Abstract
In animal models, tissue stem cells were proposed to exhibit an unexpected level of plasticity, although issues on cell fusions have lead to some controversies. Only transplantation experiments using genetically distinct recipients and donors can unequivocally show these changes in cell fate. We have analyzed oral squamous cell carcinomas arising in 8 long-term survivors of allogeneic bone marrow transplantation, in whom chronic graft-versus-host disease greatly favors development of squamous cell carcinomas, possibly as a consequence of lichenoid mucosal inflammation. With the use of 2 independent methods, (1) combined immunostaining and fluorescent in situ hybridization (FISH) analysis for X and Y chromosomes sequences in sex-mismatched grafts and (2) comparison of microsatellite typing of laser-microdissected tumor, donor, and recipient cells, in all tumors, we showed that 4 of these 8 epithelial tumors actually arose from the engrafted allogeneic bone marrow. Thus, donor-derived bone marrow cells, whether hematopoietic or mesenchymal, recruited to sites of chronic mucosal inflammation yielded epithelial tumors. Our observations therefore show that marrow cells in humans have a major role in epithelial cancer formation after allogeneic transplantation.
Introduction
Squamous cell carcinomas (SCCs) are rare, although well-recognized, complications of bone marrow transplantation (BMT).1,2 Studies in bone marrow transplant recipients have shown the donor origin of hepatocytes, in rodents and later in humans,3 and of hepatic oval cells, skeletal muscle cells, and astrocytes in rodents (reviewed in Korbling et al3 ). Experimental studies have even shown the multiorgan, multilineage engraftment by a single bone marrow–derived stem cell of donor origin, underlining the ability of bone marrow stem cells for transdifferentiation into cell types other than blood cells.4 Yet, the possibility of cell fusion has raised serious concerns and complicated the analysis of developmental plasticity of bone marrow–derived cells,5,6 yielding considerable controversies. Recently, mesenchymal stem cells have been characterized in humans. These cells with important differentiation capacities are coinfused with the bone marrow grafts.7,8
Molecular studies of chimerism after BMT allowed the detection of rare, but authentic, leukemia of donor origin. Since the original description in 1971, more than 50 donor cell leukemias have been reported and considered as the result of oncogenic transformation of apparently normal donor hematopoietic cells in the transplant recipient.9 With the use of fluorescent in situ hybridization (FISH), the contribution of donor human bone marrow cells to solid organ cancers after BMT has also been recently shown.10,11 A recent murine study suggested that bone marrow–derived cells (BMDCs) contribute to cancer arising from the stomach lining.12 Transplantation experiments performed in mice with chronic gastritis resulting from Helicobacter infection showed that resultant gastric carcinomas contained marrow-derived dysplastic and neoplastic glands. This study primarily emphasizes the importance of chronic inflammation in recruiting BMDCs.
Here, we addressed the question of the donor or recipient origin of oral squamous cell carcinomas developed after allogeneic BMT. These rare human tumors, without a corresponding experimental model to date, have peculiar characteristics, with an aggressive behavior and poor prognosis, and a strong association with prior chronic lichenoid lesions of the oral mucosa.13 In the context of BMT, recognizing the donor or recipient origin of oral SCCs could lead to a better characterization of marrow stem cell transdifferentiation in humans and could possibly have implications for therapeutic management of engrafted patients.
Methods
Patients
From June 1976 to December 2006, 26 patients with allogeneic BMT were diagnosed with SCC. Eight of them had available frozen surgical samples. Five patients received a transplant in a sex-mismatched situation, 3 in a sex-matched situation. All were full donor hematopoietic chimera, without relapse, at the time of biopsy. Controls for FISH analyses were 6 cases of SCCs in patients who did not receive a transplant and 6 cases of SCC after sex-matched allogeneic BMT. Tissue samples were formalin-fixed surgical pieces with cryopreserved parts. Histologic diagnosis had been established according to standard criteria and p53 staining.14 The study was approved by the institutional review board of the Hôpital Saint-Louis (Paris, France) and informed consent was obtained in accordance with the Declaration of Helsinki.
Combined FISH and immunostainings
Combined FISH and immunostainings were performed on the same 5-μm thick sections (as described in Meignin et al15 and Murata et al16 ). Briefly, anti–human CD45 (clone 2B11 + PD7/26; Dako UK, Cambridgeshire, United Kingdom) or p53 (clone DO7; Dako) mouse antibody were used as primary antibodies before proteinase K digestion. FISH was performed with the use of CEP X/Y DNA probes (Vysis), and followed by staining with anti–human cytokeratin (clone AE1/AE3; Roche Diagnostics, Mannheim, Germany) or CD31 (clone JC70A; Dako) mouse antibody, and AMCA-conjugated anti–mouse IgG horse secondary antibody (Vector Laboratories, Peterborough, United Kingdom).
Tissue sections were analyzed by 2 different pathologists (A.J. and H.M.) on a motorized Z-axis Olympus (Tokyo, Japan) BX 61 microscope, alternatively using bright and epifluorescent light. Microscopic pictures obtained through a UPlan FI 100×/1.3 NA objective were captured with a digital camera ColorView III using Olympus-SIS Cell F software.
For chromosomal analysis, inflammatory cells were characterized as CD45+, CD31− in the lamina propria, and CD45+, keratin− in the epithelium; endothelial cells as CD31+, CD45−; and tumor cells as keratin+, p53+. X and Y signals were counted in a minimum of 200 cells for tumor cells, inflammatory infiltrate, and nontumoral epithelial or conjunctive cells, respectively. A correction factor was calculated by analyzing XY-positive cells on male control tissues.
Laser microdissection and short-term repeated sequence polymerase chain reaction
A minimum of 1000 cells of 3 types were successively and separately laser-microdissected (PALM, Bernried, Germany) on serial 7-μm-thick sections: tumor cells, normal epidermal cells, and inflammatory cells. Short-term repeated sequence–polymerase chain reaction (STR-PCR) was performed after overnight proteinase K incubation at 56°C, without DNA extraction. Seventeen highly polymorphic STR sequences were amplified: D1S225, D1S2892, D2S138, D3S1573, D6S264, D7S490, D8S261, D8S1820, D9S162, D11S860, D11S1356, D13S171, D16S496, D17S855, D17S1879, D18S61, and P53CA.
Results
From June 1976 to December 2006, 26 patients were diagnosed at Saint-Louis Hospital with oral SCCs after allogeneic BMT. Tumors were identified by both microscopic morphologic examination and immunostaining for p53, which we have previously shown to be stabilized in the majority of this type of tumors.14 Five patients who had received a transplant in a sex-mismatched situation (donor and recipient from opposite sex), and 3 patients with a donor of the same sex (sex-matched) had available frozen surgical specimens. All these patients had received nonmanipulated, non–T cell–depleted, bone marrow grafts.
In the sex-mismatched situation, combined FISH XY and immunohistochemical staining on the same tissue section of SCC samples showed that tumor cells of 2 of the 5 sex-mismatched patients had a sexual genotype consistent with the donor (Table 1). One patient was a male with tumor cells of female genotype. The other patient was a female with tumor cells of male genotype. A thorough enquiry showed that she had never been pregnant, thus ruling out the possibility of chimeric male cells seeded during a previous male pregnancy (Table 2). We combined immunohistochemical stainings with FISH XY to perform our genotype analysis on well-characterized cell populations. Reliable distinction, on tumor microscopic sections, of epithelial cells from endothelial and inflammatory cells, was of major importance because it has been reported that some endothelial cells of the tumor vasculature can, as inflammatory cells, differentiate from the donor hematopoietic stem cells.17 Altogether, for the first case, a male recipient who received a transplant with female bone marrow, the tumor cells were of female genotype, as were inflammatory cells, whereas the normal epidermis, as capillary cells, were of male genotype (Figure 1). In the second case, a female recipient who received a transplant with male bone marrow, the tumor cells and inflammatory cells were of male genotype, whereas the endothelial cells and normal epidermal cells were of female genotype (Figure 2).
Combined FISH and immunostainings in patients with SCC after sex-mismatched, sex-matched allogeneic BMT and in patients not receiving a transplant
| Sex: donor/recipient . | Tumor cells . | Inflammatory cells . | Nontumoral epithelial or conjunctival cells . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XX . | XY . | Total . | Positive cells, % . | Positive cells corrected, %* . | XX . | XY . | Total . | Positive cells, % . | Positive cells corrected, %† . | XX . | XY . | Total . | Positive cells, % . | Positive cells corrected, %‡ . | |
| Patient with donor-type cancer no. I | |||||||||||||||
| F/M | 153 | 0 | 210 | 72.9 | 97.6 | 140 | 0 | 207 | 67.6 | 99.4 | 0 | 136 | 210 | 64.8 | 91.3 |
| Patient with donor-type cancer no. II | |||||||||||||||
| M/F | 0 | 151 | 208 | 72.6 | 97.3 | 0 | 133 | 208 | 63.9 | 94.0 | 149 | 0 | 202 | 73.8 | 100 |
| M/F | 0 | 140 | 202 | 69.3 | 92.9 | 0 | 144 | 212 | 67.9 | 99.8 | 160 | 0 | 208 | 76.9 | 100 |
| F/M | 165 | 0 | 220 | 75.0 | 100 | 152 | 0 | 218 | 69.7 | 100 | 0 | 143 | 207 | 69.1 | 97.4 |
| Oral squamous cell carcinomas after sex-matched allogeneic BMT (control I) | |||||||||||||||
| M/M | 0 | 149 | 208 | 71.6 | 0 | 134 | 210 | 63.8 | 0 | 133 | 208 | 63.9 | |||
| M/M | 0 | 162 | 212 | 76.9 | 0 | 141 | 208 | 67.8 | 0 | 148 | 205 | 72.2 | |||
| M/M | 0 | 145 | 210 | 69.0 | 0 | 154 | 205 | 75.1 | 0 | 157 | 210 | 74.8 | |||
| M/M | 0 | 166 | 205 | 81.0 | 0 | 126 | 211 | 59.7 | 0 | 133 | 215 | 61.9 | |||
| F/F | 151 | 0 | 207 | 72.9 | 143 | 0 | 207 | 69.1 | 144 | 0 | 206 | 69.9 | |||
| F/F | 163 | 0 | 209 | 78.0 | 139 | 0 | 210 | 66.2 | 145 | 0 | 204 | 71.1 | |||
| Oral squamous cell carcinomas without transplantation (control II) | |||||||||||||||
| M | 0 | 158 | 205 | 77.1 | 0 | 143 | 210 | 68.1 | 0 | 135 | 205 | 65.9 | |||
| M | 0 | 155 | 212 | 73.1 | 0 | 148 | 212 | 69.8 | 0 | 160 | 210 | 76.2 | |||
| M | 0 | 159 | 220 | 72.3 | 0 | 158 | 240 | 65.8 | 0 | 167 | 232 | 72.0 | |||
| M | 0 | 162 | 208 | 77.9 | 0 | 157 | 218 | 72.0 | 0 | 168 | 212 | 79.2 | |||
| F | 148 | 0 | 215 | 68.8 | 144 | 0 | 221 | 65.2 | 170 | 0 | 230 | 73.9 | |||
| F | 153 | 0 | 204 | 75.0 | 154 | 0 | 230 | 67.0 | 146 | 0 | 224 | 65.2 | |||
| Sex: donor/recipient . | Tumor cells . | Inflammatory cells . | Nontumoral epithelial or conjunctival cells . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XX . | XY . | Total . | Positive cells, % . | Positive cells corrected, %* . | XX . | XY . | Total . | Positive cells, % . | Positive cells corrected, %† . | XX . | XY . | Total . | Positive cells, % . | Positive cells corrected, %‡ . | |
| Patient with donor-type cancer no. I | |||||||||||||||
| F/M | 153 | 0 | 210 | 72.9 | 97.6 | 140 | 0 | 207 | 67.6 | 99.4 | 0 | 136 | 210 | 64.8 | 91.3 |
| Patient with donor-type cancer no. II | |||||||||||||||
| M/F | 0 | 151 | 208 | 72.6 | 97.3 | 0 | 133 | 208 | 63.9 | 94.0 | 149 | 0 | 202 | 73.8 | 100 |
| M/F | 0 | 140 | 202 | 69.3 | 92.9 | 0 | 144 | 212 | 67.9 | 99.8 | 160 | 0 | 208 | 76.9 | 100 |
| F/M | 165 | 0 | 220 | 75.0 | 100 | 152 | 0 | 218 | 69.7 | 100 | 0 | 143 | 207 | 69.1 | 97.4 |
| Oral squamous cell carcinomas after sex-matched allogeneic BMT (control I) | |||||||||||||||
| M/M | 0 | 149 | 208 | 71.6 | 0 | 134 | 210 | 63.8 | 0 | 133 | 208 | 63.9 | |||
| M/M | 0 | 162 | 212 | 76.9 | 0 | 141 | 208 | 67.8 | 0 | 148 | 205 | 72.2 | |||
| M/M | 0 | 145 | 210 | 69.0 | 0 | 154 | 205 | 75.1 | 0 | 157 | 210 | 74.8 | |||
| M/M | 0 | 166 | 205 | 81.0 | 0 | 126 | 211 | 59.7 | 0 | 133 | 215 | 61.9 | |||
| F/F | 151 | 0 | 207 | 72.9 | 143 | 0 | 207 | 69.1 | 144 | 0 | 206 | 69.9 | |||
| F/F | 163 | 0 | 209 | 78.0 | 139 | 0 | 210 | 66.2 | 145 | 0 | 204 | 71.1 | |||
| Oral squamous cell carcinomas without transplantation (control II) | |||||||||||||||
| M | 0 | 158 | 205 | 77.1 | 0 | 143 | 210 | 68.1 | 0 | 135 | 205 | 65.9 | |||
| M | 0 | 155 | 212 | 73.1 | 0 | 148 | 212 | 69.8 | 0 | 160 | 210 | 76.2 | |||
| M | 0 | 159 | 220 | 72.3 | 0 | 158 | 240 | 65.8 | 0 | 167 | 232 | 72.0 | |||
| M | 0 | 162 | 208 | 77.9 | 0 | 157 | 218 | 72.0 | 0 | 168 | 212 | 79.2 | |||
| F | 148 | 0 | 215 | 68.8 | 144 | 0 | 221 | 65.2 | 170 | 0 | 230 | 73.9 | |||
| F | 153 | 0 | 204 | 75.0 | 154 | 0 | 230 | 67.0 | 146 | 0 | 224 | 65.2 | |||
Correction factor of 1.34.
Correction factor of 1.47.
Correction factor of 1.41, calculated on normal epithelium.
Patient and transplant characteristics of the 4 patients with donor-derived cancer
| Patient . | Diagnosis . | Age at diagnosis, y . | Treatment before transplantation . | Conditioning regimen . | GVHD prophylaxis . | Recipient . | Donor sex/relationship . | Acute GVHD . | Chronic GVHD . | Cancer . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex . | Pregnancy . | Grade . | Treatment . | Grade . | Treatment . | Location . | Time after transplantation, y . | Follow-up time interval* . | |||||||
| I | CML | 33 | Hydroxyurea, splenectomy | Cy, TBI | TCD, CsA | M | F/sibling | III | ATG, steroids | Ext | CsA, steroids | Oral cavity | 22 | Died, cancer, 6 mo | |
| II | SAA | 21 | Androgens | Cy, TBI | MTX | F | 0 | M/sibling | I | CsA, steroids | Ext | CsA, steroids | Oral cavity | 13 | Died, cancer, 8 mo |
| III | Fanconi | 4 | Steroids, androgens | Cy | MTX | F | 0 | F/sibling | II | CsA, steroids | Ext | CsA, steroids | Oral cavity | 5 | Died, cancer, 12 mo |
| IV | ALL | 43 | Chemotherapy | Cy, VP16, TBI | MTX, CsA | M | M/sibling | II | CsA, steroids | Ext | CsA, steroids | Oral cavity | 7 | Died, cancer, 7 mo | |
| Patient . | Diagnosis . | Age at diagnosis, y . | Treatment before transplantation . | Conditioning regimen . | GVHD prophylaxis . | Recipient . | Donor sex/relationship . | Acute GVHD . | Chronic GVHD . | Cancer . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex . | Pregnancy . | Grade . | Treatment . | Grade . | Treatment . | Location . | Time after transplantation, y . | Follow-up time interval* . | |||||||
| I | CML | 33 | Hydroxyurea, splenectomy | Cy, TBI | TCD, CsA | M | F/sibling | III | ATG, steroids | Ext | CsA, steroids | Oral cavity | 22 | Died, cancer, 6 mo | |
| II | SAA | 21 | Androgens | Cy, TBI | MTX | F | 0 | M/sibling | I | CsA, steroids | Ext | CsA, steroids | Oral cavity | 13 | Died, cancer, 8 mo |
| III | Fanconi | 4 | Steroids, androgens | Cy | MTX | F | 0 | F/sibling | II | CsA, steroids | Ext | CsA, steroids | Oral cavity | 5 | Died, cancer, 12 mo |
| IV | ALL | 43 | Chemotherapy | Cy, VP16, TBI | MTX, CsA | M | M/sibling | II | CsA, steroids | Ext | CsA, steroids | Oral cavity | 7 | Died, cancer, 7 mo | |
CML indicates chronic myelogenous leukemia; Cy, cyclophosphamide; TBI, total body irradiation; TCD, T-cell depletion; CsA, cyclosporine; ATG, antithymocyte globulin; Ext, extensive; SAA, severe aplastic anemia; MTX, methotrexate; ALL, acute lymphoblastic leukemia; and VP16, vepeside.
Time interval between diagnosis and death.
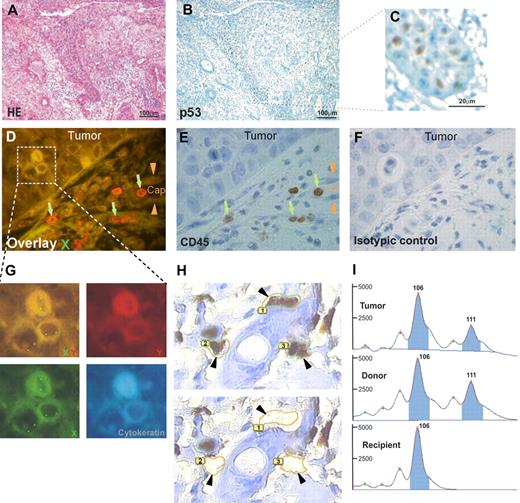
Donor cell origin of an oral squamous cell carcinoma occurring after allogeneic bone marrow transplantation. Tumor of XX genotype occurring in a male patient who received a BM transplant from a female donor. (A) Hematoxylin and eosin staining. Epithelial tumor cells invading the lamina propria of the oral mucosa. (B) Immunostaining with an antibody directed against p53. Nuclei of the basal layers of the squamous cell carcinoma are strongly positive (panel C being higher magnification of the delimited area on panel B). (D-G) Combined immunostainings with FISH methods for X chromosome (green signal) and Y chromosome (red signal). Tumor cells have a XX genotype on overlay D, as shown at higher magnification in panel G, and they are not stained with the Y chromosome–specific probe. Capillary cells (Cap, arrowheads on panels D and E) have a XY genotype on overlay D. Mononuclear CD45+ cells (E) have a XX genotype on overlay D, as shown by green arrows. (H) High magnification of p53-positive tumor cells ( ) before (top picture) and after (bottom picture) laser microdissection. (I) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and epidermal cells (from the recipient) from blocks of the same surgical piece. Microsatellite analysis at the D2S138 locus shows that the tumor microdissected cells, because the microdissected inflammatory cells of the donor are heterozygous at this locus (106 and 111 base peaks), whereas the microdissected normal epidermal cells of the recipient is homozygous (106 base peak). Microsatellite analysis of this locus another one (D17S1879, not shown) show the donor origin of the tumor. Images in panels A through C were viewed with an Olympus AX70 microscope (Olympus, Tokyo, Japan) using Olympus UPlan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Images in panels D through G were viewed with a motorized Z-axis Olympus BX 61 microscope using an Olympus UPlan Fl 100×/1.3 NA objective and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel H was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
) before (top picture) and after (bottom picture) laser microdissection. (I) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and epidermal cells (from the recipient) from blocks of the same surgical piece. Microsatellite analysis at the D2S138 locus shows that the tumor microdissected cells, because the microdissected inflammatory cells of the donor are heterozygous at this locus (106 and 111 base peaks), whereas the microdissected normal epidermal cells of the recipient is homozygous (106 base peak). Microsatellite analysis of this locus another one (D17S1879, not shown) show the donor origin of the tumor. Images in panels A through C were viewed with an Olympus AX70 microscope (Olympus, Tokyo, Japan) using Olympus UPlan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Images in panels D through G were viewed with a motorized Z-axis Olympus BX 61 microscope using an Olympus UPlan Fl 100×/1.3 NA objective and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel H was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
Donor cell origin of an oral squamous cell carcinoma occurring after allogeneic bone marrow transplantation. Tumor of XX genotype occurring in a male patient who received a BM transplant from a female donor. (A) Hematoxylin and eosin staining. Epithelial tumor cells invading the lamina propria of the oral mucosa. (B) Immunostaining with an antibody directed against p53. Nuclei of the basal layers of the squamous cell carcinoma are strongly positive (panel C being higher magnification of the delimited area on panel B). (D-G) Combined immunostainings with FISH methods for X chromosome (green signal) and Y chromosome (red signal). Tumor cells have a XX genotype on overlay D, as shown at higher magnification in panel G, and they are not stained with the Y chromosome–specific probe. Capillary cells (Cap, arrowheads on panels D and E) have a XY genotype on overlay D. Mononuclear CD45+ cells (E) have a XX genotype on overlay D, as shown by green arrows. (H) High magnification of p53-positive tumor cells ( ) before (top picture) and after (bottom picture) laser microdissection. (I) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and epidermal cells (from the recipient) from blocks of the same surgical piece. Microsatellite analysis at the D2S138 locus shows that the tumor microdissected cells, because the microdissected inflammatory cells of the donor are heterozygous at this locus (106 and 111 base peaks), whereas the microdissected normal epidermal cells of the recipient is homozygous (106 base peak). Microsatellite analysis of this locus another one (D17S1879, not shown) show the donor origin of the tumor. Images in panels A through C were viewed with an Olympus AX70 microscope (Olympus, Tokyo, Japan) using Olympus UPlan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Images in panels D through G were viewed with a motorized Z-axis Olympus BX 61 microscope using an Olympus UPlan Fl 100×/1.3 NA objective and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel H was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
) before (top picture) and after (bottom picture) laser microdissection. (I) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and epidermal cells (from the recipient) from blocks of the same surgical piece. Microsatellite analysis at the D2S138 locus shows that the tumor microdissected cells, because the microdissected inflammatory cells of the donor are heterozygous at this locus (106 and 111 base peaks), whereas the microdissected normal epidermal cells of the recipient is homozygous (106 base peak). Microsatellite analysis of this locus another one (D17S1879, not shown) show the donor origin of the tumor. Images in panels A through C were viewed with an Olympus AX70 microscope (Olympus, Tokyo, Japan) using Olympus UPlan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Images in panels D through G were viewed with a motorized Z-axis Olympus BX 61 microscope using an Olympus UPlan Fl 100×/1.3 NA objective and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel H was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
Donor cell origin of an oral squamous cell carcinoma occurring after bone marrow transplantation. Tumor of XY genotype occurring in a female patient who received a BM transplant from a male donor (no previous male pregnancy). (A) Combined FISH XY and immunostainings on the same tissue section with antibodies directed against p53 (brown) and cytokeratin (AE1/AE3; blue), showing strong stainings of the tumor cells. Tumor indicates tumor area, delimited through a large broken line; Ca, capillary lumen in the dermis; inflammatory cells, surrounded by short broken lines, in the dermis. The p53/cytokeratin-positive tumor cells are of XY genotype, as shown with green and red arrows on the enlarged overlaid area. (B) High magnification of p53-positive tumor cells ( ), surrounded by a yellow line before microdissection in the top picture. The holes left after single cell laser microdissection of the same tumor cells are surrounded by the same yellow line and shown by arrowheads in the lower picture. (C,D) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and normal epidermal cells (from the recipient) from blocks of the same surgical pieces shows the profile of tumor cells of donor origin with the use of D17S1879 (C) and D1S2892 (D) STR sequences. For the D17S1879 locus (C), the microdissected tumor cells are homozygous, because of the inflammatory cells of donor origin (158 base peak), whereas the microdissected epidermal cells of recipient origin are heterozygous (154 and 158 base peaks). For the D1S2892 locus (D), the microdissected tumor cells and inflammatory cells of donor origin are similarly heterozygous (102 and 117 base peaks), whereas microdissected normal epidermal cells of recipient origin are differently heterozygous (102 and 125 base peaks). Image in panel A was viewed with an Olympus AX70 microscope using Olympus UPLan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel B was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
), surrounded by a yellow line before microdissection in the top picture. The holes left after single cell laser microdissection of the same tumor cells are surrounded by the same yellow line and shown by arrowheads in the lower picture. (C,D) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and normal epidermal cells (from the recipient) from blocks of the same surgical pieces shows the profile of tumor cells of donor origin with the use of D17S1879 (C) and D1S2892 (D) STR sequences. For the D17S1879 locus (C), the microdissected tumor cells are homozygous, because of the inflammatory cells of donor origin (158 base peak), whereas the microdissected epidermal cells of recipient origin are heterozygous (154 and 158 base peaks). For the D1S2892 locus (D), the microdissected tumor cells and inflammatory cells of donor origin are similarly heterozygous (102 and 117 base peaks), whereas microdissected normal epidermal cells of recipient origin are differently heterozygous (102 and 125 base peaks). Image in panel A was viewed with an Olympus AX70 microscope using Olympus UPLan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel B was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
Donor cell origin of an oral squamous cell carcinoma occurring after bone marrow transplantation. Tumor of XY genotype occurring in a female patient who received a BM transplant from a male donor (no previous male pregnancy). (A) Combined FISH XY and immunostainings on the same tissue section with antibodies directed against p53 (brown) and cytokeratin (AE1/AE3; blue), showing strong stainings of the tumor cells. Tumor indicates tumor area, delimited through a large broken line; Ca, capillary lumen in the dermis; inflammatory cells, surrounded by short broken lines, in the dermis. The p53/cytokeratin-positive tumor cells are of XY genotype, as shown with green and red arrows on the enlarged overlaid area. (B) High magnification of p53-positive tumor cells ( ), surrounded by a yellow line before microdissection in the top picture. The holes left after single cell laser microdissection of the same tumor cells are surrounded by the same yellow line and shown by arrowheads in the lower picture. (C,D) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and normal epidermal cells (from the recipient) from blocks of the same surgical pieces shows the profile of tumor cells of donor origin with the use of D17S1879 (C) and D1S2892 (D) STR sequences. For the D17S1879 locus (C), the microdissected tumor cells are homozygous, because of the inflammatory cells of donor origin (158 base peak), whereas the microdissected epidermal cells of recipient origin are heterozygous (154 and 158 base peaks). For the D1S2892 locus (D), the microdissected tumor cells and inflammatory cells of donor origin are similarly heterozygous (102 and 117 base peaks), whereas microdissected normal epidermal cells of recipient origin are differently heterozygous (102 and 125 base peaks). Image in panel A was viewed with an Olympus AX70 microscope using Olympus UPLan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel B was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
), surrounded by a yellow line before microdissection in the top picture. The holes left after single cell laser microdissection of the same tumor cells are surrounded by the same yellow line and shown by arrowheads in the lower picture. (C,D) Comparison of the profiles of laser-microdissected tumor cells, inflammatory cells (from the donor), and normal epidermal cells (from the recipient) from blocks of the same surgical pieces shows the profile of tumor cells of donor origin with the use of D17S1879 (C) and D1S2892 (D) STR sequences. For the D17S1879 locus (C), the microdissected tumor cells are homozygous, because of the inflammatory cells of donor origin (158 base peak), whereas the microdissected epidermal cells of recipient origin are heterozygous (154 and 158 base peaks). For the D1S2892 locus (D), the microdissected tumor cells and inflammatory cells of donor origin are similarly heterozygous (102 and 117 base peaks), whereas microdissected normal epidermal cells of recipient origin are differently heterozygous (102 and 125 base peaks). Image in panel A was viewed with an Olympus AX70 microscope using Olympus UPLan Fl 4×/0.13 NA and 40×/0.17 NA objectives, and taken with a ColorView III digital camera using Olympus-SIS Cell F software. Image in panel B was viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
To control these FISH studies by a completely different method, which also associated molecular markers and morphologic selection of cell populations, we performed a microsatellite analysis of laser-microdissected tumor cells from Cryo-Cut sections of surgical pieces. We compared the profiles with those from laser-microdissected normal epidermis (from the recipient) and laser-microdissected inflammatory cells (from the donor) from other blocks of the same surgical pieces. We had to choose highly polymorphic short tandem repeat (STR) sequences for this STR-PCR analysis because all patients had received an allogeneic BM transplant from their siblings. In the 2 patients with sex-mismatched BMT and donor-derived tumors, the laser-microdissected tumor cells were again established to be of donor genotype on 3 and 2 different alleles, respectively (Figures 1,2).
Three other patients who underwent BMT from a donor of the same sex were also studied by STR-PCR. In 2 of the 3 patients studied, respectively 3 and 5 STR-PCR profiles from laser-microdissected cells were identical for the tumor and the donor, but they were distinct from the recipient (Figure 3), showing the donor-derived origin of the epithelial cancer. Altogether, we provide conclusive evidence of epithelial tumor of donor cell origin in 4 patients with bone marrow transplantation.
Donor cell origin of an oral squamous cell carcinoma occurring after bone marrow transplantation shown by STR-PCR of laser-microdissected cells in 2 sex-matched transplant recipients. (A top) Case III microscopic pictures of sequential cell-by-cell laser-microdissection of p53-positive tumor cells (↙). p53-positive tumor cells are surrounded by a yellow line before laser microdissection (left); the laser microdissection has been performed for the 2 upper tumor cells (middle); the laser microdissection has been performed for all surrounded p53-positive tumor cells (right). Following are microsatellite analyses that used D8S261, D8S1820, and p53CA STR sequences. For the locus D8S261, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (134 and 136 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (128 and 140 base peaks). For the locus D8S1820, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (104 and 106 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (106 and 113 base peaks). For the locus p53CA, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (122 and 124 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (103 and 118 base peaks). (B) Case IV microscopic pictures of p53-positive tumor sheets invading the lamina propria (left), and at high magnification with successive steps of laser-microdissection (middle and right). Following are microsatellite analyses that used D7S490, D17S855, and D9S162 STR sequences. For the locus D7S490, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (93 and 103 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (106 and 117 base peaks). For the locus D17S855, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (154 and 156 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (145 and 154 base peaks). For the locus D9S162, the microdissected tumor cells and inflammatory cells of donor origin were similarly homozygous (181 base peak), whereas microdissected epidermal cells of recipient origin were heterozygous (181 and 191 base peaks). Images in panels A and B were viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
Donor cell origin of an oral squamous cell carcinoma occurring after bone marrow transplantation shown by STR-PCR of laser-microdissected cells in 2 sex-matched transplant recipients. (A top) Case III microscopic pictures of sequential cell-by-cell laser-microdissection of p53-positive tumor cells (↙). p53-positive tumor cells are surrounded by a yellow line before laser microdissection (left); the laser microdissection has been performed for the 2 upper tumor cells (middle); the laser microdissection has been performed for all surrounded p53-positive tumor cells (right). Following are microsatellite analyses that used D8S261, D8S1820, and p53CA STR sequences. For the locus D8S261, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (134 and 136 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (128 and 140 base peaks). For the locus D8S1820, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (104 and 106 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (106 and 113 base peaks). For the locus p53CA, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (122 and 124 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (103 and 118 base peaks). (B) Case IV microscopic pictures of p53-positive tumor sheets invading the lamina propria (left), and at high magnification with successive steps of laser-microdissection (middle and right). Following are microsatellite analyses that used D7S490, D17S855, and D9S162 STR sequences. For the locus D7S490, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (93 and 103 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (106 and 117 base peaks). For the locus D17S855, the microdissected tumor cells and inflammatory cells of donor origin were similarly heterozygous (154 and 156 base peaks), whereas microdissected epidermal cells of recipient origin were differently heterozygous (145 and 154 base peaks). For the locus D9S162, the microdissected tumor cells and inflammatory cells of donor origin were similarly homozygous (181 base peak), whereas microdissected epidermal cells of recipient origin were heterozygous (181 and 191 base peaks). Images in panels A and B were viewed with a PALM laser catapulted microdissector system (PALM, Bernried, Germany) on an Olympus IX81 microscope using an Olympus LUCPlanFl 40×/0.6 NA objective and taken with a digital camera using PALM Robo software version 3.
We could perform this study on an exceptional set of patients, because SCCs after BMT are rare, occurring in 1 of 500 long-term survivors.1 They develop on oral mucosa, with a highly aggressive behavior, leading to death within a few months despite early and large surgical removal. This is the case for the 4 patients we studied who developed SCC 5 to 22 years after BMT and died 6 to 12 months after oral SCC was diagnosed (Table 2). None had habits of smoking or alcohol abuse, in contrast to common oral SCCs in patients not receiving a graft. However, they all had a previous history of extensive chronic graft-versus-host disease (GVHD) with oral mucosa involvement, and they had needed a prolonged immunosuppressive therapy (range, 18-42 months; mean, 36 months).
Finally, we wondered whether donor cell–derived cancer could occur in another solid tumor type (ie, breast cancer). Among 6 such cases diagnosed in our institution, pathologic material was available for only one case whose STR-PCR showed a recipient origin (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Discussion
Lichenoid lesions of oral mucosa are a characteristic feature of chronic GVHD after BMT.18,19 At the tissular level, apoptosis of basal keratinocytes, the target cells of the alloimmune reaction, is associated with large, actively synthesizing keratinocytes at the upper levels of the epithelium.20 Such an association of cell damage and tissue repair features within the epithelium is characteristic of a lichenoid reaction.21 Kinetic studies that used cell BrdU labeling have suggested that lichenoid reactions could be a form of squamous epithelial reaction to a chronic basal damage, whether immune-mediated, drug-induced, or idiopathic.22 Moreover, the ulcerated form of idiopathic oral lichen planus is associated with a high risk of malignant transformation. In the context of chronic GVHD, the lichenoid lesion of the oral mucosa could favor malignant transformation. One of the 4 patients with donor-derived SCC also had Fanconi anemia, a disease characterized by an increased risk of GVHD23 and SCC.24 Of interest, microsatellite instability (MSI) at tetra-nucleotide repeats was detected in laser-microdissected colonic crypts and in buccal smears of 75% and 42%, respectively, of patients who received an allograft.25 MSI in clinically intact oral mucosa was more frequently found at later time points after hematopoietic cell transplantation (HCT). MSI was also found in 3 posttransplantation squamous cell cancers.25
The occurrence of oral SCC, an epithelial tumor, of donor origin after BMT implies a supplementary step of transdifferentiation of marrow stem cells in normal epidermal cells.3,16 The homing of these stem cells in the oral lesion of chronic GVHD could reflect their attraction to a zone of chronic inflammation and tissue repair.26,27 Moreover, the epithelial stem/progenitor cells might become exhausted by severe or chronic injury and be replaced by circulating bone marrow–derived ones.16,28,29
Interestingly, the skin is also the main site of development of cancers in patients after solid organ transplantation.30 As in patients after BMT, these cancers are aggressive and always of SCC type.31 A single SCC of donor origin after kidney transplantation was reported.32 If the local environmental factors favor homing and subsequent transformation of marrow stem cells in the oral mucosa, a central immune dysfunction could also favor the development of SCC after BMT. Chronic GVHD is a syndrome characterized by alloreactivity and immunodeficiency, conditions that clearly favor tumor development. Immune deficiency linked to chronic GVHD is further enhanced by the treatment of chronic GVHD,1 and the risk of tumor is higher with prolonged immunosuppression.33 GVHD-associated immunosuppression also increases the susceptibility to viral pathogens.34 We previously reported that SCCs in this setting could not reliably be linked to viruses either to herpesviruses or papilloma viruses.14 This is in contrast to Epstein-Barr virus–associated posttransplantation lymphoproliferative disorders (PTLD)35 (reviewed in Flynn and Kaufman9 ). Although we have characterized donor-derived endothelial cells in the context of acute GVHD16 and others have reported donor-derived endothelial cells in other solid tumor type,17 we failed to detect donor-derived endothelial cells in the 8 SCC cases we studied.
Relevant to our clinical observations is the experimental model of epithelial gastric cancer originating from bone marrow–derived cells in the context of chronic infection with H pylori. In this model BMDCs repopulate the gastric epithelium chronically infected by H pylori progress through metaplasia to intraepithelial cancer.12 It has also been shown that bone marrow cells contribute to epithelial neoplasias of the small bowel, colon, and lung, but not of the skin.11 To further assess the contribution of bone marrow cells to epithelial cancer, those investigators used mouse models of intestinal and lung neoplasias, which showed that the hematopoietic stem cell and its progeny incorporate within cancer. In this study 3 patients had SCC (1 lung, 2 skin), and all 3 had history of GVHD. The relative contribution of donor cells to SCC varied from 0% (skin cancers) to 20% (lung cancer). The discrepancy with our results could be attributed to different locations (oral mucosa versus skin and lung) and to different degrees of associated chronic inflammation. Arai et al36 also reported a case of donor-derived SCC of the oral cavity after peripheral stem cell transplantation and chronic GVHD (assessed by FISH). In these 2 latter reports it should be noted that FISH only was used to assess the donor versus recipient origin of the cancer cells. In this study, we controlled the FISH results by a completely different method, which also associated molecular markers and morphologic selection of cell populations, and performed a microsatellite analysis of laser-microdissected tumor cells.
However, the caveat of this study is that, as expected from human materials, we cannot show which bone marrow cell type gave rise to these cancers. One interpretation of our data could be the fusion of marrow stem cells to epithelial cells.37 The possible fusion of marrow stem cells to SCC cancer stem cells, or alternatively epithelial stem cells, could be followed by “reduction division,” leading to cells that are less than tetraploid (although perhaps not purely diploid) and contain a mosaic of genetic elements of both donor and recipient. However, in our patients the issue on reduction division is unlikely because we did not found a mosaic of genetic elements (mixed origin). PCR of short tandem repeats, which are highly polymorphic markers, allowed a clear discrimination of the patient and of his or her sibling donors. Furthermore, in the 4 tumors, more than 1 (2, 3, 3, and 5) STR was found to be of donor origin, and we never observed any STR of recipient origin. With the use of the same STR analyses, substantial amounts (9%-72%) of donor DNA were found in fingernail clippings from 9 of 21 recipients 2 years and more after transplantation.38 Through microdissection and PCR of STRs, Flemming et al39 also proved the donor origin of a de novo hepatocellular carcinoma in 2 patients after liver transplantation. Finally, another possibility is that these tumors might stem from mesenchymal stem cells that are coinfused with hematopoietic stem cells within the marrow graft. We cannot, however, test this hypothesis in human beings. Finally, because we recently described that breast cancers after transplantation are not associated with chronic GVHD,40 we studied with the same methods the only case with available archived tissues and proved the recipient origin of this breast cancer. However, it must be emphasized that a single case does not allow any general conclusion (ie, development of cancer in donor cells only in the setting of chronic GVHD and of SCC type).
The characterization of these donor-derived oral epithelial tumors in patients with chronic GVHD has theoretical implications for the model of cancer progression and clinical implications in the context of widening BMT indications, prolonged immunosuppression, and longer survival of the patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mrs Stéphanie Belhadj for excellent technical assistance, and the Service d'Infographie de l'IUH.
This work was supported by grants from Association pour la Recherche sur le Cancer (ARC), Commissariat à l'Energie Atomique (CEA), Programme Hospitalier de Recherche Clinique (PHRC), Institut National d Cancer (INCa), Agence de Biomédecine, Cancéropôle Ile de France.
Authorship
Contribution: G.S. and A.J. designed the study and wrote the manuscript; A.J., H.M., and C.L. analyzed the morphologic data; H.M., C.L., J.-M.C., L.L., A.D., M.V., and P.R. performed the experimental studies; E.G. provided clinical data; and H.d.T., P.B., and J.S. provided essential support for study design and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gérard Socié, Service d'Hématologie Greffe and Inserm U728, Hôpital Saint-Louis, 1 Av Vellefaux, 75010 Paris, France; e-mail: gerard.socie@sls.aphp.fr.