Prussian blue to detect iron deposition.
Hepatocyte suspensions were generated by mincing and straining liver tissue through a 75-μm mesh. Enterocytes were obtained by scraping the mucosa off the inverted duodenum and mincing and straining through a 75-μm mesh. Total RNA was isolated and cDNA synthesized using the μMACS One-Step cDNA Synthesis kit (Miltenyi Biotec, Auburn, CA) as instructed and as previously described.11 Quantitative polymerase chain reaction (PCR) was carried out using the comparative Ct method as described by Livak and Schmittgen12 and as used previously.13 Gene expression assays (Applied Biosystems, Foster City, CA) were used to analyze expression of the murine hepcidin gene hamp1 (Mm00519025_m1) and FPN1 (fpn1; Mm00489837_m1). Murine β-actin (Mm0060793_s1) served as endogenous control. Tissues from each dietary group that did not undergo transplantation with T lymphocytes were used as reference for gene expression, and experimental results were expressed as values relative to the reference.
Sections of duodenum for immunohistochemistry for Fpn were prepared by standard procedures. The deparaffinized and rehydrated sections were blocked, labeled with primary rabbit antibody, washed, and labeled with secondary swine anti–rabbit antibody (Dako, Carpinteria, CA) as described by Canonne-Hergaux et al.14 Nonimmune rabbit serum served as control. The procedure was carried out on a Leica MicroSystems Bond Polymer Refine Detection kit (Bannockburn, IL).
Results and discussion
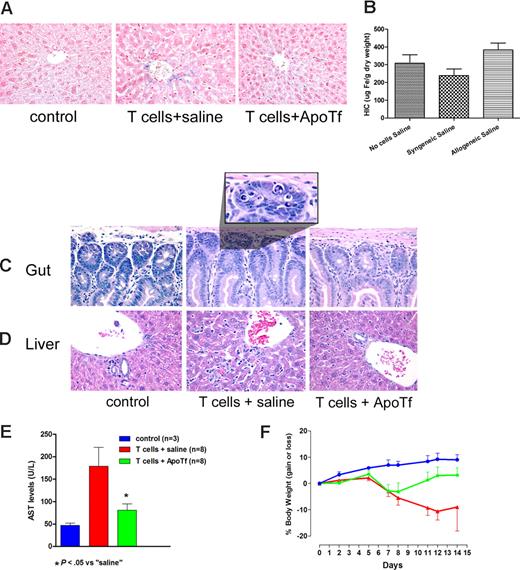
NOD/SCID mice were used to allow for engraftment of donor cells without cytotoxic conditioning of the recipient, which could induce interleukin 6 (IL-6) and affect regulation of hepcidin. In NOD/SCID mice fed normal chow and infused with 3.0 × 107 histoincompatible T lymphocytes, liver sections obtained on day 14 showed iron deposition in hepatocytes (Figure 1A). In contrast, no iron deposition was observed in mice infused with histocompatible syngeneic T cells or in control mice not given T lymphocytes (Figure 1A). Quantitative analysis of liver tissue confirmed elevated levels of hepatic iron in mice that underwent transplantation with histoincompatible T cells (Figure 1B). Mice given histoincompatible T cells also showed histologic evidence of injury of the gut mucosa (Figure 1C) and the liver (Figure 1D), associated with serum transaminase elevations (Figure 1E). Control mice showed slight increases in body weight over 14 days, whereas the weight declined progressively in mice given histoincompatible T cells, as classically observed in murine GVHD.15 The weight loss was prevented or reversed in mice pretreated with apoTf (Figure 1F). We had shown previously that Fas-initiated hepatocyte injury was attenuated or prevented by apotransferrin.13,16 Since alloactivated T lymphocytes express Fas ligand, and Fas signaling is involved in GVHD,17,18 we postulated that pretreatment of NOD/SCID mice with apotransferrin before histoincompatible T-cell infusion would interfere with hepatic injury and liver iron deposition. Results illustrated in Figure 1C through F support this hypothesis by showing substantial reduction in hepatocyte apoptosis and serum transaminase elevations. Thus, chelation of iron by apotransferrin,19 or possibly transferrin-mediated antiapoptotic signals,16 counteracted iron overload and protected tissues against allogeneic histoincompatible T cell–dependent injury. These data support earlier reports of a GVHD prophylactic effect of apoTf.20,21
Protective effect of apotransferrin against tissue injury induced by allogeneic T cells. Iron accumulation in the liver and hepatic and intestinal injury after transplantation of allogeneic T cells to NOD/SCID mice. (A) NOD/SCID mice (H-2d) underwent transplantation with 3 × 107 allogeneic lymphocytes from C57BL6 mice (H-2b), following injection of saline (middle panel) or human apotransferrin (1 mg/mouse; right panel) 24 and 2 hours before T-cell infusion. Livers from untreated mice served as additional controls (left panel). (B) Hepatic iron content (HIC) as determined by colorimetric assay (mean ± 1 SD) on day 14 after the infusion of saline and either no cells or syngeneic or allogeneic cells, respectively (3-8 mice per group). (C) Histology of the duodenum and (D) liver, representative for the 3 groups of mice described in panel A. (E) Serum transaminase levels (mean ± 1 SD) in controls (blue column) and mice on day 14 after infusion of allogeneic T lymphocytes after pretreatment with saline (red column) or apotransferrin (green column). (F) Weight changes in mice from panel E over the 14-day course of the experiment. Beyond day 7 body weight was significantly higher in ApoTf pretreated than in saline pretreated mice (P < .05 to P < .02 by Student t test, calculated on days 7 and 14).
Protective effect of apotransferrin against tissue injury induced by allogeneic T cells. Iron accumulation in the liver and hepatic and intestinal injury after transplantation of allogeneic T cells to NOD/SCID mice. (A) NOD/SCID mice (H-2d) underwent transplantation with 3 × 107 allogeneic lymphocytes from C57BL6 mice (H-2b), following injection of saline (middle panel) or human apotransferrin (1 mg/mouse; right panel) 24 and 2 hours before T-cell infusion. Livers from untreated mice served as additional controls (left panel). (B) Hepatic iron content (HIC) as determined by colorimetric assay (mean ± 1 SD) on day 14 after the infusion of saline and either no cells or syngeneic or allogeneic cells, respectively (3-8 mice per group). (C) Histology of the duodenum and (D) liver, representative for the 3 groups of mice described in panel A. (E) Serum transaminase levels (mean ± 1 SD) in controls (blue column) and mice on day 14 after infusion of allogeneic T lymphocytes after pretreatment with saline (red column) or apotransferrin (green column). (F) Weight changes in mice from panel E over the 14-day course of the experiment. Beyond day 7 body weight was significantly higher in ApoTf pretreated than in saline pretreated mice (P < .05 to P < .02 by Student t test, calculated on days 7 and 14).
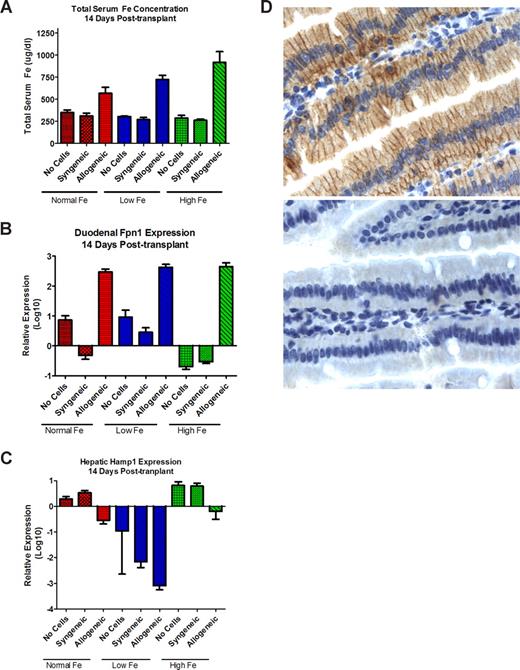
To determine whether NOD/SCID mice properly regulated intestinal iron absorption, we tested the effect of diets with different iron content on iron uptake in the absence of T-cell transplantation. Serum iron levels on all diets remained in the range of 260 μg/dL to 350 μg/dL (Figure 2). There was a slight increase in duodenal Fpn mRNA expression (relative to housekeeping genes) in mice on a low-iron diet, and a significant decrease in mice on a high-iron diet. In comparison to mice on normal chow, Hamp expression in the liver decreased in mice on a low-iron diet, and increased in mice on a high-iron diet (Figure 2B,C). This pattern was consistent with appropriate responses of NOD/SCID mice to variations in dietary iron content.
Effects of T-lymphocyte transplantation on iron homeostasis. Serum iron (mean ± 1 SD; A), duodenal ferroportin 1 (Fpn; B), and hepatic hepcidin (hamp; C) mRNA expression, on day 14 after T-cell infusion. Mice not injected with T lymphocytes served as controls. Mice were fed normal chow (normal Fe) or low Fe or high Fe diets. Values for panels B and C were obtained in each mouse by expressing the levels of Fpn1 and hamp, respectively, relative to the levels of β-actin; shown are the means plus or minus 1 SD of the relative values in 3 mice (normal Fe) or 5 mice (low and high Fe groups). (D) Fpn protein expression in the duodenum of mice on a high-iron diet. The top panel shows intense staining of enterocytes, most prominently in basolateral localization, in a mouse that underwent transplantation with allogeneic histoincompatible T cells; the bottom panel from a mouse that underwent transplantation with histocompatible (syngeneic) T cells shows only very faint staining for Fpn. Image for panel D created using Leica Dm 3000 (Wetzlar, Germany). The type, magnification, and numerical aperture of the objective lenses temperature are as follows: N plan dry 63×/0.80 NA, LED lighting. The imaging medium is as follows: digital camera photograph of paraffin-embedded, formalin-fixed glass slide, photos 64% (RGB/ 8; no fluorochromes). The camera make and model are as follows: Leica DCF 280 camera (Herrbrug, Switzerland). Acquisition software is as follows: Leica Microsystems, Leica Application Suite LAS 3.1.0 (build: 1983) CMS Adobe, Photoshop Elements 4 (San Jose, CA; 95 110-2704.)
Effects of T-lymphocyte transplantation on iron homeostasis. Serum iron (mean ± 1 SD; A), duodenal ferroportin 1 (Fpn; B), and hepatic hepcidin (hamp; C) mRNA expression, on day 14 after T-cell infusion. Mice not injected with T lymphocytes served as controls. Mice were fed normal chow (normal Fe) or low Fe or high Fe diets. Values for panels B and C were obtained in each mouse by expressing the levels of Fpn1 and hamp, respectively, relative to the levels of β-actin; shown are the means plus or minus 1 SD of the relative values in 3 mice (normal Fe) or 5 mice (low and high Fe groups). (D) Fpn protein expression in the duodenum of mice on a high-iron diet. The top panel shows intense staining of enterocytes, most prominently in basolateral localization, in a mouse that underwent transplantation with allogeneic histoincompatible T cells; the bottom panel from a mouse that underwent transplantation with histocompatible (syngeneic) T cells shows only very faint staining for Fpn. Image for panel D created using Leica Dm 3000 (Wetzlar, Germany). The type, magnification, and numerical aperture of the objective lenses temperature are as follows: N plan dry 63×/0.80 NA, LED lighting. The imaging medium is as follows: digital camera photograph of paraffin-embedded, formalin-fixed glass slide, photos 64% (RGB/ 8; no fluorochromes). The camera make and model are as follows: Leica DCF 280 camera (Herrbrug, Switzerland). Acquisition software is as follows: Leica Microsystems, Leica Application Suite LAS 3.1.0 (build: 1983) CMS Adobe, Photoshop Elements 4 (San Jose, CA; 95 110-2704.)
Responses differed strikingly in mice infused with histoincompatible allogeneic T lymphocytes. Whereas mice that underwent transplantation with histocompatible syngeneic T cells showed a pattern of serum iron levels, and hepcidin and Fpn expression that was similar to that in mice not injected with T lymphocytes, the infusion of histoincompatible T cells resulted in significant dysregulation: mice on all diets showed significant increases in serum iron levels, which was most marked in mice on a high-iron diet (P < .001; Figure 2A). Fpn expression in the duodenal mucosa increased 10- to 100-fold following the infusion of allogeneic cells, even in mice on a high-iron diet (Figure 2B). Up-regulation of Fpn message was reflected in enhanced expression of Fpn protein in enterocytes, as illustrated in Figure 2D. Conversely, Hamp expression in the liver declined consistently, including mice on a high-iron diet and documented liver iron overload (Figure 2C).
These findings suggest that alloreactive (histoincompatible) T cells cause dysregulation of intestinal iron absorption, and thereby contribute to liver iron accumulation. Both the strikingly increased Fpn expression in the duodenum and the suppressed hepcidin expression in the liver would be expected to considerably increase intestinal iron uptake,7 in line with the increased serum iron levels observed in the NOD/SCID mice after histocompatible allogeneic T-cell transplantation.
These observations provide novel data on iron dysregulation and support the notion that histoincompatible allogeneic transplantation may contribute to iron overload. They also raise new questions. For example, does the immunodeficient condition of NOD/SCID mice contribute to iron accumulation? Is the sterile milieu in which these mice are raised a contributing factor? Studies in immunocompetent mice with the same genetic background and investigations in wild-type mice rendered gnotobiotic should provide some answers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stuart Raaka for technical assistance, Dr G. McDonald for thoughtful comments, and Helen Crawford and Bonnie Larson for help with manuscript preparation. This work was supported in part by grants HL036444 and HL082941, National Institutes of Health (NIH), Bethesda, MD. J.P. was also supported by grants from the Sigrid Juselius Foundation and the Academy of Finland, Helsinki, Finland.
National Institutes of Health
Authorship
Contribution: S.B. carried out in vivo experiments and wrote the manuscript; E.S. assisted with in vivo experiments and carried out molecular analysis; J.P. co-designed the experiments and co-wrote the manuscript; H.M.S. carried out all histologic analyses; V.L. co-designed experiments, assisted with in vivo and in vitro studies, and provided the apotransferrin data; M.B. carried out immunohistochemistry studies; F.C.-H. provided anti-Fpn antibody and technical expertise on its use; K.V.K. critically evaluated the experimental design and determined liver iron content; and H.J.D. co-designed the experiments, evaluated all results, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: jdeeg@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal