Abstract
In mice, interleukin-18 (IL-18) regulates Th1- or Th2-type immune responses depending on the cytokine environment and effector cells involved, and the ST2-ligand, IL-33, primarily promotes an allergic phenotype. Human basophils, major players in allergic inflammation, constitutively express IL-18 receptors, while ST2 surface expression is inducible by IL-3. Unexpectedly, freshly isolated basophils are strongly activated by IL-33, but, in contrast to mouse basophils, do not respond to IL-18. IL-33 promotes IL-4, IL-13 and IL-8 secretion in synergy with IL-3 and/or FcϵRI-activation, and enhances FcϵRI-induced mediator release. These effects are similar to that of IL-3, but the signaling pathways engaged are distinct because IL-33 strongly activates NF-κB and shows a preference for p38 MAP-kinase, while IL-3 acts through Jak/Stat and preferentially activates ERK. Eosinophils are the only other leukocyte-type directly activated by IL-33, as evidenced by screening of p38-activation in peripheral blood cells. Only upon CD3/CD28-ligation, IL-33 weakly enhances Th2 cytokine expression by in vivo polarized Th2 cells. This study on primary human cells demonstrates that basophils and eosinophils are the only direct target leukocytes for IL-33, suggesting that IL-33 promotes allergic inflammation and Th2 polarization mainly by the selective activation of these specialized cells of the innate immune system.
Introduction
Cytokines of the interleukin (IL)–1 family are major pro-inflammatory and immunoregulatory mediators that act through receptors of the Toll-like/IL-1-receptor (TLR/IL-1R) superfamily. Due to homologous intracellular Toll/interleukin-1 receptor (TIR) domains, IL-1 family members and TLR-ligands activate very similar signaling pathways leading to NF-κB- and MAPK-activation.1 IL-1α and IL-1β, the prototypic family members, are generated in response to exogenous and endogenous danger signals and act as chief inflammatory mediators in many inflammatory conditions.2 Recent studies indicate that IL-1 may also participate in inflammatory pathologies and auto-immune diseases involving Th17-type T-helper cells.3-5 By contrast, IL-18 is best known for its role in Th1-type immune responses because it strongly amplifies IFN-γ production in natural killer (NK) cells and Th1 cells in synergy with IL-12.2 However, several lines of evidence mainly derived from mouse models indicate that IL-18 can also play a role in allergic diseases and defense against helminths.6,7 For example, in the absence of IFN-γ signaling, IL-18 increases immunoglobulin E (IgE) levels and promotes a Th2-type pathology.8 It has been suggested that this is due to the antigen-independent action of IL-18 on cells of the “innate allergic response,” basophils and mast cells.7 Bone marrow-derived c-Kit−/FcϵRI+ mouse “basophil-like” cells express IL-18 receptors (IL-18R), and IL-18 induces IL-4 and IL-13 expression in an antigen-independent manner as efficiently as IL-3.8 In the human system, IL-18 primes basophilic KU812 cells for enhanced leukotriene C4 (LTC4) production.9 Furthermore, a recent screen of novel CD antigens revealed that blood basophils express IL-18R and IL-18R-accessory protein (IL-18Rap),10 indicating that IL-18 may also act on human basophils.
A soluble form of an IL-1R family member ST2 (sST2; also called T1) has been cloned many years ago. sST2 is formed by many cells and increased sST2 levels are found in inflammatory conditions, including allergic asthma.11-13 The expression of transmembrane ST2-receptor (ST2L), however, is largely restricted to cells of hematopoietic origin. ST2L is expressed by mouse bone marrow-derived mast cells (BMMCs) and is a stable marker of mouse, but not human, Th2-lymphocytes.14-17 In the absence of a known ligand, the biologic roles of ST2 have been extensively studied in several mouse models using ST2-antibodies, sST2-constructs, and ST2-KO-mice.14,18 Although the interpretations were sometimes conflicting, most studies indicate an important contribution of ST2 in Th2-type immune responses and allergic inflammation. The biology of ST2 is further complicated by a possible direct antiinflammatory action of sST2, and by the fact that the TIR domain of ST2L appears to be a negative regulator of IL-1 and TLR signaling.19,20 Research on ST2 strongly gained momentum by the recent discovery of its ligand, the novel IL-1 family member IL-33.21 Like IL-1α, the human IL-33 precursor (proIL-33) is a nuclear protein and was first identified as a gene (termed NF-HEV) differentially expressed by high endothelial venules of lymphatic tissues.22,23 Like IL-1β and IL-18, human and mouse proIL-33 can be cleaved by caspase-1, at least in vitro. Infusion of IL-33 in mice leads to increased IL-4, IL-5 and IL-13 expression, high IgE levels, eosinophilia, and mucosal pathologies in vivo,21,24 even in the absence of an adaptive immune system. After binding to ST2 and recruitment of the IL-1Rap as accessory receptors, IL-33 activates the classical TIR/IRAK signaling pathway.21,24,25 It induces the production of proinflammatory cytokines and IL-13 in mouse BMMCs, and enhances the release of anti-CD3/CD28-induced IL-5 and IL-13 (but not IL-4) by in vitro polarized Th2 cell lines.21,24-27
Only little is known about the biology of IL-33 and ST2 in the human system. A role of IL-33 in human allergic diseases is suggested because of the association of functional SNPs in the promotor of the ST2 gene with atopic dermatitis.13 Recent studies in immature cord blood–derived mast cells (MCs) indicate that IL-33 may affect human tissue MCs. Similar to IL-1, IL-33 promotes the secretion of IL-5, IL-13, and certain chemokines in synergy with thymic stromal lymphopoietin (TSLP), and enhances the stem cell factor (SCF)–induced maturation of MC-progenitors.28-30 Furthermore, IL-33 appears to increase the frequency of cells with a polarized shape in freshly reactivated Th2 cell lines, suggesting that IL-33 may be involved in the trafficking of T cells to an inflammatory site.31 However, it is unknown whether IL-33 can activate certain primary human cell types isolated from human blood or tissues, and the direct target cells for IL-33 in the human system remain to be identified.
There is increasing evidence for a central role of mouse c-Kit−/FcϵRI+ “basophil-like” cells and human basophil granulocytes in the initiation and amplification of Th2-type immune responses.32-35 Despite important differences between mouse and human basophils, both are able to rapidly produce the key Th2 cytokines IL-4 and IL-13 in response to antigen-dependent and -independent activation. Human basophils also release other important mediators of allergic inflammation, such as histamine and LTC4. Although several cytokines, such as nerve growth-factor (NGF), IL-5, and granulocyte macrophage colony-stimulating factor (GM-CSF), can transiently prime basophils for enhanced mediator release,36-38 several studies from our and other groups showed that IL-3 is a unique and potent regulator of the phenotype and function of mature basophils.39-48 Among a large number of ligands studied, including the priming cytokines and IL-1, IL-3 is the only known cytokine that directly induces secretion of low levels of IL-4 and IL-13, and most efficiently promotes cytokine expression in synergy with C5a or FcϵRI-activation.39,43,44
Here, we investigated receptor expression, the signaling pathways, and biologic effects of IL-33 and IL-18 in blood basophils and also examined which cell types in peripheral blood are the direct targets of IL-33.
Methods
Cell isolation and culture of primary human cells
Approval was obtained from the local ethical committee for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki. Basophils, eosinophils, and neutrophils were isolated as described with a purity ranging from 95% to 99%.39 Memory T-helper subpopulations were purified from peripheral blood mononuclear cells (PBMCs) using negative selection with the Memory CD4+ T-Cell Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), staining with anti–CD4-APC, anti–CCR5-FITC (BD Biosciences, San Jose, CA) and anti–CRTH2-PE (Miltenyi Biotec), followed by sorting of CD4+/CRTH2+/CCR5− and CD4+/CRTH2−/CCR5+ subpopulations with a FacsVantage cell sorter (BD Biosciences).
All primary leukocyte types were cultured at a cell density of 1 to 2 × 106/mL in RPMI-1640 medium containing 2 mM l-glutamine, 25 mM HEPES (Gibco-Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal calf serum (FCS) and antibiotics (100 IU/mL penicillin, 100 μg/mL streptomycin). Isolated T cells subpopulations were activated by plate-bound anti-CD3 (OKT3; Otho Biotech, Bridgewater, NJ) and anti-CD28 (BD Biosciences).
Cell stimuli and measurements of mediators and cytokines
Ligands were obtained as follows: rhIL-3, rhGM-CSF (Novartis, Basel, Switzerland); rhIL-5, anti-FcϵRIα mAb 29C6 (Roche, Basel, Switzerland); rhIL-1β, (PeproTech, London, United Kingdom); rhTNF-α (Bender MedSystems, Vienna, Austria); rhIL-33 (Alexis, Lausen, Switzerland). IL-18 was from 2 commercial sources (PeproTech and MBL, Woburn, MA), and both were active on human T cells. Human C5a was purified from complement activated plasma.36 Cytokines in cell-free supernatants were quantified as follows: IL-4, IL-13, IL-5, and IFN-γ using Eli-Pair kits (Diaclone, Stamford, CT), IL-8 using the MiniKit from Endogen (Rockford, IL) and sST2 using the Kit purchased from MBL. In some experiments, cytokines were also measured with a multiplex assay (Bioplex; Bio-Rad, Hercules, CA). LTC4 was measured using the CAST-ELISA (Buehlmann Laboratories, Allschwil, Switzerland). Histamine was measured by fluorometry as described.36 Anti–human-ST2 mAbs 2A5, FB9, HB12 were from MBL and affinity-purified polyclonal antibodies (AF523) from R&D Systems (Minneapolis, MN).
Protein sample preparation and Western blot analysis
Sample preparation and Western blotting was performed as described39,47 using primary antibodies at a 1 to 1000 dilution. More details and the list of antibodies used are provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Blots were developed using peroxidase-labeled secondary antibodies and enhanced cheminluminescence detection system (ECL-Kit; Pierce Chemical, Rockford, IL). Immunoreactive bands were analyzed using the Image Analyzer LAS-3000 and AIDA software (Fujifilm, Tokyo, Japan). To allow an accurate comparison of the signals under the different experimental conditions, the blots of each figure were obtained from the same representative experiment. All experiments were repeated at least 3 times with an identical pattern of signals.
RT-PCR
RNA isolation, reverse transcription, and quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) were performed as described.39 Validated TaqMan primers and probes for ST2L (long, IL1RL1 isoform1; Hs00249389_m1), sST2 (soluble, IL1RL1 isoform 2/3; Hs01073297_m1), ST2 all (all isoforms, IL1RL1 isoform 1/2/3; Hs00545033_m1) and β2-microglobulin (B2M; Hs00187842_m1) were from Applied Biosystems. The relative expression of ST2 in comparison to the reference of the house-keeping gene B2M was calculated by the formula POWER (2,-dCT) and given as RQ (relative quantity).
Flow cytometry
Cell surface staining was performed in PBS (phosphate-buffered saline)/2% FCS. Receptor expression was analyzed using anti–ST2-FITC (MBL), anti–IL-18R-PE (R&D Systems) and isotype matched control Abs (BD Bioscience). For phosphoprotein analysis, freshly isolated PBMCs were stained before activation with anti–CRTH2-Alexa Fluor 647 (BD-Bioscience), resuspended in prewarmed RPMI-1640 containing 1 mg/mL BSA (Calbiochem, San Diego, CA) and stimulated for 10 minutes. After fixation with formaldehyde (CytofixTM; BD-Bioscience), cells were permeabilized with Perm Buffer III (BD Bioscience) and stained with anti–CD3-PE and anti–P-p38-Alexa Fluor 488 (BDBioscience). Flow cytometry was performed with a FACSCalibur (BD Bioscience). Representative flow cytometry experiments are shown in the figures. All experiments were repeated 5 to 10 times with identical results.
Immunofluorescence microscopy
Basophils plated on poly-L-lysine–coated glass slides were fixed and stained as described previously39 using the following primary antibodies: anti–granzyme B mAb (2C5) sc-8022 (Santa Cruz Biotechnology, Santa Cruz, CA), final concentration of 2 μg/mL; anti-ST2, a mixture of 3 mAb (2A5, FB6, H121; MBL), final concentration of 1.5 μg/mL each.
Results
IL-3 induces expression of the soluble and transmembrane form of ST2
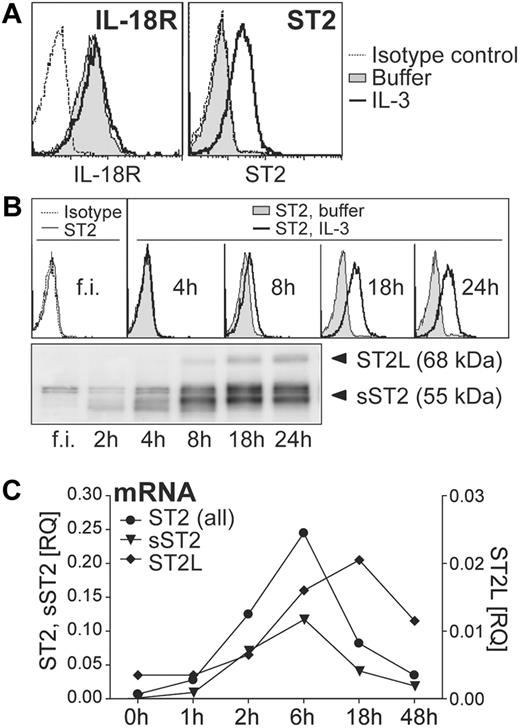
To investigate whether IL-33 and IL-18 may regulate functional responses of human blood basophils, we first analyzed surface expression of ST2 and IL-18–receptors on basophils cultured with or without IL-3. Consistent with published data,10 basophils constitutively express high levels of IL-18 receptors (IL18R1 and IL18Rap; Figure 1A, and data not shown). By contrast, ST2 was detectable only on the surface of IL-3–stimulated cells. Time course analysis of total and surface ST2 expression induced by IL-3 revealed that freshly isolated cells weakly express sST2 but no detectable membrane ST2L (Figure 1B). IL-3 promoted a rapid induction of sST2 already after 2 hours of stimulation, whereas ST2L became detectable after approximately 8 hours (Figure 1B). Measurement of mRNA levels of ST2 isoforms using real-time RT-PCR indicated that sST2 and ST2L are transcriptionally regulated by IL-3 (Figure 1C).
IL-3 up-regulates ST2 in basophils, while IL-18 receptors are constitutively expressed. (A) Constitutive and inducible expression of IL-18R and ST2. Basophils were cultured overnight in the absence (nil) or presence of IL-3 (10 ng/mL) and analyzed by flow cytometry for cell surface IL-18R and ST2 expression. The histograms show an overlay of unstimulated (nil) and IL-3–stimulated basophils including isotype control. (B) Time course of IL-3–induced ST2 expression. Freshly isolated (f.i.) basophils were analyzed immediately or cultured for the time indicated without (nil) or with IL-3. Cell surface ST2 expression was analyzed by flow cytometry (top panel) and total protein expression of IL-3–treated cells by Western blotting (bottom panel). (C) Kinetics of induction of mRNA of ST2-isoforms. Basophils were cultured with IL-3 for the time indicated and mRNA was quantified by real-time RT-PCR using specific primers for ST2-isoforms: transmembrane/long, ST2L; soluble, sST2; all isoforms ST2, ST2 (all). The expression of ST2 in comparison to the reference gene β2M is given as RQ (relative quantity). Mean values of duplicates are shown. Representative experiments are shown in all panels.
IL-3 up-regulates ST2 in basophils, while IL-18 receptors are constitutively expressed. (A) Constitutive and inducible expression of IL-18R and ST2. Basophils were cultured overnight in the absence (nil) or presence of IL-3 (10 ng/mL) and analyzed by flow cytometry for cell surface IL-18R and ST2 expression. The histograms show an overlay of unstimulated (nil) and IL-3–stimulated basophils including isotype control. (B) Time course of IL-3–induced ST2 expression. Freshly isolated (f.i.) basophils were analyzed immediately or cultured for the time indicated without (nil) or with IL-3. Cell surface ST2 expression was analyzed by flow cytometry (top panel) and total protein expression of IL-3–treated cells by Western blotting (bottom panel). (C) Kinetics of induction of mRNA of ST2-isoforms. Basophils were cultured with IL-3 for the time indicated and mRNA was quantified by real-time RT-PCR using specific primers for ST2-isoforms: transmembrane/long, ST2L; soluble, sST2; all isoforms ST2, ST2 (all). The expression of ST2 in comparison to the reference gene β2M is given as RQ (relative quantity). Mean values of duplicates are shown. Representative experiments are shown in all panels.
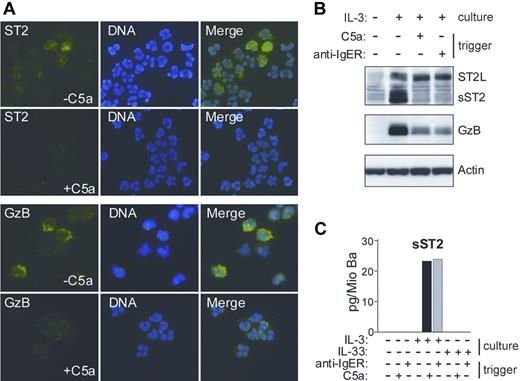
IL-3 has been shown to induce a prominent change in the content of basophil granules.39 The observation that a secreted form of ST2 protein remains cell-associated in IL-3–stimulated basophils prompted us to examine whether it localizes to the granule compartment. Figure 2 demonstrates that de novo formed sST2 is sorted to basophil granules and can be mobilized by degranulation in response to IgE-dependent or - independent stimuli. This granular localization of sST2 in basophils was an unexpected finding, because, in contrast to Granzyme B (GzB), which is a known granule protein, sST2 (like ST2L) contains a signal sequence for transport through the cellular membrane.
IL-3–induced sST2 is sorted to basophils granules and released by triggers of exocytosis. (A) Immunofluorescence analysis of ST2 expression and release in human basophils. Cytospin preparations of basophils cultured for 20 hours in the presence of IL-3, with or without triggering degranulation by 10 nM C5a for 30 minutes, were stained with anti-ST2 mAb (green, top left panel) and anti-GzB mAb (green, bottom left panel). DNA was stained with Hoechst (blue, middle panels). Images were acquired using constant settings on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 60×/1.4 NA oil-immersion objective lens using FITC and DAPI filter sets. A Nikon DXM 1200 digital camera was used to capture images. Merged images (right panels) were created in Adobe Photoshop 8.0.1 (Adobe Systems, San Jose, CA) without any alteration of the original digital images. (B,C) Degranulation of basophils triggers the release of sST2. (B) Protein extracts derived from basophils cultured overnight with or without IL-3 followed by stimulation with C5a or anti-FcϵRIα mAb (100 ng/mL; anti-IgER) for 30 minutes as indicated were analyzed for the presence of ST2 and Granzyme B (GzB) by Western blotting. (C) Purified basophils were cultured in medium, IL-3 or IL-33 (50 ng/mL) for 20 hours and subsequently stimulated with C5a or anti-FcϵRIα mAb as indicated. sST2 was measured in cell supernatants by specific ELISA. Mean values of triplicates are shown. The representative data shown in panels A, B, and C were from separate experiments with cells from different donors.
IL-3–induced sST2 is sorted to basophils granules and released by triggers of exocytosis. (A) Immunofluorescence analysis of ST2 expression and release in human basophils. Cytospin preparations of basophils cultured for 20 hours in the presence of IL-3, with or without triggering degranulation by 10 nM C5a for 30 minutes, were stained with anti-ST2 mAb (green, top left panel) and anti-GzB mAb (green, bottom left panel). DNA was stained with Hoechst (blue, middle panels). Images were acquired using constant settings on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 60×/1.4 NA oil-immersion objective lens using FITC and DAPI filter sets. A Nikon DXM 1200 digital camera was used to capture images. Merged images (right panels) were created in Adobe Photoshop 8.0.1 (Adobe Systems, San Jose, CA) without any alteration of the original digital images. (B,C) Degranulation of basophils triggers the release of sST2. (B) Protein extracts derived from basophils cultured overnight with or without IL-3 followed by stimulation with C5a or anti-FcϵRIα mAb (100 ng/mL; anti-IgER) for 30 minutes as indicated were analyzed for the presence of ST2 and Granzyme B (GzB) by Western blotting. (C) Purified basophils were cultured in medium, IL-3 or IL-33 (50 ng/mL) for 20 hours and subsequently stimulated with C5a or anti-FcϵRIα mAb as indicated. sST2 was measured in cell supernatants by specific ELISA. Mean values of triplicates are shown. The representative data shown in panels A, B, and C were from separate experiments with cells from different donors.
IL-33, but not IL-18, is an efficient and direct activator of blood basophils
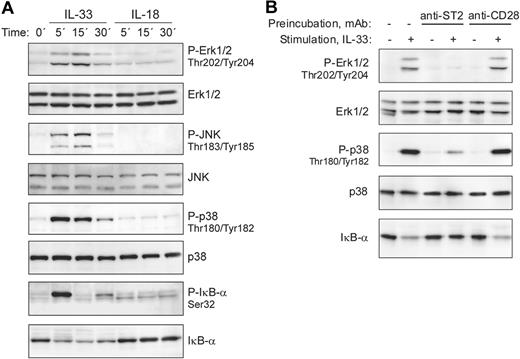
Ligand binding to receptors of the IL-1/TLR family, including IL-33 stimulation of mouse ST2-expressing cells, leads to the recruitment of MyD88, IRAK1, IRAK4, and TRAF6 with formation of a signaling complex that results in downstream activation of NF-κB and MAP-kinases.1,21 We therefore analyzed whether IL-33 is able to stimulate these signaling pathways in freshly isolated human basophils. Figure 3A demonstrates that IL-33 rapidly activated ERK1/2, JNK, p38, and NF-κB signaling cascades, as evidenced by phosphorylation of MAP kinases and phosphorylation and degradation of the NF-κB inhibitor IκB-α. In contrast to mouse cells, in which IL-33 has been reported to induce prominent ERK activation,21 it stongly activates p38 MAPK in human basophils. The ability of IL-33 to stimulate freshly isolated basophils expressing no detectable ST2L suggested a ST2-independent mechanism of activation. To investigate the involvement of ST2L in the bioactivity of IL-33, we first tested in pilot experiments the capacity of commercially available anti–human-ST2 monoclonal and polyclonal antibodies to block p38 activation in the human mast cell-line HMC-1 that we found to constitutively express ST2L. The most efficient, albeit incomplete, inhibition of IL-33–induced p38 activation in HMC-1 cells was obtained by combining 3 mAbs (data not shown). Figure 3B shows that pre-incubation of basophils with this mixture of anti-ST2 mAbs specifically blocked IL-33 signaling, demonstrating that IL-33 activates basophils through ST2L, even though the expression levels are below the detection limit.
IL-33, but not IL-18, activates MAP-kinase and NF-kB signaling pathways in blood basophils. (A) IL-33 rapidly activates signaling pathways of the TLR/IL-1R family in freshly isolated blood basophils. Cells were stimulated with IL-33 or IL-18 (50 ng/mL each) for the indicated time periods. Western blot analysis of cell extracts shows the rapid activation of the MAP-kinases, Erk, JNK, and p38, and the phosphorylation and degradation of IκB-α in response to IL-33, but not to IL-18. The lack of responsiveness of human basophils to IL-18 was confirmed with different preparations of IL-18 from 2 different suppliers. (B) Anti-ST2 antibodies block IL-33–induced basophil activation. Basophils were left untreated, or pre-incubated with anti-ST2 antibodies (a mixture of 3 mAbs, clones 2A5, FB9, and HB12; 3 μg/mL each) or anti-CD28 (control mAb; at 10 μg/mL) for 15 minutes at 37°C. Cells were stimulated with 2 ng/mL IL-33 for 15 minutes as indicated. Erk and p38 activation and the degradation of IκB-α were assessed by Western blotting.
IL-33, but not IL-18, activates MAP-kinase and NF-kB signaling pathways in blood basophils. (A) IL-33 rapidly activates signaling pathways of the TLR/IL-1R family in freshly isolated blood basophils. Cells were stimulated with IL-33 or IL-18 (50 ng/mL each) for the indicated time periods. Western blot analysis of cell extracts shows the rapid activation of the MAP-kinases, Erk, JNK, and p38, and the phosphorylation and degradation of IκB-α in response to IL-33, but not to IL-18. The lack of responsiveness of human basophils to IL-18 was confirmed with different preparations of IL-18 from 2 different suppliers. (B) Anti-ST2 antibodies block IL-33–induced basophil activation. Basophils were left untreated, or pre-incubated with anti-ST2 antibodies (a mixture of 3 mAbs, clones 2A5, FB9, and HB12; 3 μg/mL each) or anti-CD28 (control mAb; at 10 μg/mL) for 15 minutes at 37°C. Cells were stimulated with 2 ng/mL IL-33 for 15 minutes as indicated. Erk and p38 activation and the degradation of IκB-α were assessed by Western blotting.
Despite abundant IL-18 receptors, and in marked contrast to mouse basophils,8 IL-18 did not activate in human basophils signaling pathways known to be used by the IL-18R (Figure 3A). The lack of bioactivity of IL-18 on basophils was confirmed in a large set of experiments using IL-18 alone or in combination with IL-3 and/or IgE receptor (IgER) crosslinking and/or C5a using several functional readouts (exocytosis, LTC4 formation, IL-4, IL-13 and IL-8 secretion; Figure 4; and data not shown). Although one cannot completely exclude the possibility that IL-18 regulates other responses through an unconventional signaling pathway, our data indicate that IL-18 neither activates nor modulates the function of basophils, revealing an important difference between the mouse and human system.
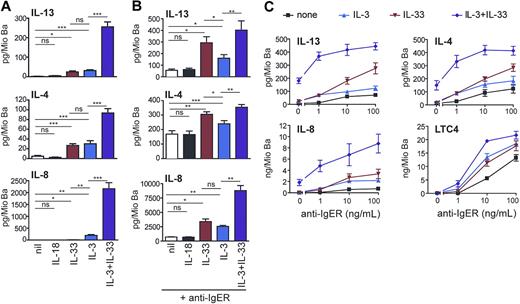
IL-33 promotes cytokine production and enhances IgE receptor–induced mediator release as efficiently as IL-3. (A) Direct and synergistic induction of secretion of Th2 cytokines and IL-8 by IL-3 and IL-33. Freshly isolated basophils were cultured in medium alone or with IL-18 (50 ng/mL), IL-33 (50 ng/mL), IL-3 (10 ng/mL) or in combination of IL-3 + IL-33 for 8 hours. Cytokine secretion was measured in the cell supernatants. Means plus SEM of 8 independent experiments are shown. (B,C) IL-33 augments IL-4, IL-13, IL-8, and LTC4 production in basophils activated by IgE receptor crosslinking. (B) Cells were activated by a maximally effective concentration of anti-FcϵRIα mAb (anti-IgER; 100 ng/mL) alone or with IL-18 (50 ng/mL), IL-33 (50 ng/mL), IL-3 (10 ng/mL), or IL-33 + IL-3, and cytokines were measured in cell supernatants after culture for 8 hours. Mean plus SEM of 5 experiments with cells from different donors are shown. (C) Basophils were stimulated with increasing concentration of anti-IgER in the absence or presence of IL-3, IL-33, or IL-3 + IL-33 for 8 hours. Mean values plus or minus SEM of 2 independent experiments performed in duplicates are shown. Statistical analysis: The statistical significance of differences between the different experimental conditions (marked by lines for each pair of conditions) was analyzed using the Student t test (ns, not significant, *P < .05, **P < .01, ***P < .001).
IL-33 promotes cytokine production and enhances IgE receptor–induced mediator release as efficiently as IL-3. (A) Direct and synergistic induction of secretion of Th2 cytokines and IL-8 by IL-3 and IL-33. Freshly isolated basophils were cultured in medium alone or with IL-18 (50 ng/mL), IL-33 (50 ng/mL), IL-3 (10 ng/mL) or in combination of IL-3 + IL-33 for 8 hours. Cytokine secretion was measured in the cell supernatants. Means plus SEM of 8 independent experiments are shown. (B,C) IL-33 augments IL-4, IL-13, IL-8, and LTC4 production in basophils activated by IgE receptor crosslinking. (B) Cells were activated by a maximally effective concentration of anti-FcϵRIα mAb (anti-IgER; 100 ng/mL) alone or with IL-18 (50 ng/mL), IL-33 (50 ng/mL), IL-3 (10 ng/mL), or IL-33 + IL-3, and cytokines were measured in cell supernatants after culture for 8 hours. Mean plus SEM of 5 experiments with cells from different donors are shown. (C) Basophils were stimulated with increasing concentration of anti-IgER in the absence or presence of IL-3, IL-33, or IL-3 + IL-33 for 8 hours. Mean values plus or minus SEM of 2 independent experiments performed in duplicates are shown. Statistical analysis: The statistical significance of differences between the different experimental conditions (marked by lines for each pair of conditions) was analyzed using the Student t test (ns, not significant, *P < .05, **P < .01, ***P < .001).
IL-33 promotes Th2 cytokine secretion and enhances IgER-induced mediator release as efficiently as IL-3
It is now well established that IL-3 is a very effective and unique regulator of cytokine expression in human basophils, and no other ligand with a similar efficacy has been identified so far.43-45 Figure 4 demonstrates that IL-33 can promote pro-inflammatory and immunoregulatory basophil functions as effectively as IL-3. Both stimuli induce the release of similar amounts of IL-4 and IL-13 (Figure 4A) although basophils from individual donors can respond to one agonist more strongly than to the other (Figure S1). Figure 4A also shows that IL-3 and IL-33 synergistically induce the secretion of Th2 cytokines and IL-8 at levels reaching (IL-4) or even exceeding (IL-13, IL-8) that observed in cells after optimal IgER activation (Figure 4B). Similar to the suppression of IL-33–induced early signaling events (Figure 3B), anti-ST2 antibodies strongly inhibited the enhancement of cytokine release promoted by IL-33, further demonstrating the requirement of ST2L for the bioactivity of IL-33 in human basophils (Figure S2A).
The IgER-induced secretion of cytokines was also strongly enhanced by IL-33, and in this respect, IL-33 was even more effective than IL-3, particularly at higher concentrations of IgER-crosslinking Abs (Figure 4B,C). The production of cytokines induced by IL-33 alone or in synergy with other agonists was rapid, reaching maximal levels after 8 hours of incubation (not shown), and occurred dose dependently at 1 to 100 ng/mL of IL-33 (Figure S3). Alone or in combination with IL-3, IL-33 did not promote degranulation or lipid mediator formation. However, it enhanced IgER-induced histamine release (not shown) and LTC4 generation (Figure 4C). Taken together, our data indicate that IL-33 is a potent regulator of basophil functions, particularly when acting in concert with other stimuli.
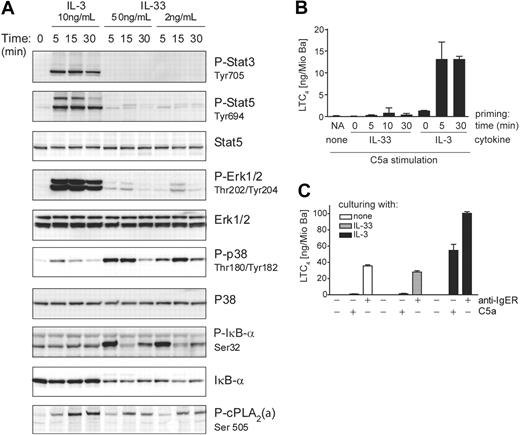
IL-3 and IL-33 activate distinct signaling pathways in blood basophils
Despite the similar bioactivities and efficacies in promoting cytokine secretion and enhancing IgER-induced mediator release, the signaling pathways activated by IL-3 and IL-33 in freshly isolated basophils differ markedly (Figure 5A). While IL-3 (but not IL-33) activates the Jak/Stat-pathway leading to the phosphorylation of Stat3/Stat5, IL-33 (but not IL-3) strongly activates NF-κB as evidenced by the phosphorylation and degradation of IκB-α. Furthermore, IL-3 and IL-33 exhibit a preference in activating the MAP-kinases Erk and p38, respectively (Figures 5A, S4, S5). The marked difference between IL-3 and IL-33 in promoting the activating phosphorylation of ERK and p38, respectively, was not only evident at different time points after activation with maximally effective concentrations of the cytokines (Figure 5A), but was also observed at lower doses of ligands (Figure S4). We also examined whether IL-3–induced up-regulation of ST2 altered the signaling pathways or signaling strength in response to IL-33. However, while culture of basophils with IL-3 diminished Stat3/5 phosphorylation in response to restimulation with IL-3, it hardly influenced IL-33 signaling (Figure S5).
IL-3 and IL-33 activate distinct signal transduction pathways leading to differential regulation of LTC4 synthesis. (A) IL-3 and IL-33 activate distinct signaling pathways in basophils. Cells were stimulated with IL-3 or IL-33 at concentrations and for the time periods indicated. Activation of MAP-kinases, Erk, and p38, and transcriptional regulators, Stat3/Stat5 and IκB-α, and phosphorylation of cPLA2 (P-cPLA2) were analyzed by Western blotting. A representative experiment is shown. Analysis by densitometry of Western blots of 5 independent experiments showed that ERK activation by IL-3 was at least 9 times higher as compared with that induced by IL-33, while p38 activation in response to IL-33 was at least 4 times higher than that promoted by IL-3. The ratio of cPLA2 phosphorylation of cells treated with IL-3 versus cells treated with IL-33 ranged between 1.5 and 2.1. (B) IL-33 does not efficiently prime basophils for C5a-induced LTC4 formation. Cells were primed with 10 ng/mL IL-3 or 50 ng/mL IL-33 for the time periods indicted (priming time) followed by stimulation with 10 nM C5a for 30 minutes. NA / none: nonprimed cells stimulated with C5a alone. (C) Culture with IL-33 does not potentiate IgE-dependent or -independent mediator release. Cells were cultured for 24 hours with IL-3 or IL-33 as indicated, followed by stimulation for 30 minutes with C5a or by IgER-crosslinking using anti-IgER mAb. LTC4 levels (mean values + SEM of 3 experiments) in the cell supernatants are shown in panels B and C.
IL-3 and IL-33 activate distinct signal transduction pathways leading to differential regulation of LTC4 synthesis. (A) IL-3 and IL-33 activate distinct signaling pathways in basophils. Cells were stimulated with IL-3 or IL-33 at concentrations and for the time periods indicated. Activation of MAP-kinases, Erk, and p38, and transcriptional regulators, Stat3/Stat5 and IκB-α, and phosphorylation of cPLA2 (P-cPLA2) were analyzed by Western blotting. A representative experiment is shown. Analysis by densitometry of Western blots of 5 independent experiments showed that ERK activation by IL-3 was at least 9 times higher as compared with that induced by IL-33, while p38 activation in response to IL-33 was at least 4 times higher than that promoted by IL-3. The ratio of cPLA2 phosphorylation of cells treated with IL-3 versus cells treated with IL-33 ranged between 1.5 and 2.1. (B) IL-33 does not efficiently prime basophils for C5a-induced LTC4 formation. Cells were primed with 10 ng/mL IL-3 or 50 ng/mL IL-33 for the time periods indicted (priming time) followed by stimulation with 10 nM C5a for 30 minutes. NA / none: nonprimed cells stimulated with C5a alone. (C) Culture with IL-33 does not potentiate IgE-dependent or -independent mediator release. Cells were cultured for 24 hours with IL-3 or IL-33 as indicated, followed by stimulation for 30 minutes with C5a or by IgER-crosslinking using anti-IgER mAb. LTC4 levels (mean values + SEM of 3 experiments) in the cell supernatants are shown in panels B and C.
IL-33 does not efficiently prime basophils for C5a-induced LTC4 generation
IL-3, IL-5, GM-CSF, and NGF all rapidly prime basophils for C5a-induced LTC4 synthesis.36-38 Figure 5B shows that, compared with IL-3, priming of cells with IL-33 leads to the generation of little LTC4 in response to C5a. This might be due to insufficient activation of cytosolic phospholipase A2 (cPLA2), whose activating phosphorylation is indeed weaker in response to IL-33 as compared with IL-3 (Figure 5A). This difference in cPLA2 phosphorylation is possibly related to the type of MAP-kinases that are preferentially activated by the 2 cytokines.49,50 Consistent with this interpretation, we find a complete block of C5a-induced LTC4 synthesis in IL-3–primed cells by the specific MEK1/2 inhibitor U0126 acting upstream of ERK (data not shown). Particularly large quantities of leukotrienes are formed in response to diverse agonists when basophils are cultured with IL-3 for 18 hours or longer, an effect that is unique for this cytokine, and has been termed late priming.39,42 Figure 5C demonstrates that cells cultured with IL-33 do not generate LTC4 in response to C5a. Furthermore, there was no enhancement of LTC4 formation in response to IgER-crosslinking after prolonged culture with IL-33. The observation that IL-33 is not capable of promoting late priming, therefore, reveals another important difference between the bioactivity of IL-3 and IL-33. Finally, neither short nor prolonged preincubation with IL-33 enhanced C5a-induced histamine release, in contrast to the known priming effects of IL-3 (Figure S6).
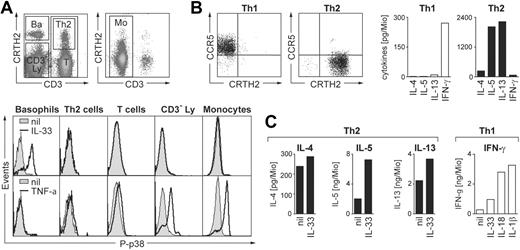
Basophils are the only cell type within mononuclear cells that directly respond to IL-33
Basophil granulocytes are present in the fraction of mononuclear cells and display similar light scatter characteristic as lymphocytes. To search for other direct human leukocyte targets of IL-33, freshly isolated PBMCs were stimulated with IL-33 and analyzed by flow cytometry for p38 phosphorylation. Figure 6A demonstrates that, in contrast to basophils, monocytes or different lymphocyte populations, including CRTH2+ Th2-polarized T-helper cells, do not respond to IL-33 stimulation. By contrast, monocytes and lymphocyte subsets are activated to variable degrees by TNF-α used as a positive control, while basophils do not respond to this pro-inflammatory cytokine.
Biologic activities of IL-33 on leukocyte subpopulations in PBMCs. (A) Basophils are the only direct target cells for IL-33 within the mononuclear leukocyte fraction. Freshly isolated PBMCs were stimulated for 10 minutes with IL-33 (50 ng/mL) or TNF-α (10 ng/mL) and analyzed for p38 phosphorylation by flow cytometry. The top panels show the gating of leukocyte subpopulations in total PBMCs stained with anti-CD3 and anti-CRTH2 Abs in the lymphocyte (left) and monocyte (right) light scatter gates: Ba, CRTH2high/CD3− basophils; Th2, CD3+/CRTH2+ Th2 cells; T, CD3+ total T cells; CD3−Ly, CRTH2−/CD3− cells in the lymphocyte scatter gate mainly composed of B cells and NK cells; Mo, CD3− cells in the monocyte scatter gate. Bottom panels: Histograms show overlays of phospho-p38 (P-p38) staining in the gated subpopulations in unstimulated (nil, shaded area) and stimulated (solid line) cells. Only basophils are activated by IL-33 while all other subpopulations, except basophils, respond to TNF-α stimulation to variable degrees. A representative experiment is shown. Identical results were obtained with cells from 10 different donors. (B,C) IL-33 moderately enhances anti-CD3/CD28–induced cytokine release in primary in vivo polarized Th2 cells. Sorted CD4+/CRTH2−/CCR5+ (Th1) and CD4+/CRTH2+/CCR5− (Th2) memory T cells were stimulated for 24 hours with plate-bound anti-CD3/CD28 mAbs (1 μg/mL each) in the presence of IL-33, IL-1β or IL-18 (all at 50 ng/mL) as indicated, and IL-4, IL-5, IL-13, and IFN-γ was measured in the cell supernatants. Panel B shows an example of CCR5/CRTH2 plots of the sorted Th1 and Th2 (left panels) cells and the cytokine profile in response to CD3/CD28-ligation (right panels; mean of 5 experiments). Panel C shows the effects of IL-33 on anti-CD3/CD28–induced secretion of IL-4, IL-5, and IL-13 by Th2 cells and of IL-33, IL-1β or IL-18 on IFN-γ release by Th1 cells. Shown are the mean of 5 independent experiments with cells isolated from different donors. Data from individual experiments and statistical analysis are shown in Figure S7.
Biologic activities of IL-33 on leukocyte subpopulations in PBMCs. (A) Basophils are the only direct target cells for IL-33 within the mononuclear leukocyte fraction. Freshly isolated PBMCs were stimulated for 10 minutes with IL-33 (50 ng/mL) or TNF-α (10 ng/mL) and analyzed for p38 phosphorylation by flow cytometry. The top panels show the gating of leukocyte subpopulations in total PBMCs stained with anti-CD3 and anti-CRTH2 Abs in the lymphocyte (left) and monocyte (right) light scatter gates: Ba, CRTH2high/CD3− basophils; Th2, CD3+/CRTH2+ Th2 cells; T, CD3+ total T cells; CD3−Ly, CRTH2−/CD3− cells in the lymphocyte scatter gate mainly composed of B cells and NK cells; Mo, CD3− cells in the monocyte scatter gate. Bottom panels: Histograms show overlays of phospho-p38 (P-p38) staining in the gated subpopulations in unstimulated (nil, shaded area) and stimulated (solid line) cells. Only basophils are activated by IL-33 while all other subpopulations, except basophils, respond to TNF-α stimulation to variable degrees. A representative experiment is shown. Identical results were obtained with cells from 10 different donors. (B,C) IL-33 moderately enhances anti-CD3/CD28–induced cytokine release in primary in vivo polarized Th2 cells. Sorted CD4+/CRTH2−/CCR5+ (Th1) and CD4+/CRTH2+/CCR5− (Th2) memory T cells were stimulated for 24 hours with plate-bound anti-CD3/CD28 mAbs (1 μg/mL each) in the presence of IL-33, IL-1β or IL-18 (all at 50 ng/mL) as indicated, and IL-4, IL-5, IL-13, and IFN-γ was measured in the cell supernatants. Panel B shows an example of CCR5/CRTH2 plots of the sorted Th1 and Th2 (left panels) cells and the cytokine profile in response to CD3/CD28-ligation (right panels; mean of 5 experiments). Panel C shows the effects of IL-33 on anti-CD3/CD28–induced secretion of IL-4, IL-5, and IL-13 by Th2 cells and of IL-33, IL-1β or IL-18 on IFN-γ release by Th1 cells. Shown are the mean of 5 independent experiments with cells isolated from different donors. Data from individual experiments and statistical analysis are shown in Figure S7.
IL-33 enhances CD3/CD28-mediated cytokine expression in primary in vivo polarized Th2 cells
Ligation of CD3/CD28 in Th2 (but not Th1) human T-cell lines was reported to up-regulate ST2 mRNA, predominantly the soluble isoform.15 Recent studies showed an enhanced cellular migration of T-cell receptor (TCR)–activated in vitro polarized human Th2 cell lines in response to IL-33, indicating that IL-33 can also regulate functions of human T-helper cells.31 To get a first insight into the biologic effects of IL-33 on human blood T-cell subpopulations, we sorted CRTH2+/CCR5−/CD4+ Th2 and CCR5+/CRTH2−/CD4+ Th1 cells. Figure 6B shows that the cytokine secretion pattern of the purified T-helper cell populations is in line with published data.51,52 Addition of IL-33 to CD3/CD28-activated CRTH2+ Th2 cells enhanced the expression of IL-4, IL-5, and IL-13 (Figure 6C). The effect of IL-33 was statistically significant for all 3 cytokines and most prominent for IL-5 (Figure S7). However, the enhancement of Th2 cytokine expression was rather weak compared with the more than 10-fold increased secretion of IFN-γ caused by IL-1 or IL-18 in CCR5+ Th1 cells. In contrast to the IL-1 family members IL-1 and IL-18, IL-33 did not enhance in a statistically significant manner the secretion of IFN-γ in CCR5+ Th1 cells (Figures 6C, S7). These data demonstrate that the engagement of CD3/CD28 renders primary human Th2 cells responsive to stimulation with IL-33. However, they also suggest that compared with the well established and potent costimulatory effect of IL-1 or IL-18 on Th1 cells, the regulation of Th2 cells may not be the most biologically relevant activity of IL-33 in the human system.
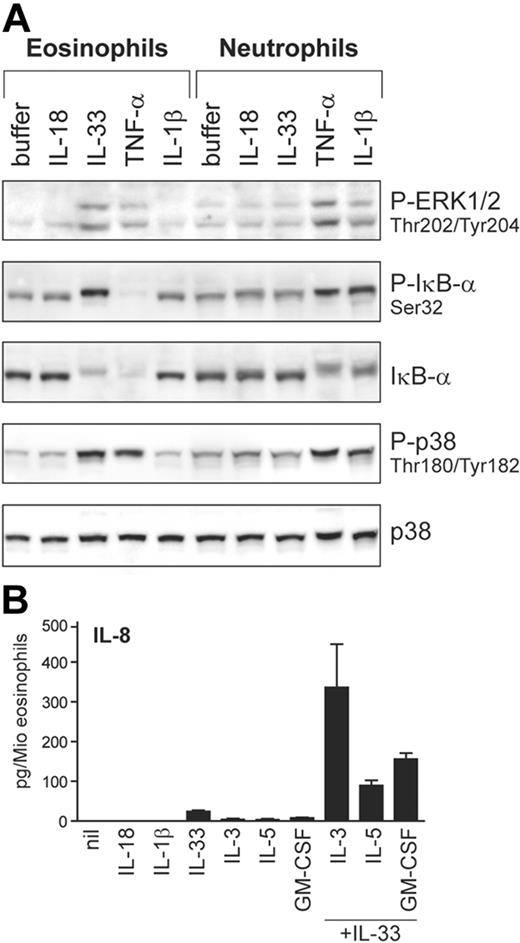
Apart from basophils, IL-33 also activates eosinophils, but not neutrophils
Next, we investigated whether IL-33 may be capable of activating granulocyte types other than basophils. For comparison, we also examined the activation of classical IL-1R–signaling pathways in response to other IL-1 family members and TNF-α (Figure 7A). Although ST2L expression is undetectable in either neutrophils or eosinophils (not shown), IL-33 selectively activates eosinophils while IL-1β acts on neutrophils. Both granulocyte types are activated by TNF-α, in contrast to basophils (see Figure 6A). Thus, these results indicate that the different granulocyte types are regulated by distinct sets of these pro-inflammatory cytokines. Figure 7B shows that stimulation of eosinophils by IL-33 leads to IL-8 production, a response that is strongly enhanced by cytokines of the GM-CSF family, demonstrating a first biologic activity of IL-33 on the function of human eosinophils. The synergistic induction of IL-8 by IL-3 plus IL-33 is strongly and specifically suppressed by anti-ST2 antibodies, demonstrating that ST2 is involved in the IL-33 mediated activation also in eosinophils. Taken together, our data demonstrate that basophils and eosinophils, “innate” cells of allergic Th2-type inflammatory conditions, are the prime, and possibly only, direct target leukocytes of IL-33 in humans.
IL-33 activates blood eosinophils. (A) Differential activation of eosinophils and neutrophils by IL-1 family members. Neutrophil and eosinophil granulocytes isolated from the same donors were stimulated with IL-1β, IL-18, IL-33 (all at 50 ng/mL), or TNF-α (100 ng/mL) for 10 minutes. Activation of ERK and p38 and the phosphorylation and degradation of IκB-α was analyzed by Western blotting. (B) IL-33 promotes IL-8 secretion in human eosinophils. Cells were incubated with the cytokines (all at 50 ng/mL) alone or in combination as indicated. After 24 hours, IL-8 was measured in the cell supernatants. Western blots of a representative experiment (of 4) are shown in A, and the mean plus or minus SEM of 4 independent experiments with cells from different donors is shown in panel B. The direct induction of IL-8 by IL-33 alone (control vs IL-33) as well as the synergistic induction by IL-33 in combination with cytokines of the GM-CSF family (IL-33 alone or 1 of the GM-CSF family members alone vs IL-33 combined with IL-3 or IL-5 or GM-CSF) were all statistically significant (P < .01 or P < .001) as assessed by the Student t test.
IL-33 activates blood eosinophils. (A) Differential activation of eosinophils and neutrophils by IL-1 family members. Neutrophil and eosinophil granulocytes isolated from the same donors were stimulated with IL-1β, IL-18, IL-33 (all at 50 ng/mL), or TNF-α (100 ng/mL) for 10 minutes. Activation of ERK and p38 and the phosphorylation and degradation of IκB-α was analyzed by Western blotting. (B) IL-33 promotes IL-8 secretion in human eosinophils. Cells were incubated with the cytokines (all at 50 ng/mL) alone or in combination as indicated. After 24 hours, IL-8 was measured in the cell supernatants. Western blots of a representative experiment (of 4) are shown in A, and the mean plus or minus SEM of 4 independent experiments with cells from different donors is shown in panel B. The direct induction of IL-8 by IL-33 alone (control vs IL-33) as well as the synergistic induction by IL-33 in combination with cytokines of the GM-CSF family (IL-33 alone or 1 of the GM-CSF family members alone vs IL-33 combined with IL-3 or IL-5 or GM-CSF) were all statistically significant (P < .01 or P < .001) as assessed by the Student t test.
Discussion
Here we show that blood basophils and eosinophils, 2 related granulocyte types involved in allergic inflammatory diseases and in Th2-type immune responses to helminth infections, are the prime direct target leukocytes of the novel IL-1 family member IL-33. The characterization of the bioactivity of IL-33 on human basophils forms the main body of investigations presented here. Our study was initiated by the observation that IL-3, a major regulator of the phenotype and functions of basophils, strongly induces the IL-1R family member ST2. Both soluble as well as transmembrane isoforms of ST2 are induced at the mRNA and protein levels. In basophils, sST2 remains cell-associated, is stored in a granule compartment, and can be released by triggering degranulation. Thus, sST2 represents another example for the alteration in the basophil granule content that is induced by IL-3, as reported previously for GzB.39
Most surprising was the finding that IL-33 potently activates freshly isolated basophils in a ST2-dependent manner, despite undetectable ST2L protein expression levels. A weak expression of transmembrane ST2L mRNA is, however, detectable by RT-PCR in freshly isolated blood basophils. Moreover, activation of basophils with IL-33 is inhibited by anti-ST2 Abs, demonstrating that IL-33 interacts with ST2 receptors. Thus, IL-33 is able to transmit strong activation signals by engaging only very few receptors. In contrast to mouse basophils, which release IL-4 and IL-13 upon stimulation with IL-18,6-8 human basophils do not respond to IL-18 even though they constitutively express abundant IL-18 receptors. These observations indicate that genomic or proteomic receptor expression profiles of leukocyte subsets do not always provide a reliable guide for predicting the responsiveness of cells to the corresponding ligands. We have no explanation for these intriguing findings other than to suggest that some unknown ligand-specific receptor or signaling component(s) may also dictate the responsiveness of cells to IL-18 or IL-33, respectively.
Although IL-3 and IL-33 activate different signaling pathways resulting in certain differences in bioactivity (eg, priming for C5a-induced LTC4 production), they share the ability to promote the secretion of IL-4, IL-13, and IL-8, and to enhance IgER-induced mediator release and cytokine production. Therefore, IL-33 emerges as a second major regulator of these important basophil functions. IL-3 and IL-33 also induce cytokine expression in a synergistic manner, providing an efficient IgE-independent mechanism for the secretion of Th2 cytokines and chemokines in human basophils. Our finding that IL-33 can promote cytokine expression in human basophils is in part in agreement with more preliminary experiments with human basophils published very recently.53 However, in marked contrast to the prominent induction of IL-5 reported in this study,53 we never observed any secretion of IL-5 (detection limit of 5 pg IL-5 per million basophils) under any experimental condition (our unpublished observations). It should also be noted that in contrast to the well established capacity of basophils to produce IL-4 and IL-13, the secretion of IL-5 by human basophils has never been reported and thus requires further confirmation.
The synergistic induction of immunoregulatory and pro-inflammatory cytokines by IL-33, IL-3 and IgER activation shown here provides a mechanism by which allergen-dependent and tissue-derived factors may cooperate to activate basophils at sites of inflammation. Although the regulation of expression and release of biologically active IL-33 in vitro and in vivo is still largely unknown,22,54 the expression of mRNA of proIL-33 seems to be restricted to resident tissue cells, in contrast to proIL-1 that is also strongly expressed in hematopoietic cells.2,21,55 Thus, IL-33 derived from inflamed tissue, IL-3 derived from activated mast cells or Th2 lymphocytes, and exposure to allergen, may all contribute to variable degrees to the activation state of basophils in chronic allergic inflammation. Our findings in blood basophils are reminiscent of the very recently reported synergistic induction of IL-5, IL-13 and proinflammatory cytokines in response to TSLP and IL-1 or IL-33 in human cord blood (CB)–derived mast cells,28,30 indicating that tissue MCs may be another target cell for IL-33. TSLP is a tissue cell–derived cytokine structurally related to other interleukins and hematopoietins, and TSLP receptors and IL-3 receptors use very similar signal transduction pathways.56 The synergistic induction of cytokines by TSLP and IL-1 or IL-33 in CB-derived MCs and by IL-3 and IL-33 in basophils is, therefore, likely to involve similar mechanisms. It is still unknown, however, whether IL-33 also activates mature tissue MCs, and whether the anatomical location or the cytokine environment influences their responsiveness to IL-33.
Because ST2L expression appeared to be an unreliable guide for the identification of cell types that may be activated by IL-33, p38 activation was used to search for other IL-33–responsive cell types present in peripheral human blood. We believe that testing the activation of common and conserved early signaling events represents a particularly reliable and rapid screening method for defining the target cell profile of a novel cytokine. This experimental approach revealed that, apart from basophils, the related eosinophil granulocytes are the only other cells in peripheral blood that directly respond to IL-33. Although eosinophils do not express detectable ST2L protein, IL-33 activates MAP-kinases, in particular p38, and the canonical NF-κB pathway. As a first biologic function of IL-33 in human eosinophils, we demonstrate a direct induction of IL-8, a response that is synergistically enhanced by GM-CSF–family cytokines. As in basophils, this response is strongly inhibited by anti-ST2 antibodies (Figure S2), indicating that IL-33 activates eosinophils in a ST2-dependent manner. Our finding that IL-33 is a potent and direct activator of human eosinophils has been recently confirmed and extended by 2 independent studies that showed that IL-33 also promotes other cellular functions such as adhesion, CD11b expression, exocytosis, and superoxide production.57,58
The lack of p38 activation in PBMCs other than basophils indicates that monocytes and different lymphocyte populations, including in vivo polarized CRTH2+ Th2 cells, do not directly respond to IL-33. After CD3/CD28-ligation of CRTH2+ cells, however, we find a modestly increased secretion of Th2 cytokines in the presence of IL-33, indicating that T-cell–receptor activation renders primary human Th2 cells responsive to IL-33. In this context, it is noteworthy that the regulation of the function of lymphoid cells by IL-33 may be rather complex and context-dependent, and that under certain conditions IL-33 may even favor Th1-polarization and IFN-γ expression, as suggested by a very recent study.53 This paper also showed that culture of NK cells with IL-12 induces ST2 and renders these cells responsive to IL-33 leading to IFN-γ expression,53 and in this regard, the bioactivity of IL-33 is similar to that of IL-18. Thus, although our data indicate that lymphoid cells are not directly activated by IL-33, certain lymphocyte subsets may nevertheless become IL-33–responsive, depending on antigenic stimulation or exposure to the appropriate cytokine.
The present study on primary human leukocytes together with recent findings from other laboratories support the concept that IL-33 primarily acts on cells of the “innate allergic response,” basophils, eosinophils, and mast cells, directly leading to a “Th2-type” inflammatory pathology without the necessary involvement of the adaptive immune system. The effect of IL-33 is further regulated and amplified by costimulatory cytokines, such as TSLP in MCs, IL-3 in basophils, and GM-CSF, IL-3, and IL-5 in eosinophils. Consistent with this interpretation are pharmacologic studies in mice, demonstrating that infusion of IL-33 leads to a pronounced allergic phenotype in the absence of antigenic stimulation21,24 and even in the absence of an adaptive immune system.59 Our data may also help to explain why allergic asthma can develop into a chronic disease that becomes largely independent on the exposure to allergens. In this setting, the activation of cells of the innate immune system by tissue cell–derived cytokines such as TSLP and IL-33 may play a particularly important role.60,61
In conclusion, our study provides a first definition of IL-33–responsive cells within the different leukocyte types of peripheral blood in humans. We present a detailed analysis of the bioactivity of IL-33 on blood basophils, demonstrating that this cytokine is, apart from IL-3, a second major regulator of basophil functions. The data indicate that basophils and eosinophils represent the only direct leukocyte target of IL-33, suggesting a prominent role for these cells of the innate immune system in the biology of IL-33.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swiss National Science Foundation and the Stiftung 3R.
Authorship
Contribution: T.P.-P. and S.A.D. performed and analyzed most experiments and participated in writing; S.K. performed and analyzed experiments on basophils and eosinophils; N.S. performed and analyzed research on PBMCs and T cells; and C.A.D. designed and supervised the studies and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clemens A. Dahinden, Institute of Immunology, University Hospital Bern, CH-3010 Bern, Switzerland; e-mail: clemens.dahinden@iib.unibe.ch.
References
Author notes
*T.P.-P. and S.A.D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal