Abstract
Tetraspan TM4SF5 is highly expressed in a diverse number of tumor types. Here we explore the mechanistic roles of TM4SF5 in angiogenesis. We found that TM4SF5 overexpression correlates with vascular endothelial growth factor (VEGF) expression in SNU449 hepatocytes and with vessel formation in clinical hepatocarcinoma samples. Conditioned media from TM4SF5-expressing cells enhanced viability and tube formation of primary human umbilical vein endothelial cells, and outgrowth of endothelial cells from aorta ring segments, which was abolished by treatment with an anti-VEGF antibody. TM4SF5 retained integrin α5 on the cell surface for VEGF induction, and preincubation with anti–integrin α5 antibody abolished TM4SF5-mediated VEGF expression and secretion. TM4SF5-mediated effects required integrin α5, c-Src, and signal transducer and activator of transcription 3 (STAT3). In addition, tumors from nude mice injected with TM4SF5-expressing cells and from clinical human hepatocarcinoma tissues showed enhanced integrin α5 expression, vessel formation, and signaling activity, which were inhibited by administration of anti–integrin α5 or -VEGF antibody. This study suggests that TM4SF5 facilitates angiogenesis of neighboring endothelial cells through VEGF induction, mediated by cooperation between TM4SF5 and integrin α5 of epithelial cells.
Introduction
All viable tumors must undergo angiogenesis from preexisting vasculature to acquire oxygen and nutrients for continued growth and metastatic dissemination.1 Angiogenesis requires the production and release of soluble factors, including vascular endothelial growth factor (VEGF), which interacts with cognate receptors resulting in proliferation and invasion of endothelial cells.2-5 Induction of VEGF can be facilitated either by hypoxia6 or oncoprotein activation.7,8 The identification and function of pro- or antiangiogenic factors enabling tumor cells to modulate endothelial cell responses has been intensively studied. However, little is known about how signals of cancer cells regulate tumor angiogenesis.9
Integrins are cell-adhesion receptors that activate diverse intracellular signaling molecules to reorganize actin filaments10,11 and to regulate cell proliferation and gene regulation.12,13 When integrins interact with extracellular matrix at focal adhesions, the cytoplasmic tails of integrin subunits recruit focal adhesion molecules, including focal adhesion kinase (FAK), paxillin, p130Cas, and c-Src.14 Because integrins are linked to actin filaments through protein complexes at their cytoplasmic tails,11 the assembly and activation of focal adhesion molecules result in an efficient response to the extracellular environments for regulation of cell functions, including gene expression and protein secretion. Communications between cancer cells and neighboring endothelial cells occur through angiogenic factor expression/secretion and receptor liganding.15 Integrins transduce signaling by interaction with growth factor receptors16,17 or TM4SF.18
TM4SF proteins (also known as tetraspanins or tetraspans) are a group of hydrophobic proteins with 4 transmembrane domains, 2 extracellular loops, and 2 short cytoplasmic tails.19 TM4SFs form complexes with integrins to create massive tetraspanin-web or tetraspanin-enriched microdomain (TERM) structures to collaboratively perform roles in cell adhesion, proliferation, and motility.18,20,21 TM4SF5 (also known as L6H) is a homolog of tumor-associated antigen L6 (TM4SF1 or L6-Ag) and forms a 4-transmembrane L6 superfamily with L6, IL-TMP, and L6D.22 TM4SF5 is highly expressed in diverse tumor types, including hepatocarcinoma.23,24 We observed that TM4SF5 causes actin reorganization through regulation of FAK and RhoA activity, epithelial-mesenchymal transition, and loss of contact inhibition leading to multilayer growth.24 TM4SF5 ectopically expressed in fibroblasts interacts with integrin α2 to regulate focal adhesion turnover and actin reorganization.25 Therefore, it is probable that TM4SF5 cooperates with the integrins to regulate cellular functions.
In this study, TM4SF5 overexpression in human hepatocarcinoma patients occurred together with VEGF expression and aggressive vascularization. Ectopic expression of TM4SF5 in hepatocytes resulted in higher surface retention of integrin α5 and subsequent activation of FAK, c-Src, and signal transducer and activator of transcription 3 (STAT3), leading to VEGF expression and secretion, and enhanced angiogenic activity of human umbilical vein endothelial cells (HUVECs). Therefore, TM4SF5 and integrin α5 enhanced VEGF expression via the c-Src-FAK/STAT3–signaling linkage, indicating that TM4SF5 is proangiogenic.
Methods
Cells
Stable clones (control SNU449Cp and TM4SF5-expressing SNU449Tp or SNU449T16 cell clones) were described previously.24 HUVECs were maintained until the sixth passage in M199 media containing 20% fetal bovine serum (FBS; JBI, Daegu, Korea), 5 U/mL heparin (Sigma-Aldrich, St Louis, MO), and 5 ng/mL basic fibroblast growth factor on 0.3% gelatin-precoated culture dishes.
Transfections
Cells were transfected for 2 days with the indicated expression vectors: mock construct, pcDNA3-TM4SF5, myc-(His)6–TM4SF5, pcDM–wild-type (WT) integrin α5, pECE-tailless integrin α526 (with deletion of the cytoplasmic 27 amino acids), pRc/CMV-(HA)3-WT FAK, pBS-FRNK, pKH3-WT c-Src, pKH3-inactive Y416F c-Src mutant,27 pRc/CMV-WT STAT3, or pRc/CMV–dominant-negative Y705F STAT3.28 SNU449Tp or TM4SF5-positive Huh729 cells were microporated with control short, hairpin RNA (shRNA) against GFP or TM4SF5 (shTM4SF5),25 small, interfering RNA (siRNA) against c-Src (QIAGEN, Valencia, CA, targeting against cgg ctt gtg ggt gat gtt tga) or STAT3 (Dharmacon RNA Technologies, Lafayette, CO, targeting against gag att gac cag cag tat att caa cat gtc att tgc tga att cca aca atc cca aga atg ttt and caa cag att gcc tgc att gtt).
Western blots
Stable cells were treated with pharmacologic inhibitors, including 20 μM U0126, 40 μM PD98056 (LC Laboratories, Woburn, MA), 40 μM PP2, 40 μM PP3 (A.G. Scientific, San Diego, CA), 30 μM AG490, or 15 μM Y27632 (Calbiochem, San Diego, CA) for 24 hours before lysate preparation. Whole-cell lysates were prepared as previously described.25 SNU449Cp and SNU449Tp cells were suspended in 10 mM ethylenediaminetetraacetic acid and washed with phosphate-buffered saline (PBS), then incubated with normal human IgG or function blocking anti–integrin α5 (P1D6) antibody (30 μg/mL; Chemicon International, Temecula, CA). Cells were reseeded into tissue-culture dishes, and incubated at 37°C and 5% CO2 for 10 hours before collecting conditioned media and lysate preparation. Control or tumor tissues were frozen immediately after surgery using liquid N2, homogenized in liquid N2 and extracted using radioimmunoprecipitation assay (RIPA) buffer containing 0.1% sodium dodecyl sulfate, before centrifugation at 13 000g for 30 minutes at 4°C. Standard Western blots were performed using antibodies against phospho-Y397FAK, phospho-Y577FAK, phospho-Y861FAK, and phospho-Y925FAK (Invitrogen, Carlsbad, CA), integrin αv, α2, α3, α4, α5, β1, β3, β5 (Chemicon International), phospho-Y705STAT3, STAT3, HA (AbGent), VEGF-A (sc-152 or sc-7269), phospho-Y416Src, phospho-Y527Src, c-Src (Santa Cruz Biotechnology, Santa Cruz, CA), FAK (BD Biosciences, San Jose, CA), α-tubulin (Sigma-Aldrich), phospho-Erk1/2, Erk1/2 (Cell Signaling Technology, Danvers, MA), or TM4SF5.24
VEGF promoter assay
SNU449 cells were transiently microporated for 48 hours with pGL3-human VEGF promoter, pBabe–β-galactosidase, and the indicated expression vectors (human integrin α5 WT, tailless integrin α5 mutant, FAK WT, FRNK, c-Src WT, inactive Y416F c-Src mutant, STAT3 WT, or dominant negative [DN; Y705F] STAT3). Luciferase activity was analyzed and normalized using β-galactosidase activity for transfection efficiency.28
Angiogenic antibody array
Subconfluent SNU449Cp and SNU449Tp cells were serum-deprived for 24 hours. Conditioned media were collected from each cell culture. For functional-blocking experiments, conditioned media were collected from SNU449Tp cells that had been suspended and incubated for 20 minutes with either normal mouse IgG or antihuman integrin α5 (P1D6) monoclonal antibody (30 μg/mL) before reseeding onto normal culture dishes for 10 hours. Angiogenic factors in the conditioned media were determined using RayBiotech Human Angiogenesis Antibody Array (Norcross, GA), following the manufacturer's protocol.
Determination of surface integrin α5
SNU449 cells were microporated with pcDNA3 or pcDNA3-myc-(His)6-TM4SF5 plasmid. After 1 day, cells were suspended in 10 mM ethylenediaminetetraacetic acid, centrifuged, and resuspended in PBS. The same number of cells was incubated with various concentration of trypsin (1-1000 units/mL PBS) at room temperature for 15 minutes. Digestion was stopped by addition of 1 mM phenylmethylsulfonyl fluoride and cells were washed twice with cold PBS and lysed with RIPA buffer, before immunoblots using either anti–α5ecto (BD Bioscience) or -α5cyto (Chemicon International) antibody, recognizing an extracellular or the cytoplasmic region of integrin α5, respectively. Alternatively, the transfected cells were collected and an equal number of cells in 2 sets were analyzed for integrin α5 on cell surface by flow cytometry using monoclonal anti–integrin α5 antibody (clone P1D6; Chemicon International).26 One set was nonpermeabilized, whereas the other set was permeabilized with 0.5% Triton X-100 in PBS before fixation and antibody incubation. Data were analyzed using WinMDI software (The Scripps Research Institute, San Diego, CA).
Coimmunoprecipitation
Subconfluent TM4SF5-null SNU449 or SNU398 cells were cotransfected for 48 hours with mock, mock plus myc-(His)6-TM4SF5, or myc-(His)6-TM4SF5 plus either WT integrin α5, tailless α5 (α5/1), or chimeric α5 tail where the α5 cytoplasmic tail replaced the tail of integrin α4 (X4C5).30 Whole-cell lysates were immunoprecipitated with anti-(His)6 antibody, before immunoblots using anti–integrin α5ecto (BD Bioscience) or α5cyto (Chemicon International) antibodies.
HUVEC tube formation assay
Media for SNU449 cell lines (RPMI 1640 media containing 10% FBS) were changed to M199 media containing 5% FBS for a 48-hour incubation. Media supernatant was cleared with a 0.45-μm syringe filter. Before the tube formation assay, HUVECs were serum-starved by incubating in M199 media containing 1% FBS for 8 hours. Matrigel (BD Biosciences) was polymerized (200 μL/well of a 24-well culture plate) for 30 minutes at 37°C. Serum-starved HUVECs were resuspended in 1.2 mL of the conditioned media and reseeded in Matrigel-coated wells (105 cells/well). After incubation without or with anti-VEGF antibody (40 μg/mL) for the indicated periods, images of capillary-like networks were recorded randomly using a phase-contrast microscope (CKX41; Olympus, Tokyo, Japan).
Aorta ring assay
Aorta segments were prepared from a 6-week-old male BALB/c mouse (Orient, Seungnam, Korea). Aorta ring pieces were embedded between 2 layers of Matrigel in 24-well culture plates, in the presence of the conditioned media. Normal IgG or anti-VEGF antibody (40 μg/mL) was mixed with the conditioned media, 20 minutes before application. Four days later, images of sprouts indicating endothelial cell growth were obtained from 5 randomly isolated fields using a phase-contrast microscope (CKX41; Olympus).
Mouse and tumor xenografts
Four- or 5-week-old female nude (BALB/cAnNCrjBgi-nu) mice were maintained following the procedures in the Seoul National University Laboratory Animal Maintenance Manual and institutional review board approval. Mice were injected subcutaneously in the flank with 5 × 106 of viable SNU449Cp or SNU449Tp cells in PBS. Tumor volumes were measured with a caliper and calculated using the formula: volume = (a × b2)/2, where a was the width at the widest point of the tumor and b was the maximal width perpendicular to a. Anti–integrin α5 (P1D6 clone) or -VEGF (Upstate Biotechnology) antibody or normal IgG at 2.5 mg/kg was administered directly to tumors twice a week after the tumor dimension reached approximately 100 mm3.
Human liver tissue samples
Human liver tissues (normal or with tumors) were obtained from patients in the Kyungpook National University Hospital (Daegu, South Korea) with informed consent obtained in accordance with the Declaration of Helsinki and institutional review board approval. Normal tissues were cut from an area grossly free of tumor (> 5 times of tumor size distal from the tumor's visible edge). Biologic and clinical information was available for the samples.
Tumor histology
Mouse tumor tissues were removed approximately 6 weeks after cell injection. Mouse tumor or clinical hepatocarcinoma tissues were fixed with formalin and embedded in paraffin. Serial sections (6 μm thick) were stained with anti-CD31 or –integrin α5 before visualization by fluorescent microscopy (BX51; Olympus). Slides were viewed with an Olympus BX51 research microscope for images at DIC (Nomarski) units (UPLFLN 10-100×) using a digital camera (KY-1030BU; JVC, Yokohama, Japan), which were processed with Ky-Link software (JVC) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
Viability assay
HUVECs were seeded into 24-well culture plates (105 cells/well). One day later, triplicate cultures were treated with conditioned media from SNU449, SNU449Cp, SNU449Tp, and SNU449T16 cells. During conditioned media incubation, viable cells were counted by trypan blue staining.
Statistical methods
Immunohistochemistry images for CD31 were photographed (× 40) and scanned in Adobe Photoshop (San Diego, CA). The CD31-positive area from at least 5 independent images was quantified with Image-Pro Plus (Media Cybernetics, Silver Spring, MD). Student t tests were performed, where P values less than .05 were considered significant.
Results
Correlation between TM4SF5, VEGF expression, and vessel formation in human liver carcinoma
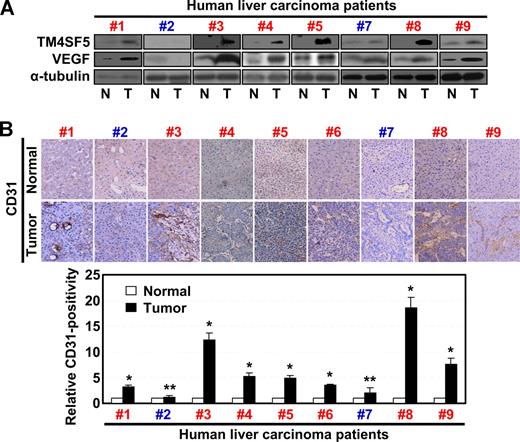
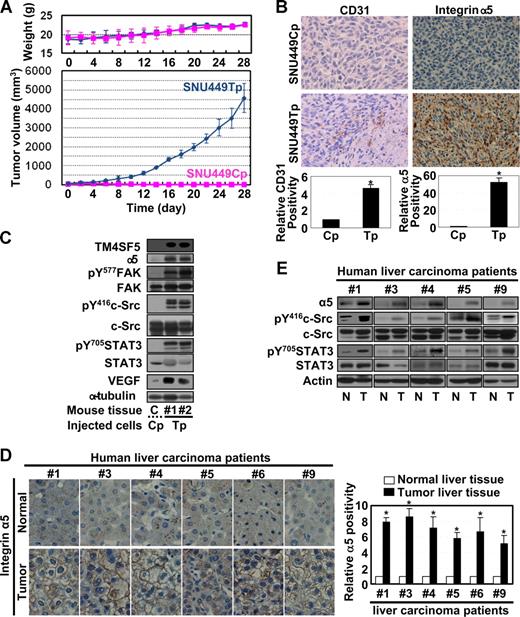
We recently reported that TM4SF5 expression in hepatocytes causes epithelial-mesenchymal transition and loss of contact inhibition.24 To clarify the role of TM4SF5 in angiogenesis, we examined whether the pattern of TM4SF5 expression in normal or tumor liver tissues correlated with VEGF expression and/or vessel formation. Immunoblots of tissue lysates revealed that 7 hepatocarcinoma tissues (cases 1, 3-6, 8, and 9, Figure 1) showed enhanced levels of TM4SF5 and VEGF compared with normal tissues. Two hepatocarcinoma samples (cases 2 and 7) did not show a correlation between TM4SF5 and VEGF expression (Figure 1). Immunohistochemical detection for vessel formation further showed that 7 of 9 hepatocarcinoma tissues were positive for the endothelial cell marker CD31 (cases 1, 3-6, 8, and 9, Figure 1B). Therefore, the TM4SF5-positive tumors showed a tendency for enhanced VEGF expression and vessel formation, compared with normal tissues, indicating that TM4SF5 may function in tumor angiogenesis.
TM4SF5 overexpression correlates with VEGF expression and vessel formation in human hepatocarcinoma. Normal and tumor liver tissues separately obtained from 9 Korean hepatocarcinoma patients were analyzed by standard Western blot for the indicated proteins (A) or by immunohistochemistry for human CD31 at × 100 magnification (B). Western blot for the sixth patient was not shown because the sample was not available for immunoblots. However, a previous report showed that TM4SF5 was overexpressed in tumor tissue of the sixth patient by immunohistochemistry.24 VEGF expression in 7 of 9 cases (cases 1, 3-6, 8, and 9) correlated to TM4SF5 overexpression. Case 2 was negative for TM4SF5, VEGF expression, or vessel formation, and case 7 did not show clear correlations between the parameters. In addition, we could not find any histologic differences in TM4SF5-positive and -negative samples. P values less than .05 were considered significant: *P < .05; **P > .05. Data are representative of 3 independent experiments.
TM4SF5 overexpression correlates with VEGF expression and vessel formation in human hepatocarcinoma. Normal and tumor liver tissues separately obtained from 9 Korean hepatocarcinoma patients were analyzed by standard Western blot for the indicated proteins (A) or by immunohistochemistry for human CD31 at × 100 magnification (B). Western blot for the sixth patient was not shown because the sample was not available for immunoblots. However, a previous report showed that TM4SF5 was overexpressed in tumor tissue of the sixth patient by immunohistochemistry.24 VEGF expression in 7 of 9 cases (cases 1, 3-6, 8, and 9) correlated to TM4SF5 overexpression. Case 2 was negative for TM4SF5, VEGF expression, or vessel formation, and case 7 did not show clear correlations between the parameters. In addition, we could not find any histologic differences in TM4SF5-positive and -negative samples. P values less than .05 were considered significant: *P < .05; **P > .05. Data are representative of 3 independent experiments.
TM4SF5-mediated VEGF expression and secretion
The correlation between TM4SF5 and VEGF expression in hepatocarcinoma tissues suggests that TM4SF5 may mediate VEGF expression. Therefore, we examined the effects of transient TM4SF5 expression on VEGF promoter activity in hepatocytes. When TM4SF5-null SNU449 hepatocytes were transfected with TM4SF5 plasmids, VEGF promoter activity was enhanced by an average of 3- to 4-fold, compared with that of mock control-transfected cells (Figure 2A). Consistently, expression of VEGF (presumably 121, 165, and 189 amino acid human VEGF-A splice variants) was enhanced in transiently TM4SF5-transfected SNU449 or stably transfected SNU449Tp or T16 cells (Figure 2B,C). We then examined whether the TM4SF5-expressing cells secreted VEGF. Conditioned media were prepared from stable SNU449Cp cells lacking TM4SF5 and SNU449Tp cells expressing TM4SF5 and arrayed with human angiogenic antibodies. Interestingly, the conditioned media from TM4SF5-expressing cells showed a significant amount of secreted VEGF in the culture media compared with negative controls (Figure 2D). These observations indicate that TM4SF5 expression results in effective VEGF induction and secretion.
Ectopic expression of TM4SF5 in SNU449 cells enhanced VEGF expression and secretion. (A) Parental SNU449 cells were transfected with pGL3-human VEGF promoter and pBabe–β-galactosidase together with a mock construct, pcDNA3-TM4SF5, or Myc-(His)6-TM4SF5. After 2 days, lysates were prepared and luminescence was measured. Transfection efficiency was normalized by β-galactosidase activity. Data are mean plus or minus SD of 3 independent experiments. RLU indicates relative luciferase activity units. (B,F) TM4SF5-null SNU449 cells were transiently transfected with either the mock construct or pcDNA3-TM4SF5. One day later, whole-cell lysates were prepared, normalized, and analyzed by standard Western blots for the indicated proteins. (C) Whole-cell extracts prepared from parental SNU449 (P), control SNU449Cp (Cp), or stably TM4SF5-expressing SNU449 cell lines (Tp or T16) were analyzed for VEGF expression using 2 different anti-VEGF antibodies (rabbit anti-VEGF polyclonal and mouse anti–VEGF-A [c-1] monoclonal antibody from Santa Cruz Biotechnology, B) or the indicated molecules (F). (D) Subconfluent SNU449Cp and SNU449Tp cells were rinsed and incubated with serum-free media for 24 hours before collection of conditioned media. Using conditioned media from each cell line, angiogenic factors were analyzed using RayBiotech human angiogenesis antibody array. Notably, only VEGF was elevated in conditioned media from TM4SF5-expressing SNU449Tp cells. (E) One day after SNU449Cp or SNU449Tp cells were seeded, subconfluent cells were treated with dimethyl sulfoxide or various pharmacologic inhibitors (U0126, 20 μM; PD98056, 40 μM; PP2, 40 μM; PP3, 40 μM; AG490, 30 μM; Y27632, 15 μM) for 24 hours. Whole-cell extracts were normalized and used in standard Western blots for the indicated molecules. Notably, c-Src family kinase inhibitor PP2 mostly reduced VEGF expression in SNU449Tp cells. (G-J) TM4SF5-positive SNU449Tp (G-I) or Huh7 (J) cells were transfected with shRNA against GFP (shGFP) or TM4SF5 (shTM4SF5) (G,J), or siRNA against GFP, c-Src (H), or STAT3 (I). After 48 hours, whole-cell lysates were prepared, normalized, and used in standard Western blots for the indicated molecules. Immunoblotting with anti-VEGF antibody (pAb) resulted in multiple bands, presumably isotypes. Data shown were representative from 3 independent experiments.
Ectopic expression of TM4SF5 in SNU449 cells enhanced VEGF expression and secretion. (A) Parental SNU449 cells were transfected with pGL3-human VEGF promoter and pBabe–β-galactosidase together with a mock construct, pcDNA3-TM4SF5, or Myc-(His)6-TM4SF5. After 2 days, lysates were prepared and luminescence was measured. Transfection efficiency was normalized by β-galactosidase activity. Data are mean plus or minus SD of 3 independent experiments. RLU indicates relative luciferase activity units. (B,F) TM4SF5-null SNU449 cells were transiently transfected with either the mock construct or pcDNA3-TM4SF5. One day later, whole-cell lysates were prepared, normalized, and analyzed by standard Western blots for the indicated proteins. (C) Whole-cell extracts prepared from parental SNU449 (P), control SNU449Cp (Cp), or stably TM4SF5-expressing SNU449 cell lines (Tp or T16) were analyzed for VEGF expression using 2 different anti-VEGF antibodies (rabbit anti-VEGF polyclonal and mouse anti–VEGF-A [c-1] monoclonal antibody from Santa Cruz Biotechnology, B) or the indicated molecules (F). (D) Subconfluent SNU449Cp and SNU449Tp cells were rinsed and incubated with serum-free media for 24 hours before collection of conditioned media. Using conditioned media from each cell line, angiogenic factors were analyzed using RayBiotech human angiogenesis antibody array. Notably, only VEGF was elevated in conditioned media from TM4SF5-expressing SNU449Tp cells. (E) One day after SNU449Cp or SNU449Tp cells were seeded, subconfluent cells were treated with dimethyl sulfoxide or various pharmacologic inhibitors (U0126, 20 μM; PD98056, 40 μM; PP2, 40 μM; PP3, 40 μM; AG490, 30 μM; Y27632, 15 μM) for 24 hours. Whole-cell extracts were normalized and used in standard Western blots for the indicated molecules. Notably, c-Src family kinase inhibitor PP2 mostly reduced VEGF expression in SNU449Tp cells. (G-J) TM4SF5-positive SNU449Tp (G-I) or Huh7 (J) cells were transfected with shRNA against GFP (shGFP) or TM4SF5 (shTM4SF5) (G,J), or siRNA against GFP, c-Src (H), or STAT3 (I). After 48 hours, whole-cell lysates were prepared, normalized, and used in standard Western blots for the indicated molecules. Immunoblotting with anti-VEGF antibody (pAb) resulted in multiple bands, presumably isotypes. Data shown were representative from 3 independent experiments.
TM4SF5-mediated VEGF induction involves signaling activities downstream of integrins
We next investigated which signaling molecules are responsible for TM4SF5-mediated VEGF induction. We treated SNU449Cp and SNU449Tp cells with pharmacologic inhibitors for 24 hours before lysate preparation for immunoblots. Pharmacologic reagents included dimethyl sulfoxide (as a control vehicle), U0126 and PD98056 (as specific MEK/Erk1/2 inhibitors), PP2 (a specific c-Src family kinase inhibitor), PP3 (a negative control compound of PP2), AG490 (a specific JAK inhibitor), and Y27632 (a specific ROCK inhibitor). TM4SF5-enhanced VEGF induction was inhibited by PP2 treatment, but not by treatment with the other reagents (Figure 2E). This observation indicates that TM4SF5-mediated VEGF induction requires c-Src family kinase activity, but not MEK/Erk1/2 and JAK activities.
We then analyzed proteins involved in the signaling networks upstream or downstream of c-Src family kinase by immunoblots. In particular, we examined FAK and c-Src levels, which are major signaling mediators downstream of integrins,12,13 that may participate in cross-talk with TM4SFs and/or tetraspans.18 We previously reported that phosphorylation of FAK Tyr577 (pY577FAK) correlated with c-Src phosphorylation (pY416c-Src) in TM4SF5-expressing cells,24 and that STAT3 downstream of c-Src can induce VEGF promoter activity.28 TM4SF5 expression resulted in enhanced pY577FAK, pY861FAK (but not pY397FAK or pY925FAK), pY416c-Src, pY705STAT3, and VEGF protein levels. Expression levels of major integrin subunits were not altered by transient transfection of TM4SF5 (Figure 2F). These enhanced signaling activities and VEGF levels were blocked by transfection with shRNA against TM4SF5 (Figure 2G), or with siRNA against c-Src (Figure 2H) or STAT3 (Figure 2I), but not with shRNA against GFP protein, without subsequent alteration of expression of major integrin subunits. Furthermore, shTM4SF5 introduction to the TM4SF5-endogenously expressing Huh7 cell line showed similar results (Figure 2J).
Conditioned media from TM4SF5-expressing cells enhanced angiogenic activities of primary endothelial cells
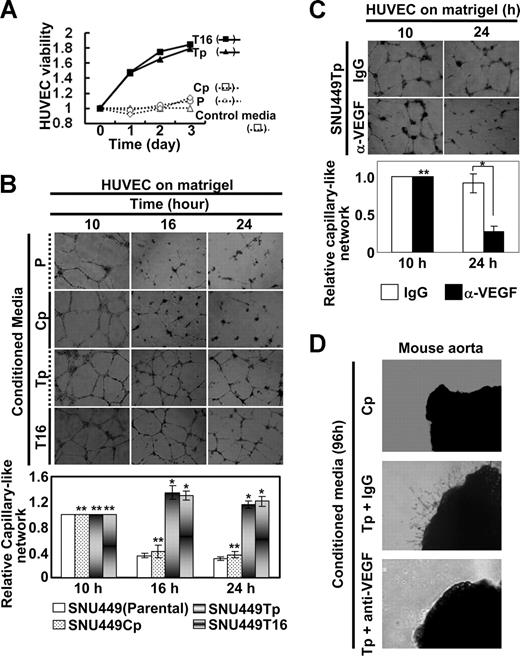
We next examined whether conditioned media from TM4SF5-expressing cells could enhance viability and angiogenic activity of primary HUVECs. Conditioned media prepared from cultures of diverse cells were added to primary HUVEC cultures. HUVEC viability increased substantially after treatment with conditioned media from TM4SF5-postive cells, whereas conditioned media from TM4SF5-null SNU449 or SNU449Cp cells did not have an effect (Figure 3A). We examined whether vessel-like tube formation by primary HUVECs on Matrigel is influenced by conditioned media from TM4SF5-expressing cells. Primary HUVECs efficiently formed vessel-like tubes on Matrigel. When HUVECs were treated with conditioned media from either TM4SF5-null or TM4SF5-positive cells for 10 hours, similar vessel-like tubes formed (Figure 3B left). However, maintenance of the tube-like structure was longer (up to 24 hours) with treatment with conditioned media from TM4SF5-positive cells relative to treatment with media from TM4SF5-null cells (Figure 3B right). This sustaining effect was blocked by preincubation of cells with anti-VEGF antibody, but not with normal IgG (Figure 3C).
Angiogenic properties in conditioned media from TM4SF5-expressing cells. SNU449 cell lines were seeded in complete RPMI 1640 media containing 10% FBS, rinsed, and incubated in M199 media with 5% FBS (control media) for 48 hours, before collection of conditioned media for HUVECs. The media was changed to a M119 media system, where HUVECs were prepared and maintained. (A) HUVECs were serum-starved for 8 hours, trypsinized, collected, and counted. A total of 5 × 104 cells per well (of 12-well plate) were resuspended with SNU449 cell line–derived conditioned media and seeded in triplicates. The total numbers of cells were counted every 24 hours, and relative cell viability was plotted compared with cells that received control media. (B,C) A total of 105 HUVECs per well (of 24-well plate) were resuspended in conditioned media from SNU449 (P), SNU449Cp (Cp), SNU449Tp (Tp), or SNU449T16 (T16) cell cultures and seeded on Matrigel-coated plates. In case, normal IgG or anti-VEGF antibody (40 μg/mL) was added to the plates (C). Images of capillary-like networks were recorded from 5 randomly isolated fields using a phase-contrast microscope at indicated times (original magnification × 40). After 16 hours of incubation, tube-like structures started to collapse in cultures treated with conditioned media from TM4SF5-null cells, whereas structural integrity was maintained in cultures treated with conditioned media prepared from TM4SF5-expressing cells. (D) Aortic ring segments were prepared and embedded into 2 layers of Matrigel. Normal IgG or anti-VEGF antibody (40 μg/mL) was added to conditioned media from SNU449Tp for 20 minutes. After incubation for 96 hours with conditioned media collected from either SNU449Cp (Cp) or SNU449Tp (Tp) cell cultures, images of endothelial cell outgrowth forming branched cords from the vessel segment margins were obtained using a phase-contrast microscope (original magnification × 40). P values less than .05 were considered significant: *P < .05; **P > .05. Data shown are representative of 3 different experiments.
Angiogenic properties in conditioned media from TM4SF5-expressing cells. SNU449 cell lines were seeded in complete RPMI 1640 media containing 10% FBS, rinsed, and incubated in M199 media with 5% FBS (control media) for 48 hours, before collection of conditioned media for HUVECs. The media was changed to a M119 media system, where HUVECs were prepared and maintained. (A) HUVECs were serum-starved for 8 hours, trypsinized, collected, and counted. A total of 5 × 104 cells per well (of 12-well plate) were resuspended with SNU449 cell line–derived conditioned media and seeded in triplicates. The total numbers of cells were counted every 24 hours, and relative cell viability was plotted compared with cells that received control media. (B,C) A total of 105 HUVECs per well (of 24-well plate) were resuspended in conditioned media from SNU449 (P), SNU449Cp (Cp), SNU449Tp (Tp), or SNU449T16 (T16) cell cultures and seeded on Matrigel-coated plates. In case, normal IgG or anti-VEGF antibody (40 μg/mL) was added to the plates (C). Images of capillary-like networks were recorded from 5 randomly isolated fields using a phase-contrast microscope at indicated times (original magnification × 40). After 16 hours of incubation, tube-like structures started to collapse in cultures treated with conditioned media from TM4SF5-null cells, whereas structural integrity was maintained in cultures treated with conditioned media prepared from TM4SF5-expressing cells. (D) Aortic ring segments were prepared and embedded into 2 layers of Matrigel. Normal IgG or anti-VEGF antibody (40 μg/mL) was added to conditioned media from SNU449Tp for 20 minutes. After incubation for 96 hours with conditioned media collected from either SNU449Cp (Cp) or SNU449Tp (Tp) cell cultures, images of endothelial cell outgrowth forming branched cords from the vessel segment margins were obtained using a phase-contrast microscope (original magnification × 40). P values less than .05 were considered significant: *P < .05; **P > .05. Data shown are representative of 3 different experiments.
Because we observed that TM4SF5 expression resulted in VEGF induction and secretion (Figure 2) and that conditioned media from TM4SF5-expressing cells enhanced angiogenic activity of the HUVECs (Figure 3A,B), we next examined whether conditioned media from TM4SF5-expressing cells contains sufficient amounts of VEGF to promote angiogenesis using a mouse aorta ring assay. Aorta ring segments embedded in Matrigel were incubated with conditioned media from TM4SF5-expressing and control cells (Figure 3D top and middle). Enhanced outgrowth of endothelial cells was observed after treatment with the conditioned media from TM4SF5-expressing cells but was blocked by preincubation of the conditioned media with anti-VEGF antibody (Figure 3D bottom). These results indicate that secreted VEGF indeed facilitates endothelial cell outgrowth.
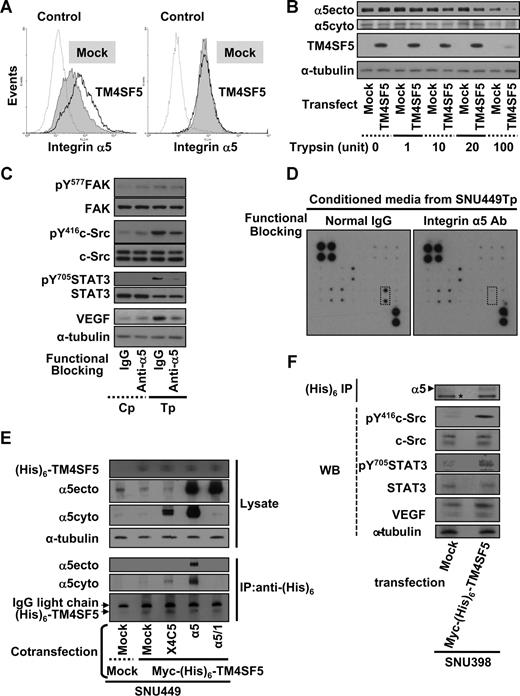
TM4SF5 causes retention of integrin α5 on cell surface
The expression levels of major integrin subunits were not changed by transient expression of TM4SF5 during immunoblotting (Figure 2). Because integrin-mediated signaling appeared to be involved in TM4SF5-mediated VEGF induction, we next explored cell-surface expression of integrins by flow cytometric analysis, using cells mock- or TM4SF5-transfected cells without or with permeabilization before antibody incubation. Integrin α5 levels on TM4SF5-transfected cells were higher than on mock-transfected cells, under conditions where the membrane is not permeabilized, thus allowing detection solely of surface expressed integrins. Meanwhile, total integrin α5 levels visualized after permeabilization were similar between mock- and TM4SF5-transfected cells (Figure 4A). Increased integrin α5 expression on the surface of TM4SF5-transfected cells would result in more extracellular domain-cleaved integrin α5 on protease treatment. To test this possibility, equal numbers of cells were treated with various concentrations of trypsin for 15 minutes at room temperature. As more trypsin was treated, immunoblot analysis using an antibody that recognizes an extracellular integrin α5 region (ie, α5ecto) showed lower levels of integrin α5 in TM4SF5-transfected cells, compared with mock-transfected cells. This indicates that intact and intracellular integrin α5 were recognized by α5ecto antibody more in mock-transfected cells. Immunoblots using an anti–α5cyto antibody to recognize the cytoplasmic tail of integrin α5 revealed similar levels of integrin α5 in both mock- and TM4SF5-transfected cells (Figure 4B). These observations indicate that TM4SF5 expression increased integrin α5 on the cell surface. We next examined whether functional-blocking antibody of integrin α5 (clone P1D6) would inhibit TM4SF5-mediated VEGF induction and secretion. Cells were incubated for 20 minutes with normal mouse IgG or P1D6 integrin α5 antibody, and reseeded on normal culture dishes for an additional 10 hours. Immunoblots showed decreased TM4SF5-mediated signaling activity and VEGF induction with functional-blocking antibody (Figure 4C lanes 3 and 4). Anti-body array analysis of conditioned media revealed that VEGF secretion was inhibited when TM4SF5-positive cells were incubated with integrin α5-blocking antibody, although other angiogenic molecules detected using the array were unchanged (Figure 4D). These observations suggest that TM4SF5 expression in hepatocytes requires integrin α5 on the cell surface for VEGF induction and secretion.
TM4SF5-mediated VEGF expression involves retention of cell-surface integrin α5. (A,B) SNU449 cells were transiently transfected with a mock construct or pcDNA3-TM4SF5 for 48 hours. (A) Transfected cells were collected, and an equal number of cells were probed with antihuman integrin α5 (P1D6) and antimouse IgG-conjugated with phycoerythrin without (left) and with (right) permeabilization, before fixation and flow cytometric analysis. Note that the histogram for integrin α5 on the surface of TM4SF5-transfected cells (TM4SF5) without permeabilization is right-shifted, compared with that of mock construct-transfected cells (Mock). Control indicates no incubation with the primary antibody. (B) Transfected cells were suspended and allocated into sets with an equal number of cells, which were treated with trypsin at the indicated units for 15 minutes at room temperature. Cells were then mixed with 1 mM phenylmethylsulfonyl fluoride in PBS, washed with PBS, and lysed at 4°C, before standard Western blots for the indicated molecules. (C,D) Conditioned media were collected from SNU449Tp cells that had been suspended and incubated with either normal mouse IgG or anti–human integrin α5 (P1D6) monoclonal antibody (30 μg/mL) 20 minutes before reseeding onto normal culture dishes for 10 hours of incubation. (C,F) Whole-cell lysates were prepared and immunoblotted for the indicated molecules. (D) Conditioned media were analyzed for VEGF levels using the angiogenic antibody array, as in Figure 2D. Dotted rectangles represent array blots for VEGF. Data shown are representative of 3 different experiments. (E,F) TM4SF5-null SNU449 (E) or SNU398 (F) cells were transiently cotransfected with various plasmids. Immunoprecipitates using anti-(His)6 antibody and lysates (WCL) were blotted in parallel. α5ecto or α5cyto indicates α5 integrin that was immunoblotted by the antibody recognizing an extracellular region or the cytoplasmic domain of integrin α5, respectively. X4C5 expression was much less than integrin α5 or α5/1, but X4C5 showed increased coimmunoprecipitation with myc-(His)6-TM4SF5 than α5/1. *Nonspecific band for an internal control. Data shown are representative of 3 isolated experiments.
TM4SF5-mediated VEGF expression involves retention of cell-surface integrin α5. (A,B) SNU449 cells were transiently transfected with a mock construct or pcDNA3-TM4SF5 for 48 hours. (A) Transfected cells were collected, and an equal number of cells were probed with antihuman integrin α5 (P1D6) and antimouse IgG-conjugated with phycoerythrin without (left) and with (right) permeabilization, before fixation and flow cytometric analysis. Note that the histogram for integrin α5 on the surface of TM4SF5-transfected cells (TM4SF5) without permeabilization is right-shifted, compared with that of mock construct-transfected cells (Mock). Control indicates no incubation with the primary antibody. (B) Transfected cells were suspended and allocated into sets with an equal number of cells, which were treated with trypsin at the indicated units for 15 minutes at room temperature. Cells were then mixed with 1 mM phenylmethylsulfonyl fluoride in PBS, washed with PBS, and lysed at 4°C, before standard Western blots for the indicated molecules. (C,D) Conditioned media were collected from SNU449Tp cells that had been suspended and incubated with either normal mouse IgG or anti–human integrin α5 (P1D6) monoclonal antibody (30 μg/mL) 20 minutes before reseeding onto normal culture dishes for 10 hours of incubation. (C,F) Whole-cell lysates were prepared and immunoblotted for the indicated molecules. (D) Conditioned media were analyzed for VEGF levels using the angiogenic antibody array, as in Figure 2D. Dotted rectangles represent array blots for VEGF. Data shown are representative of 3 different experiments. (E,F) TM4SF5-null SNU449 (E) or SNU398 (F) cells were transiently cotransfected with various plasmids. Immunoprecipitates using anti-(His)6 antibody and lysates (WCL) were blotted in parallel. α5ecto or α5cyto indicates α5 integrin that was immunoblotted by the antibody recognizing an extracellular region or the cytoplasmic domain of integrin α5, respectively. X4C5 expression was much less than integrin α5 or α5/1, but X4C5 showed increased coimmunoprecipitation with myc-(His)6-TM4SF5 than α5/1. *Nonspecific band for an internal control. Data shown are representative of 3 isolated experiments.
We next examined whether TM4SF5 associates with integrin α5, using a coimmunoprecipitation approach. Whole-cell lysates obtained from SNU449 cells that were transfected with mock-, mock plus myc-(His)6-TM4SF5, or myc-(His)6-TM4SF5 plus either WT integrin α5, tailless α5,26 or chimeric α5 tail (X4C530 ). Anti-(His)6 antibody immunoprecipitates were immunoblotted for integrin α5. Interestingly, WT integrin α5 and X4C5 chimera were coimmunoprecipitated by TM4SF5, although X4C5 was coimmunoprecipitated less presumably because of less expression, whereas tailless integrin α5 was not (Figure 4E). However, anti-(His)6 immunoprecipitates did not precipitate integrin α5, when myc-(His)6–TM4SF5 was not transfected (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These TM4SF5-mediated effects were shown also in another cell line (SNU398; Figure 4F). Therefore, TM4SF5 appeared to associate at least with the cytoplasmic tail of integrin α5.
TM4SF5-mediated VEGF induction involves the integrin α5/FAK-c-Src/STAT3 signaling cascade
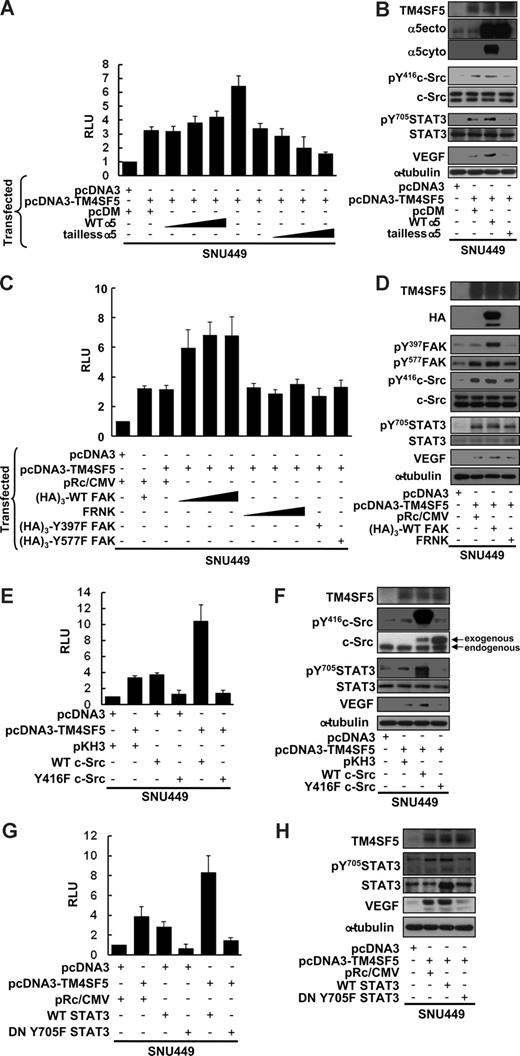
TM4SF5-mediated VEGF expression correlated with phosphorylation of FAK, c-Src, and STAT3 (Figure 2), and TM4SF5 expression resulted in retained integrin α5 on the cell surface (Figure 3). We thus investigated whether integrin α5 was required for the TM4SF5-mediated VEGF promoter activity. First, we performed the VEGF promoter assay using SNU449 cells cotransfected with TM4SF5 and either WT integrin α5 or tailless integrin α5. TM4SF5 transfection resulted in approximately 3-fold increase in VEGF promoter activity. Cotransfection with WT integrin α5 greatly enhanced the promoter activity (to ∼ 7 fold), but cotransfection with tailless integrin α5 abolished TM4SF5-mediated VEGF promoter activity (Figure 5A). When TM4SF5 alone was transfected, pY416c-Src, pY705STAT3, and VEGF protein levels were increased (Figure 5B lanes 1 and 2). Integrin α5 overexpression caused further signaling enhancement and increased VEGF protein levels, whereas tailless integrin α5 abolished the TM4SF5-mediated effects (Figure 5B). When SNU449 cells were transfected with TM4SF5 and either WT FAK, FRNK (a naturally occurring dominant-negative inhibitor of FAK), or FAK mutants (Y397F or Y577F), WT FAK positively enhanced TM4SF5-mediated VEGF promoter activity (Figure 5C) and VEGF induction-related signaling activities (Figure 5D). However, cotransfection of FRNK or FAK mutants did not further enhance VEGF promoter activity or VEGF-induction signaling and did not abolish the TM4SF5-mediated effects (Figure 5C,D).
TM4SF5-mediated VEGF induction involves integrin α5, FAK-c-Src, and STAT3 activation. SNU449 cells were transiently microporated for 48 hours with pGL3-human VEGF promoter, pBabe–β-galactosidase, and the indicated expression vectors. Total DNA quantity was compensated for an equal amount with a vector only plasmid. (A,C,E,G) Luminescence was measured, and transfection efficiency was normalized by β-galactosidase activity. Data are mean plus or minus SD of 3 independent experiments. RLU indicates relative luciferase activity units. (B,D,F,H) Whole-cell lysates were prepared, normalized, and used in standard Western blots for the indicated molecules. Data shown are representative of at least 3 independent experiments.
TM4SF5-mediated VEGF induction involves integrin α5, FAK-c-Src, and STAT3 activation. SNU449 cells were transiently microporated for 48 hours with pGL3-human VEGF promoter, pBabe–β-galactosidase, and the indicated expression vectors. Total DNA quantity was compensated for an equal amount with a vector only plasmid. (A,C,E,G) Luminescence was measured, and transfection efficiency was normalized by β-galactosidase activity. Data are mean plus or minus SD of 3 independent experiments. RLU indicates relative luciferase activity units. (B,D,F,H) Whole-cell lysates were prepared, normalized, and used in standard Western blots for the indicated molecules. Data shown are representative of at least 3 independent experiments.
We next examined the requirement of c-Src for the TM4SF5-mediated effects. c-Src cotransfection with TM4SF5 greatly enhanced TM4SF5-mediated VEGF promoter activity and pY705STAT3, whereas cotransfection with an inactive Y416F c-Src mutant abolished the TM4SF5 effects (Figure 5E,F). In addition, WT STAT cotransfection with TM4SF5 resulted in increased TM4SF5-mediated VEGF promoter activity and VEGF expression, whereas the dominant negative mutant of STAT3 (DN Y705F) almost completely abolished the TM4SF5 effects (Figure 5G,H). Together, these data indicate that TM4SF5 cooperates with integrin α5 to induce VEGF through signal transduction, including FAK–c-Src and STAT3 activation.
Enhanced angiogenesis in TM4SF5-mediated tumors in nude mice
We next investigated whether the TM4SF5-mediated effects observed in vitro also occur in vivo. We subcutaneously injected nude mice with SNU449Cp or SNU449Tp cells and measured tumor growth. As shown in Figure 6A, mice injected with TM4SF5-expressing SNU449Tp cells formed more aggressive tumors, compared with mice injected with TM4SF5-null control cells. Injection of another cell clone expressing TM4SF5 at half the level of SNU449Tp cells resulted in formation of smaller tumors, approximately 20% plus or minus 3.5% less in average volume (data not shown), indicating that the tumor formation was influenced by the level of TM4SF5 expression. TM4SF5-mediated tumor or normal epidermal tissues (from locations injected with control cells) were immunostained with anti-CD31. The SNU449 cell-derived tumor showed more vessel formation (Figure 6B). Furthermore, immunoblots of tissue extracts from the TM4SF5-mediated tumor showed enhanced levels of integrin α5, pY577FAK, pY416c-Src, pY705STAT3, and VEGF, compared with those for normal epidermal tissue (Figure 6C). In human liver tissues, integrin α5 overexpression was pronounced at the membrane surface (Figure 6D), and a close linkage exists between increased levels of TM4SF5, integrin α5, pY416c-Src, pY705STAT3, and VEGF (Figures 1A, 6E).
Mouse tumor tissues caused by TM4SF5-expressing cell injection show enhanced angiogenic properties. (A) A total of 5 × 106 SNU449Cp or SNU449Tp cells were injected subcutaneously into nude mice (n = 7). SNU449Cp cells did not form tumors. Data are reported as mean plus or minus SD. (B) Epidermal tissue around the SNU449Cp injection site or tumor tissue from mice injected with SNU449Tp cells were analyzed for CD31 or integrin α5 (original magnification × 100). (C) Epidermal tissue or tumor tissue lysates from mice injected with SNU449Cp, or SNU449Tp cells were analyzed by standard Western blots. Data shown are representative 3 independent experiments. (D,E) Immunohistochemistry for integrin α5 (original magnification × 40) (D) or immunoblots of extracts of normal or tumor liver tissues obtained from liver cancer patients. From a total of 9 cases, representative datasets with enhanced integrin α5 expression and c-Src and STAT3 signaling activity were shown. P values less than .05 were considered significant: *P < .05. Data shown are representative of 3 different experiments.
Mouse tumor tissues caused by TM4SF5-expressing cell injection show enhanced angiogenic properties. (A) A total of 5 × 106 SNU449Cp or SNU449Tp cells were injected subcutaneously into nude mice (n = 7). SNU449Cp cells did not form tumors. Data are reported as mean plus or minus SD. (B) Epidermal tissue around the SNU449Cp injection site or tumor tissue from mice injected with SNU449Tp cells were analyzed for CD31 or integrin α5 (original magnification × 100). (C) Epidermal tissue or tumor tissue lysates from mice injected with SNU449Cp, or SNU449Tp cells were analyzed by standard Western blots. Data shown are representative 3 independent experiments. (D,E) Immunohistochemistry for integrin α5 (original magnification × 40) (D) or immunoblots of extracts of normal or tumor liver tissues obtained from liver cancer patients. From a total of 9 cases, representative datasets with enhanced integrin α5 expression and c-Src and STAT3 signaling activity were shown. P values less than .05 were considered significant: *P < .05. Data shown are representative of 3 different experiments.
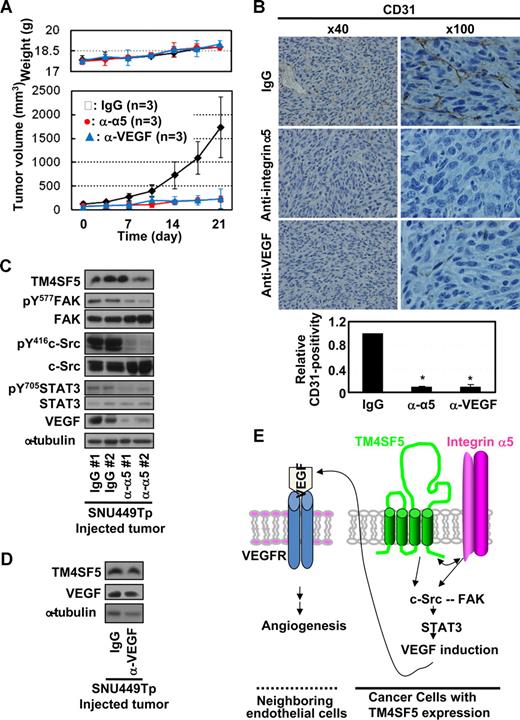
Anti–integrin α5 or -VEGF antibody administration blocked the TM4SF5-mediated effects
We next examined whether anti–integrin α5 or -VEGF antibody administration would inhibit the TM4SF5-mediated tumor and vessel formation in mice and the underlying signaling. When TM4SF5-mediated tumors reached approximately 100 mm3 in size, antibodies were injected directly to the tumors twice a week. Interestingly, the administration of the antibodies, but not normal IgG, efficiently (∼ 80%) blocked TM4SF5-mediated tumor formation (Figure 7A) and vessel formation (Figure 7B). In addition, the signaling for TM4SF5-mediated VEGF expression was also blocked by anti–integrin α5 antibody administration, whereas TM4SF5 levels were unchanged (Figure 7C). Possibly, anti-VEGF antibody-mediated sequestering of extracellular VEGF might inhibit the tumor growth, although the VEGF level in tissue extracts was unchanged (Figure 7D).
Administration of anti–integrin α5 or -VEGF antibody inhibited TM4SF5-mediated vessel formation and tumor growth in nude mice. (A) When tumors sized to approximately 100 mm3, normal IgG, anti–integrin α5 or -VEGF (2.5 mg/kg body weight) was injected directly to the tumors twice a week (n = 3) with measurements of weights and tumor sizes to calculate tumor volumes (mean ± SD). (B-D) Tumors in the mice were dissected out and paraffin-blocked for immunohistochemistry for CD31 or integrin α5 (original magnification × 100) (B) or frozen immediately using liquid N2 for immunoblots of the indicated molecules (C,D). *P < .05 was considered significant. (E) The working model: Cooperation between TM4SF5 and integrin α5 results in c-Src activation, which plays roles in intracellular signaling as a complex with FAK.8 Integrin-mediated FAK activation appears to be synergistic with TM4SF5-mediated c-Src activation, but inactivation of FAK does not block the TM4SF5-mediated c-Src activation and VEGF induction. Physical association between TM4SF5 and the cytoplasmic tail of integrin α5 may be a potential underlying mechanism. Activation of the c-Src/FAK complex can lead to activation of STAT3 by phosphorylation at Tyr705, which can in turn promote transcriptional activity of the VEGF promoter. VEGF induced by cooperation between TM4SF5 and integrin α5 is secreted from epithelial tumor cells. The secreted VEGFs can bind to VEGF receptor on neighboring endothelial cells, consequently promoting angiogenic activities, including proliferation, viability/survival, and sustained vessel-like tube formation.
Administration of anti–integrin α5 or -VEGF antibody inhibited TM4SF5-mediated vessel formation and tumor growth in nude mice. (A) When tumors sized to approximately 100 mm3, normal IgG, anti–integrin α5 or -VEGF (2.5 mg/kg body weight) was injected directly to the tumors twice a week (n = 3) with measurements of weights and tumor sizes to calculate tumor volumes (mean ± SD). (B-D) Tumors in the mice were dissected out and paraffin-blocked for immunohistochemistry for CD31 or integrin α5 (original magnification × 100) (B) or frozen immediately using liquid N2 for immunoblots of the indicated molecules (C,D). *P < .05 was considered significant. (E) The working model: Cooperation between TM4SF5 and integrin α5 results in c-Src activation, which plays roles in intracellular signaling as a complex with FAK.8 Integrin-mediated FAK activation appears to be synergistic with TM4SF5-mediated c-Src activation, but inactivation of FAK does not block the TM4SF5-mediated c-Src activation and VEGF induction. Physical association between TM4SF5 and the cytoplasmic tail of integrin α5 may be a potential underlying mechanism. Activation of the c-Src/FAK complex can lead to activation of STAT3 by phosphorylation at Tyr705, which can in turn promote transcriptional activity of the VEGF promoter. VEGF induced by cooperation between TM4SF5 and integrin α5 is secreted from epithelial tumor cells. The secreted VEGFs can bind to VEGF receptor on neighboring endothelial cells, consequently promoting angiogenic activities, including proliferation, viability/survival, and sustained vessel-like tube formation.
Discussion
Observations from this study suggest that tetraspan TM4SF5 cooperates with integrin α5 to transduce signaling through FAK-c-Src complex activation and STAT3 phosphorylation to induce VEGF in cancerous epithelial cells. Secreted VEGF promotes angiogenic activities in neighboring endothelial cells, presumably allowing efficient angiogenesis (Figure 7E).
Administration of functional-blocking integrin α5 antibody into TM4SF5-mediated tumors blocked the vessel formation and tumor growth. Functional neutralization of cell-surface integrin α5 on TM4SF5-expressing SNU449Tp cells using function blocking antibody as well as cotransfection of TM4SF5 with a cytoplasmic tailless integrin α5 mutant suggests that the integrin α5 cytoplasmic tail is required for TM4SF5-mediated VEGF expression and secretion. Consistent with these observations, tailless integrin α5 did not bind TM4SF5, whereas the cytoplasmic tail of integrin α5 in the X4C5 chimera did bind TM4SF5. TM4SF5 expression appeared to retain integrin α5 on the cell surface, presumably via TM4SF5-mediated influence on internalization and/or trafficking. This increased integrin α5 retention on TM4SF5-expressing cell surface resulted in more efficient adhesion and spreading on fibronectin (S.-A.L. and J.W.L., unpublished data, May 2008). It has been previously reported that the TM4SF5-related tetraspan L6-Ag positively modulated cell motility by regulating surface retention and endocytosis of tetraspanins CD63 and CD82 without any changes in the total expression levels of the tetraspanins.31 SNU449Tp cells and tumor tissues in nude mice injected with TM4SF5-expressing cells showed enhanced levels of integrin α5 compared with SNU449Cp cells and normal epidermal tissue around the injection site, presumably because of TM4SF5-mediated integrin α5 stabilization on the cell surface. However, the binding affinities of TM4SF5 to different integrin subunits may differ. We have observed coimmunoprecipitation between the extracellular domains of TM4SF5 and integrins α2 or β1 in SNU449Tp cells, whereas coimmunoprecipitation of TM4SF5 with endogenous integrin α5 was insignificant (S.-A.L. and J.W.L., data not shown). Moreover, the association between TM4SF5 and integrin α2 (not α5) was observed in fibroblasts ectopically expressing TM4SF5 and was abolished by growth factor signaling.25 The cytoplasmic tails of integrin α5 and TM4SF5 are short and thus recruit other signaling molecules to mediate cooperation between both receptors. Although it is unclear whether the association between TM4SF5 and integrin α5 tail is direct or indirect, it will be interesting to identify cytoplasmic mediator(s) for the cooperation that bind either receptor.
Integrin α5β1 engagement with fibronectin causes recruitment of FAK to focal adhesions through interaction between the FAK C-terminal domain and integrin β1-binding proteins, such as talin and paxillin.32 It was recently reported that integrin α5β1-mediated neuroblastoma migration involves FAK-mediated c-Src activation.33 Evidence for a reciprocal influence between c-Src and FAK has been documented; when cells adhere, FAK is autophosphorylated at Tyr397, and c-Src is recruited to form a FAK/c-Src complex, leading to c-Src activation.8 Activated c-Src bound to pY397FAK can phosphorylate other tyrosine residues, including pY576FAK and Y577FAK for full FAK activation.8 Interestingly, FAK appears to play a redundant role in TM4SF5-mediated VEGF induction. Although integrin α5 was required for TM4SF5-mediated VEGF induction and WT FAK overexpression could synergistically enhance the TM4SF5 effects, inhibition of FAK through expression of FRNK or FAK mutants did not abolish the TM4SF5-mediated transcriptional activation of VEGF promoter and induction of VEGF. Therefore, it appears that TM4SF5-mediated VEGF induction may not depend completely on FAK activity, although FAK can increase the TM4SF5 effects. It may be probable that the role of integrin α5 in c-Src activation is required and that FAK activation is redundant, for TM4SF5-mediated VEGF expression. These observations indicate that cooperative complexes formed between c-Src and FAK may be different depending on the signaling contexts including TM4SF5. Moreover, c-Src was required for the TM4SF5-mediated effects, which were abolished by introduction of siRNA against c-Src or of an inactive Y416F c-Src mutant. Therefore, it cannot be ruled out that c-Src activity emanating directly from TM4SF5 is sufficient for VEGF induction to a certain degree when endogenous integrin α5 expression on the cell surface may be insignificant. To our knowledge, ligands for most tetraspan(in)s are unknown, and their regulatory roles in cellular signal transduction and function may involve cross-talk with other receptors. It will be interesting to clarify whether TM4SF5-mediated c-Src activation occurs through the TM4SF5 signaling capacity or through cross-talk with other receptors.
TM4SF5 mRNA is known to be highly expressed in diverse tumor types, including pancreatic,23 nonendocrine lung, and corticotropin-negative bronchial carcinoid tumors.34 We recently observed that TM4SF5 protein is highly overexpressed in human hepatocarcinoma,24 and colon carcinoma tissues (S.-A.L. and J.W.L., unpublished data, July 2008). TM4SF5 belongs to a 4-transmembrane L6 superfamily consisting of L6 (or L6-Ag), IL-TMP, and L6D.22 Tetraspanins or TM4SFs have 4 transmembrane domains, 2 extracellular loops, and 2 short cytoplasmic tails.19 The extracellular loop 2 of genuine tetraspanins has a variable region that mediates specific protein-protein interactions and a conserved region that includes 4 conserved cysteines for disulfide bonding, correct folding, and homodimerization.19 However, the extracellular loop 2 loop of TM4SF5 is relatively divergent, containing only 2 cysteine residues.22 The 4 transmembrane domains are suggested to be responsible for intramolecular and intermolecular interactions during organizing the tetraspanin-web or TERM on the cell surface19 and for forming complexes with integrins to collaboratively regulate cell adhesion, proliferation, and motility.18,20 We recently reported that TM4SF5 causes morphologic changes through RhoA inactivation, epithelial-mesenchymal transition, abnormal cell-cycle progression, and loss of contact inhibition in vitro and in vivo in tumor formation in nude mice.24 Our previous study implicated the existence of cross-talk between TM4SF5 and integrin signaling pathways. In addition, ectopic expression of TM4SF5 in fibroblasts regulated actin reorganization and focal adhesion turnover via association with integrin α2, which appears to be competitively regulated by growth factor receptor signaling.25 In this study, TM4SF5 enhanced surface retention of integrin α5 without changing total protein levels. Function-blocking of surface integrin α5 using its antibody abolished TM4SF5-mediated VEGF induction and secretion. Furthermore, anti–integrin α5 antibody administrated into mice might bind integrin α5 on cell surface, so that its resultant conformation change might disrupt the intracellular association between TM4SF5 and integrin α5, leading to inhibition of c-Src/STAT3 signaling, vessel formation, and tumor growth in nude mice. Similarly, tumor-associated antigen L6 (L6-Ag), which has a sequence identity of approximately 50% with TM4SF5,22 was recruited to the TERM, and the recruitment was correlated with the promigratory activity of L6-Ag through regulation of surface retention and endocytosis of CD63 and CD82 but not of integrin β1.31 Therefore, such as genuine tetraspanins, TM4SF5, and L6-Ag, also appear to play roles in TERM by regulating networks between membrane receptors, including tetraspanins and integrins. TM4SF5 may facilitate efficient communication between cancer cells and extracellular environments (including nearby endothelial cells) through regulation of architectural integrity with diverse membrane receptors. Therefore, developing therapeutics to target TM4SF5 may be a strategy to inhibit tumorigenesis and angiogenesis.
The online version of this article contains a data supplement.
Presented in abstract form at the Keystone Symposia for Cell Migration in Invasion and Inflammation (B7), Taos, NM, February 12, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Research Program for New Drug Target Discovery (2007-03536) and a Korea Science and Engineering Foundation grant (Cell Dynamics Research Center R11-2007-007-01 004-0) to J.W.L.
Authorship
Contribution: S.C. performed experiments and wrote the manuscript; S.-A.L., T.K.K., H.J.K., and M.J.L. helped perform animal experiments; S.-K.Y., S.-H.K., and S.K. supplied materials and discussed data; J.W.L. designed the research and edited the paper; and all authors interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jung Weon Lee, Cancer Research Institute, College of Medicine, Seoul National University, Seoul 110-799, Korea; e-mail: jwl@snu.ac.kr.

![Figure 2. Ectopic expression of TM4SF5 in SNU449 cells enhanced VEGF expression and secretion. (A) Parental SNU449 cells were transfected with pGL3-human VEGF promoter and pBabe–β-galactosidase together with a mock construct, pcDNA3-TM4SF5, or Myc-(His)6-TM4SF5. After 2 days, lysates were prepared and luminescence was measured. Transfection efficiency was normalized by β-galactosidase activity. Data are mean plus or minus SD of 3 independent experiments. RLU indicates relative luciferase activity units. (B,F) TM4SF5-null SNU449 cells were transiently transfected with either the mock construct or pcDNA3-TM4SF5. One day later, whole-cell lysates were prepared, normalized, and analyzed by standard Western blots for the indicated proteins. (C) Whole-cell extracts prepared from parental SNU449 (P), control SNU449Cp (Cp), or stably TM4SF5-expressing SNU449 cell lines (Tp or T16) were analyzed for VEGF expression using 2 different anti-VEGF antibodies (rabbit anti-VEGF polyclonal and mouse anti–VEGF-A [c-1] monoclonal antibody from Santa Cruz Biotechnology, B) or the indicated molecules (F). (D) Subconfluent SNU449Cp and SNU449Tp cells were rinsed and incubated with serum-free media for 24 hours before collection of conditioned media. Using conditioned media from each cell line, angiogenic factors were analyzed using RayBiotech human angiogenesis antibody array. Notably, only VEGF was elevated in conditioned media from TM4SF5-expressing SNU449Tp cells. (E) One day after SNU449Cp or SNU449Tp cells were seeded, subconfluent cells were treated with dimethyl sulfoxide or various pharmacologic inhibitors (U0126, 20 μM; PD98056, 40 μM; PP2, 40 μM; PP3, 40 μM; AG490, 30 μM; Y27632, 15 μM) for 24 hours. Whole-cell extracts were normalized and used in standard Western blots for the indicated molecules. Notably, c-Src family kinase inhibitor PP2 mostly reduced VEGF expression in SNU449Tp cells. (G-J) TM4SF5-positive SNU449Tp (G-I) or Huh7 (J) cells were transfected with shRNA against GFP (shGFP) or TM4SF5 (shTM4SF5) (G,J), or siRNA against GFP, c-Src (H), or STAT3 (I). After 48 hours, whole-cell lysates were prepared, normalized, and used in standard Western blots for the indicated molecules. Immunoblotting with anti-VEGF antibody (pAb) resulted in multiple bands, presumably isotypes. Data shown were representative from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/8/10.1182_blood-2008-05-160671/5/m_zh80070930960002.jpeg?Expires=1769949229&Signature=WFj7cokaIFQgjrvqEq-oApoFoIFhD8I94UaD23wNsds1F0jrY2tIkhAbe7fGpjzOPh3hTlnxikQ0lON~0K6h36HyFob5~wFgyW1iBJV5n382uPF~HDsz8lXDiipOslcx-YUR~RdL77N1uJm17UJKQ3P6zH~taxqStfT2OH2pKBjUOuKYu1uEOTmDH-H~rQ-m~64mTmLiQaOAmvxPJXZoPuBOpjnG5hEXppI8Bo97MHB9Vj3K1VITQckoeuaZduFmivaoQMdEShHSjCJD8WbEze4o0doo-eJCGFwroOr1anlxckwebd9Xbz1vCqRh-8nj5Pb1A4IEldvZfb0Zq7Sldg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal