Abstract
While commonly accepted in poor-risk acute lymphoblastic leukemia (ALL), the role of allogeneic hematopoietic stem cell transplantation (allo-SCT) is still disputed in adult patients with standard-risk ALL. We evaluated outcome of patients with ALL in first complete remission (CR1), according to a sibling donor versus no-donor comparison. Eligible patients (433) were entered in 2 consecutive, prospective studies, of whom 288 (67%) were younger than 55 years, in CR1, and eligible to receive consolidation by either an autologous SCT or an allo-SCT. Allo-SCT was performed in 91 of 96 patients with a compatible sibling donor. Cumulative incidences of relapse at 5 years were, respectively, 24 and 55% for patients with a donor versus those without a donor (hazard ratio [HR], 0.37; 0.23-0.60; P < .001). Nonrelapse mortality estimated 16% (± 4) at 5 years after allo-SCT. As a result, disease-free survival (DFS) at 5 years was significantly better in the donor group: 60 versus 42% in the no-donor group (HR: 0.60; 0.41-0.89; P = .01). After risk-group analysis, improved outcome was more pronounced in standard-risk patients with a donor, who experienced an overall survival of 69% at 5 years (P = .05). In conclusion, standard-risk ALL patients with a sibling donor may show favorable survival following SCT, due to both a strong reduction of relapse and a modest nonrelapse mortality. This trial is registered with http://www.trialregister.nl under trial ID NTR228.
Introduction
Although the majority of adult patients with acute lymphoblastic leukemia (ALL) may enter remission upon standard remission-induction chemotherapy, the risk of relapse is still high and varies between 30% and 80%, depending on the presence of particular risk factors.1-5 Commonly, a standard-risk and a poor-risk category of adult ALL is distinguished. Poor risk is usually defined by the presence of unfavorable cytogenetic abnormalities, high white blood cell (WBC) count, immunophenotype, increasing age, and also a delayed response to achieve remission upon induction chemotherapy (> 4 weeks). Poor-risk ALL usually involves approximately 30 to 40% of all adult patients. The remaining patients are considered standard-risk patients. Allogeneic hematopoietic stem cell transplantation (allo-SCT) has been established as an effective treatment modality to reduce the risk of relapse in adults with ALL in first complete remission (CR1),6-15 but treatment-related mortality (TRM) may counterbalance that favorable effect. As a result, it has been difficult to demonstrate improved overall outcome in the context of prospective studies. Randomized controlled trials (RCT) are the “gold standard” for the evaluation of treatment efficacy, but genetically randomized studies have been accepted as a reliable alternative, as the presence or absence of a sibling donor can be used as a surrogate for randomization.16,17 Several donor versus no-donor studies have shown that allo-SCT reduces the risk of relapse in patients with ALL in CR1, but only a few studies showed improved outcome (reviewed by Hahn et al18 ). Moreover, following the observation by the French Adult Acute Lymphoblastic Leukemia (LALA) group that the beneficial effect may be restricted to poor-risk patients,8 allo-SCT has preferably been applied in poor-risk ALL and in standard-risk patients only after relapse. A recent meta-analysis of several larger studies had suggested better overall survival in patients with a donor compared with those without a sibling donor, but, again, the survival advantage was especially apparent in poor-risk patients.19 In contrast, the large Medical Research Council/Eastern Cooperative Oncology Group (MRC/ECOG) study recently suggested that allo-SCT may also improve outcome of adult patients with standard-risk features.20
Here we set out to address the question of whether the use of allo-SCT in patients with ALL in CR1 favorably impacts on overall survival (OS) and disease-free survival (DFS) in general as well as in commonly accepted risk categories. Hence, we compared outcome of patients with ALL in CR1 with a human leukocyte antigen (HLA) identical sibling donor with outcome of patients without such a donor. The analysis is based on patients enrolled in 2 successive prospective ALL studies conducted by the Dutch-Belgian HOVON cooperative group, in which patients without a donor were offered an autograft as ultimate consolidation therapy. In these studies, the majority of patients were evaluated for the presence of a sibling donor, irrespective of underlying risk profile, and a comparatively high percentage of patients with a sibling donor indeed received their intended allograft.
Methods
The study population for this study consists of newly diagnosed patients with precursor B-cell or precursor T-cell ALL included in the consecutive HOVON-18 ALL (HO18) and HOVON-37 ALL (HO37) studies between November 1992 and November 2005 with the following additional selection criteria: (1) age younger than 50 years for patients in HO18, because patients aged 50 years and older were not eligible for allo-SCT in that study, and age 55 years or younger for patients in the HO37 study; (2) CR reached after remission induction cycles I or II, or intensification; (3) completion of at least 2 cycles of induction and 1 cycle of intensification chemotherapy; and (4) eligible for consolidation therapy (ie, World Health Organization [WHO] performance ≤ 2, absence of severe cardiac/pulmonary/hepatic/renal dysfunction, not refractory to platelets, and informed consent).
Irrespective of whether and which type of consolidation therapy was applied, the patients from the study population were classified in 3 groups: (1) the donor group; (2) the no-donor group; and (3) a group of patients with insufficient information. Similar to the recent HOVON donor versus no-donor study in acute myeloid leukemia,21 a patient was classified in the donor group if the search for a donor resulted in a genotypically or phenotypically identical sibling, an HLA identical sibling (without further specification), a matched related donor, or a syngeneic twin. Another requirement for the donor group was that the donor was willing to serve as a donor and that there were no medical contraindications for stem cell collection. Those not fulfilling the criteria for the donor group were classified in the no-donor group. Thus the no-donor group included patients with no HLA-identical siblings or no siblings available for typing, those with siblings with one or more mismatches, as well as patients with a (mis)matched unrelated donor or those for whom a search for an unrelated donor was initiated. Patients who could neither be classified in the donor group nor in the no-donor group were classified in the no-information group. The HO18 and HO37 studies were approved by the ethics committees of the participating centers and were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study patients.
Treatment protocols
Treatment in the HO18 study involved one cycle of induction chemotherapy with prednisone, daunorubicin, vincristine, and asparaginase, followed by a second cycle of cyclophosphamide, cytarabin, and 6-mercaptopurin (6-MP). The third cycle of intensification chemotherapy consisted of high-dose cytarabin combined with etoposide, the cycle of which was to be followed by consolidation treatment with either an autologous SCT or an allo-SCT, depending on the presence of an HLA-matched sibling donor. More detailed information with respect to design and specific dosages of chemotherapy are given on the HOVON website (http://www.HOVON.nl). Treatment in the HO37 study included a similar induction scheme followed by intensification consisting of a second cycle of high-dose cytarabine combined with mitoxantrone and a third cycle of high-dose methotrexate (MTX) combined with asparaginase and 6-MP. Subsequently, patients planned to receive either an autologous SCT or allo-SCT in case of an HLA-matched sibling donor. Moreover, patients with poor-risk characteristics including t(9;22), t(4;11), or t(1;19) and lacking a sibling donor were eligible for unrelated donor SCT, if 9 or more of 10 HLA-matched unrelated donors (MUD) could be identified. In addition, patients in CR after autologous SCT were randomly assigned to continue treatment with maintenance for 1 year (6-MP, 60 mg/m2 per d; MTX, 20 mg/m2 per wk) or to receive no further treatment in the HO37 study. All patients received intrathecal chemotherapy with MTX at regular intervals until start of myeloablative conditioning. Conditioning therapy in both studies consisted of high-dose cyclophosphamide and busulfan prior to autologous SCT and high-dose cyclophosphamide combined with total body irradiation (TBI; 2 × 6 Gy) prior to allo-SCT. Patients with poor-risk ALL with t(9;22) received imatinib 600 mg daily in conjunction with scheduled chemotherapy from April 2003. Patients with mature B-cell ALL (B-ALL) were not eligible for the HOVON 18 and 37 studies and were treated according to the German Protocol, specifically developed for mature B-ALL.1,3
Risk groups
Patients were classified as poor risk if they met one of the following criteria at diagnosis: (1) cytogenetic abnormalities t(9;22), t(4;11), or t(1;19); (2) pro–B-cell immunophenotype; (3) high WBC (ie, > 30 × 109/L in case of B-ALL; > 100 × 109/L in case of T-cell ALL [T-ALL]). In addition, patients were also considered poor risk in case they would attain a late CR1 (ie, beyond 4 weeks from start induction). All other patients were classified in the standard risk group. Survival according to risk groups for all 433 patients, who were eligible for the HOVON 18 and 37 studies is presented in Figure 1. Immunophenotypical classification of all ALLs was performed according to the classification proposed by the European Group for the Immunological Characterization of Leukemias (EGIL).22
Survival of all patients registered. OS of all patients presented as from diagnosis and registration for the HOVON 18 and 37 studies (A) and according to risk status (B).
Survival of all patients registered. OS of all patients presented as from diagnosis and registration for the HOVON 18 and 37 studies (A) and according to risk status (B).
End points and statistical methods
All CR1 patients, who were classified in the donor and no-donor groups irrespective of whether or how consolidation therapy was applied, were evaluated as from the day of remission evaluation after intensification chemotherapy (IC). DFS was determined from the date of remission evaluation after IC until relapse or death in CR1, the latter being considered as nonrelapse mortality (NRM). OS was measured from evaluation of remission after IC until death from any cause. Patients still alive at the date of last contact were censored. In addition, patients who received an allogeneic transplant in first CR of an unrelated donor were censored at the date of transplantation for both end points. The cumulative risks of relapse and NRM over time were calculated as competing risks with actuarial methods, whereas patients alive in continuing first complete remission were censored at the date of last contact. Multivariate Cox regression analysis for OS, DFS, relapse, and NRM was applied on an intention-to-treat basis to calculate hazard ratios (HR) for the donor group compared with the no-donor group. All P values for tests that compare the outcomes in the donor and no-donor group were based on log likelihood ratio tests, except when explicitly stated otherwise. Log likelihood ratio tests were also used to test for interactions (ie, to test for differences in the donor effect between risk groups for each of the end points OS, DFS, relapse, and TRM). To compare our results with those previously published, we also evaluated the prognostic value of donor availability for standard-risk and poor-risk subgroups separately. As reported before23,24 only if the statistical interaction test supported subgroup analysis, conclusions were influenced. P values of these tests for interaction are only mentioned in the results section when less than .10. HR estimates with 95% confidence intervals (CI) comparing the donor group with the no-donor group were also obtained by log-rank analysis in subgroups stratified by risk. Kaplan-Meier curves were generated to illustrate differences between subgroups and compared using the log-rank test. In addition, differences between survival curves at the 5-year time point were also evaluated with χ2 testing. All reported P values are 2-sided, and a significance level α equal to .05 was used.
Results
Donor availability and consolidation treatment applied
Between November 1992 and November 2005, 459 patients were included in consecutive HOVON ALL studies HO18 and HO37, of whom 433 patients met the HO18 and 37 eligibility criteria. OS from diagnosis and study registration of these 433 patients is presented in Figure 1 for all patients and according to risk status. Results of the HO18 and HO37 studies did not differ significantly with respect to remission rates and DFS, but a (nonsignificant) trend toward improved survival was noted in the HO37 study (HR: 0.80; 0.62-1.02; P = .07). For the present donor versus no-donor comparison, 145 of these 433 patients were excluded, resulting in a study population of 288 patients. Reasons for exclusion were: age over 50 (HO18; n = 29) or 55 (HO37; n = 16); no CR after induction/intensification chemotherapy (n = 52); early relapse or death before completion of intensification chemotherapy (n = 31); or ineligible for consolidation therapy (n = 17). Detailed information as regards the presence and availability of an HLA-identical sibling donor was obtained in 257 of 288 of the eligible patients (89%). An HLA-identical sibling donor was available in 96 of 257 patients (37%), while 161 of 257 patients (63%) lacked such a donor as a result of absence of siblings, HLA incompatibility, or ineligibility of a potential donor. Information regarding the presence of siblings and/or results of HLA typing was lacking in 31 patients, which constituted the “no information” group. These 31 patients included 20 patients from one particular center that had chosen to focus on autografting and not to include allo-SCT in their treatment policy of adult ALL during the course of the HO18 study and 11 patients with insufficient information with respect to HLA typing or the presence of siblings. Outcome of these 31 patients did not differ from the 161 patients included in the no-donor group (DFS in both groups of 42% at 5 years, HR: 1.12; 0.67-1.88; P = .7). These patients are not included in the analysis, as this study concentrates entirely on the comparison between the donor and no-donor groups. At the time of data analysis, the median follow-up from diagnosis of patients still alive was 65 months (range, 7-164). Patient characteristics of the donor and no-donor groups are presented in Table 1. Both groups are comparable with respect to sex, ALL immunophenotype, WBC, cytogenetic abnormalities, number of cycles to achieve remission, prognostic risk category, and time between diagnosis or CR1 until SCT. Age, however, appeared somewhat higher in the donor group (median 31 years, range: 16-55; vs 26 years, range: 16-54; P = .01).
Patient characteristics
| Parameter . | Subgroup of patients . | |
|---|---|---|
| Donor (n = 96) . | No donor (n = 161) . | |
| Median age, y (range) | 31 (16-55) | 26 (15-54) |
| Sex | ||
| Male | 58 (60) | 96 (60) |
| Female | 38 (40) | 65 (40) |
| Immunophenotype (No.) | ||
| Precursor B cell | 65 (68) | 121 (75) |
| Precursor T cell | 28 (29) | 38 (24) |
| Not specified | 3 | 2 |
| WBC, × 109/L (No. [%]) | ||
| ≤ 30 | 64 (67) | 118 (73) |
| 30-100 | 18 (19) | 26 (16) |
| > 100 | 14 (15) | 17 (11) |
| Cytogenetics successful (No. [%]) | 79 (82) | 130 (81) |
| Cytogenetic poor-risk features* | ||
| t(9;22) | 17 (22) | 21 (16) |
| t(4;11) | 4 (5) | 4 (3) |
| t(1;19) | 1 (1) | 3 (2) |
| Cycles to CR (No. [%]) | ||
| 1 | 87 (91) | 148 (92) |
| 2-3 | 9 (9) | 13 (8) |
| Prognostic risk category (No. [%])† | ||
| Standard | 50 (52) | 88 (55) |
| Poor | 46 (48) | 73 (45) |
| Time from diagnosis until SCT, mo | ||
| Median (range) | 5.2 (2.8-10.8) | 5.4 (3.2-10.2) |
| Time from CR1 until SCT, mo | ||
| Median (range) | 3.8 (0.5-9.2) | 3.9 (0.4-8.9) |
| Parameter . | Subgroup of patients . | |
|---|---|---|
| Donor (n = 96) . | No donor (n = 161) . | |
| Median age, y (range) | 31 (16-55) | 26 (15-54) |
| Sex | ||
| Male | 58 (60) | 96 (60) |
| Female | 38 (40) | 65 (40) |
| Immunophenotype (No.) | ||
| Precursor B cell | 65 (68) | 121 (75) |
| Precursor T cell | 28 (29) | 38 (24) |
| Not specified | 3 | 2 |
| WBC, × 109/L (No. [%]) | ||
| ≤ 30 | 64 (67) | 118 (73) |
| 30-100 | 18 (19) | 26 (16) |
| > 100 | 14 (15) | 17 (11) |
| Cytogenetics successful (No. [%]) | 79 (82) | 130 (81) |
| Cytogenetic poor-risk features* | ||
| t(9;22) | 17 (22) | 21 (16) |
| t(4;11) | 4 (5) | 4 (3) |
| t(1;19) | 1 (1) | 3 (2) |
| Cycles to CR (No. [%]) | ||
| 1 | 87 (91) | 148 (92) |
| 2-3 | 9 (9) | 13 (8) |
| Prognostic risk category (No. [%])† | ||
| Standard | 50 (52) | 88 (55) |
| Poor | 46 (48) | 73 (45) |
| Time from diagnosis until SCT, mo | ||
| Median (range) | 5.2 (2.8-10.8) | 5.4 (3.2-10.2) |
| Time from CR1 until SCT, mo | ||
| Median (range) | 3.8 (0.5-9.2) | 3.9 (0.4-8.9) |
CR indicates complete remission; and WBC, white blood cell count.
Restricted to patients with successful cytogenetics.
Based on cytogenetics, WBC count, and early or late attainment of CR (see “Methods”).
Patients in complete remission after induction/intensification chemotherapy received for consolidation: high-dose cytotoxic therapy followed by an autograft (n = 126); allogeneic SCT (n = 122); maintenance chemotherapy only (n = 4); or no treatment (n = 5). Twenty recipients of autologous SCT were treated with maintenance therapy after transplantation. Maintenance after autologous SCT appeared poorly feasible, was prematurely stopped in the majority of these 20 patients, and did not affect outcome (results not shown). Among the donor group, 91 of 96 of the patients (95%) received an allo-SCT from an HLA-identical sibling, while 3 patients received an autologous SCT, followed by maintenance chemotherapy in 1 patient (Table 2). One patient did not receive consolidation treatment, and 1 other patient received maintenance therapy only. On the other hand, 123 of 161 of the patients (76%) in the no-donor group received an autologous stem cell graft, followed by maintenance in 19 patients, while 31 of 161 of the patients (19%) received an allo-SCT using stem cells of mismatched related (n = 2) or unrelated donors (n = 29). Four patients did not receive consolidation therapy, and 3 patients were treated with maintenance only (Table 2).
Consolidation therapies applied in the donor and no-donor groups
| Type of therapy . | Group of patients . | |
|---|---|---|
| Donor (n = 96) . | No donor (n = 161) . | |
| Allogeneic SCT | ||
| HLA-identical sibling | 91 (95%) | — |
| Unrelated donor | — | 29 (18%) |
| Mismatched family donor | — | 2 (1%) |
| Autologous SCT | 3 (3%) | 123 (76%) |
| Maintenance only | 1 (1%) | 3 (2%) |
| No treatment | 1 (1%) | 4 (2%) |
| Type of therapy . | Group of patients . | |
|---|---|---|
| Donor (n = 96) . | No donor (n = 161) . | |
| Allogeneic SCT | ||
| HLA-identical sibling | 91 (95%) | — |
| Unrelated donor | — | 29 (18%) |
| Mismatched family donor | — | 2 (1%) |
| Autologous SCT | 3 (3%) | 123 (76%) |
| Maintenance only | 1 (1%) | 3 (2%) |
| No treatment | 1 (1%) | 4 (2%) |
SCT indicates stem cell transplantation; and HLA, human leukocyte antigen.
Relapse, TRM, and survival
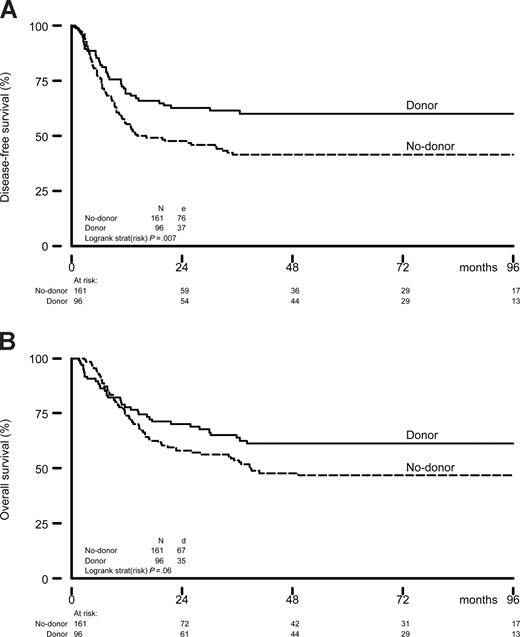
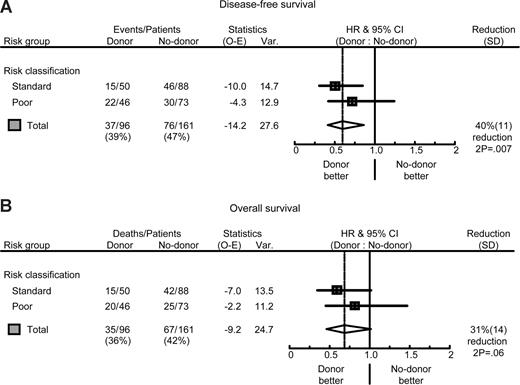
At the time of the analysis, 59 of 96 CR1 patients (61%) with a donor and 85 of 161 of patients (53%) in the no-donor group were alive in continuous complete remission. The details of the comparison of the donor and no-donor group are shown in Table 3. The risk of relapse was significantly less in the donor group (24 vs 55% at 5 years, HR: 0.37; 95% confidence interval (CI) 0.23-0.60; P < .001) (Table 3). The reduction in risk of relapse was observed especially in standard-risk patients (HR: 0.23; 95% CI 0.10-0.51; P < .001), but it was also statistically significant in poor-risk ALL (HR: 0.52; 95% CI 0.28-0.97; P = .03). Overall, NRM was significantly higher in the donor group (16 vs 3%, P = .002), and was higher both in the poor-risk patients (15 vs 4%, P = .08) and the standard-risk patients (16 vs 2%, P = .01). The combined effects of a much lower relapse rate and a somewhat higher NRM in the donor group resulted in a significantly better DFS in the donor group compared with the no-donor group (60 vs 42%, HR: 0.60; 95% CI 0.41-0.89; P = .01) (Table 3 and Figure 2). The improvement in DFS was observed especially in standard-risk patients (HR = 0.47; 95% CI 0.26-0.84; P = .007), and to a lesser extent in poor-risk patients (Table 3 and Figure 3). Comparison of DFS at the 5-year time point using χ2 yielded comparable results with P equal to .006 (all patients), P equal to .006 (standard-risk patients), and P equal to .14 (poor-risk patients), respectively. The improved DFS in the donor group translated in improved OS: 61 versus 47% OS at 5 and 8 years in the donor group compared with the no-donor group (HR: 0.70; 95% CI 0.46-1.05; P = .08) (Table 3 and Figures 2 and 3), without obvious difference in prognostic value of donor availability between standard-risk and poor-risk patients (Table 3 and Figure 3). Again, P values determined by χ2 confirmed these findings (P = .03, all patients; P = .03, standard risk; and P = .27, poor risk). After multivariate Cox regression analyses, the availability of an HLA-identical sibling donor was confirmed as a favorable risk factor for both DFS and OS. While significantly associated with outcome in univariate analysis, age and risk status did not significantly affect survival in multivariate analysis. Other multivariate Cox regression analyses with end points OS and DFS were performed by adding more variables to the model: gender, WBC (logarithm) at diagnosis, cycles of chemotherapy needed to obtain CR1 (1 vs 2 or 3), time from diagnosis to SCT, and time from CR1 to SCT. None of these were significantly associated with reduced OS and DFS. The addition of these covariates hardly changed the HR for donor availability on the end points DFS (HR: 0.58; 95% CI 0.38-0.87; P = .008) and OS (HR: 0.68; 95% CI 0.44-1.04; P = .07).
Effect of donor availability on outcome in ALL in CR1
| Outcome parameter . | Donor (n = 96) . | No donor (n = 161) . | ||||
|---|---|---|---|---|---|---|
| Total number of events (death; relapse) . | Probability of outcome at 5 y (% ± SE) . | Total number of events (death; relapse) . | Probability of outcome at 5 y (% ± SE) . | P . | Hazard ratio (95% CI) . | |
| All patients | ||||||
| DFS | 37 | 60 ± 5 | 76 | 42 ± 4 | .01 | 0.60 (0.41-0.89) |
| Relapse | 22 | 24 ± 4 | 72 | 55 ± 4 | < .001 | 0.37 (0.23-0.60) |
| NRM | 15 | 16 ± 4 | 4 | 3 ± 1 | .002 | 4.84 (1.60-14.6) |
| Survival | 35 | 61 ± 5 | 67 | 47 ± 5 | .08 | 0.70 (0.46-1.05) |
| Standard risk | 50 patients | 88 patients | ||||
| DFS | 15 | 69 ± 7 | 46 | 45 ± 5 | .007 | 0.47 (0.26-0.84) |
| Relapse | 7 | 14 ± 5 | 44 | 52 ± 5 | < .001 | 0.23 (0.10-0.51) |
| NRM | 8 | 16 ± 5 | 2 | 2 ± 2 | .01 | 5.93 (1.25-28.0) |
| Survival | 15 | 69 ± 7 | 42 | 49 ± 6 | .05 | 0.57 (0.31-1.02) |
| Poor risk | 46 patients | 73 patients | ||||
| DFS | 22 | 50 ± 8 | 30 | 35 ± 7 | .23 | 0.72 (0.41-1.24) |
| Relapse | 15 | 34 ± 7 | 28 | 61 ± 7 | .03 | 0.52 (0.28-0.97) |
| NRM | 7 | 15 ± 7 | 2 | 4 ± 3 | .08 | 3.67 (0.76-17.7) |
| Survival | 20 | 53 ± 8 | 25 | 41 ± 8 | .50 | 0.82 (0.45-1.47) |
| Outcome parameter . | Donor (n = 96) . | No donor (n = 161) . | ||||
|---|---|---|---|---|---|---|
| Total number of events (death; relapse) . | Probability of outcome at 5 y (% ± SE) . | Total number of events (death; relapse) . | Probability of outcome at 5 y (% ± SE) . | P . | Hazard ratio (95% CI) . | |
| All patients | ||||||
| DFS | 37 | 60 ± 5 | 76 | 42 ± 4 | .01 | 0.60 (0.41-0.89) |
| Relapse | 22 | 24 ± 4 | 72 | 55 ± 4 | < .001 | 0.37 (0.23-0.60) |
| NRM | 15 | 16 ± 4 | 4 | 3 ± 1 | .002 | 4.84 (1.60-14.6) |
| Survival | 35 | 61 ± 5 | 67 | 47 ± 5 | .08 | 0.70 (0.46-1.05) |
| Standard risk | 50 patients | 88 patients | ||||
| DFS | 15 | 69 ± 7 | 46 | 45 ± 5 | .007 | 0.47 (0.26-0.84) |
| Relapse | 7 | 14 ± 5 | 44 | 52 ± 5 | < .001 | 0.23 (0.10-0.51) |
| NRM | 8 | 16 ± 5 | 2 | 2 ± 2 | .01 | 5.93 (1.25-28.0) |
| Survival | 15 | 69 ± 7 | 42 | 49 ± 6 | .05 | 0.57 (0.31-1.02) |
| Poor risk | 46 patients | 73 patients | ||||
| DFS | 22 | 50 ± 8 | 30 | 35 ± 7 | .23 | 0.72 (0.41-1.24) |
| Relapse | 15 | 34 ± 7 | 28 | 61 ± 7 | .03 | 0.52 (0.28-0.97) |
| NRM | 7 | 15 ± 7 | 2 | 4 ± 3 | .08 | 3.67 (0.76-17.7) |
| Survival | 20 | 53 ± 8 | 25 | 41 ± 8 | .50 | 0.82 (0.45-1.47) |
HR indicates hazard ratio for donor compared to no donor from univariate Cox model; P value from likelihood ratio test; n, number of patients; DFS, disease-free survival; NRM, nonrelapse mortality; and SE, standard error.
All survival end points are determined from date of evaluation of intensification chemotherapy and presented as probabilities in time. Events are given as (absolute) total numbers of relapse (accounting for DFS and relapse) and/or death (accounting for OS, DFS, and NRM) that had occurred during the time of follow-up.
Outcome by donor availability. Actuarial rates of DFS (A) and OS (B) of patients with ALL in CR1 according to sibling donor availability. OS and DFS are presented as from the day after completion and recovery of intensification chemotherapy, just before the start of consolidation by autologous SCT or allo-SCT.
Outcome by donor availability. Actuarial rates of DFS (A) and OS (B) of patients with ALL in CR1 according to sibling donor availability. OS and DFS are presented as from the day after completion and recovery of intensification chemotherapy, just before the start of consolidation by autologous SCT or allo-SCT.
Risk group analysis. Forest plots of the HR for DFS (A) and OS (B) of patients with ALL in CR1; donor versus no-donor, split by risk group, and the overall estimate together with 95% CI. The percentage reduction is equal to 100 × (1 HR). Poor risk was defined by: (1) cytogenetic abnormalities t(9;22), t(4;11), or t(1;19); (2) pro–B-cell immunophenotype; (3) high WBC (ie, > 30 × 109/L in case of B-ALL; > 100 × 109/L in case of T-ALL); and (4) late CR1 (ie, beyond 4 weeks from start induction). All other patients were classified in the standard-risk group. The pooled estimates of the HR of donor availability for all patients were, respectively, 0.60 (95% CI 0.41-0.87; P = .007) for DFS and 0.69 (95% CI 0.46-1.02; P = .06) for OS.
Risk group analysis. Forest plots of the HR for DFS (A) and OS (B) of patients with ALL in CR1; donor versus no-donor, split by risk group, and the overall estimate together with 95% CI. The percentage reduction is equal to 100 × (1 HR). Poor risk was defined by: (1) cytogenetic abnormalities t(9;22), t(4;11), or t(1;19); (2) pro–B-cell immunophenotype; (3) high WBC (ie, > 30 × 109/L in case of B-ALL; > 100 × 109/L in case of T-ALL); and (4) late CR1 (ie, beyond 4 weeks from start induction). All other patients were classified in the standard-risk group. The pooled estimates of the HR of donor availability for all patients were, respectively, 0.60 (95% CI 0.41-0.87; P = .007) for DFS and 0.69 (95% CI 0.46-1.02; P = .06) for OS.
Outcome estimates by donor availability as presented in Figures 2 and 3 and Table 3 were calculated as from the day of remission evaluation after intensification chemotherapy, irrespective of type of SCT or other treatment received thereafter. That statistical plan was applied to come as close as possible to a real randomized study, which would have taken place at that point of time.21 The recent donor versus no-donor study by the MRC/ECOG collaborative group20 however, evaluated outcome of adult ALL by donor availability as from diagnosis. To compare their results with those of the present study, we performed an additional analysis by evaluating the effect of donor availability as from diagnosis. Similar to the selection applied by the MRC/ECOG, excluding induction failures (n = 60) and older (n = 35) patients, 339 patients were eligible for tissue typing, which was ultimately performed in 295 patients. It resulted in a donor group of 106 patients and a no-donor group of 189 patients. OS and event-free survival (EFS), according to donor availability and stratified by risk, are presented in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). With respect to both end points, a significant benefit of donor availability was noted, which was most pronounced in standard-risk patients. By χ2 testing of OS and EFS at 5 years,20 P values estimated .02 and .01, respectively, underlining the advantage of donor availability. Philadelphia-negative (Ph−) standard-risk patients with a donor showed a survival of 60% at 5 years (Figure S1D), compared with a 62% OS reported in the same group by MRC/ECOG.
Outcome after relapse
Most relapsing patients (n = 94) received salvage treatment (82/94, 87%), either chemotherapy (49/82, 60%), an autologous transplant (1/82, 1%) or allogeneic transplant (22/82, 27%), or donor lymphocyte infusion (10/82, 12%). Thirty-eight percent of the relapsing patients reached a second CR (CR2, n = 36), but most of these either relapsed again (21/36) or died in CR2 (7/36). At the time of analysis, 11 of the relapsed patients were still alive, of whom 8 continued in CR2. The actuarial probability of survival after relapse at 2 years was 19% in the no-donor group and 6% in the donor group (P = .42).
Alternative donor allo-SCT; Philadelphia-positive ALL
The donor versus no-donor analysis was performed on an intention-to-treat basis by HLA-matched sibling donor availability, with censoring of recipients of an unrelated donor allo-SCT at the time of SCT. Search for an alternative donor was only performed for poor-risk patients lacking a sibling donor. That policy became the standard way of care in the HO37 study as of April 1999. Among the 73 poor-risk patients in the no-donor group, 27 received a MUD transplant. These 27 recipients of an unrelated donor graft included 17 patients with Philadelphia-positive (Ph+) ALL, 4 patients with high WBC, 3 patients with a late CR, 2 patients with a pro B-cell immunophenotype of the underlying ALL, and 1 patient with t(1;19) ALL. Outcome following MUD allo-SCT was 39% DFS at 5 years, as determined by a relapse rate of 22% and a NRM of 39%. In total, 77 patients presented with Ph+ precursor B-ALL, who, by intention to treat, all followed the HO18 or HO37 treatment schedules and the ensuing biologic randomization. Forty-two Ph+ patients proceeded to consolidation by SCT, including autologous SCT in 5, allo-SCT from a sibling donor in 18, and 19 received an allo-SCT from an unrelated donor. Eighteen of these 77 patients had received imatinib in conjunction with their induction and consolidation chemotherapy. Patients having received imatinib, distributed evenly among the donor and no-donor group, were more likely to enter complete remission (17/18, 94% vs 39/59, 66%) and experienced better DFS compared with Ph+ patients treated without imatinib (the median DFS was prolonged from 8 to 20 months, HR: 0.46; 0.21-1.01; P = .04), but the group of imatinib-treated patients was rather small and had different follow-up.
Discussion
While allo-SCT is widely applied in adults with poor-risk ALL in CR1, the value of allo-SCT in patients without poor-risk features has remained questionable. Only a limited number of larger studies using an intention-to-treat analysis compared outcome of patients with a sibling donor versus those without a donor. One of the first studies suggested an advantage of allo-SCT restricted to poor-risk patients.8 It was therefore suggested that allo-SCT in first CR should be restricted to poor-risk patients, and several centers adopted the latter transplantation policy. In subsequent studies, the French LALA group and others reproduced the beneficial effect of allo-SCT in poor-risk patients,10-12 while patients without poor-risk features only qualified for allo-SCT in second CR. Given the potent antileukemic effect of allo-SCT in ALL, the paucity of prospective studies and the high relapse rate in standard-risk ALL, here we addressed the question of whether allo-SCT would favorably impact outcome in adult ALL overall, including patients with standard-risk features.
The Dutch-Belgian HOVON cooperative group has conducted 2 successive trials in previously untreated adult patients with ALL aged 60 years or younger, in which allo-SCT was consistently standard treatment for all patients achieving CR and having an HLA-identical sibling donor, irrespective of risk category. The present study shows that more than 90% of patients were indeed evaluated for the presence of a sibling donor, which subsequently resulted in a high access (95%) to allo-SCT after achieving remission. According to the intention-to-treat analysis, a strong reduction of relapse was observed in patients with a donor, which together with a modest NRM translated into improved outcome for the donor group and resulted in a net gain of 18% in DFS and 14% in OS at 5 years, respectively (Figures 2,3 and Table 3). That favorable effect was observed especially in patients with a standard-risk profile. Currently, myeloablative allo-SCT is not universally recommended for patients with ALL in CR1 without poor-risk features. This is done to avoid the unnecessary risks of transplantation procedure-related mortality and graft-versus-host disease to patients, who may be cured with chemotherapy alone and to postpone allo-SCT to an eventual relapse. In the present study, approximately 50% of standard-risk CR1 patients showed long-term DFS after chemotherapy and consolidation by autologous SCT, while the relapse rate was estimated at 52% in those patients. May patients relapsing after autologous SCT be rescued with an allogeneic transplant in second CR? In the present study, only a small minority of relapsing patients experienced favorable long-term outcome, which was mainly accounted for by patients who could proceed to unrelated donor allo-SCT. The MRC/ECOG collaborative group earlier reported results of 609 relapsing patients, who had been entered in the prospective MRC UKALL XII/ECOG trial.25 Results showed a dismal long-term survival of only 7% of relapsing patients showing long-term survival. After risk-factor analysis, it was suggested that only younger patients with a late relapse (without central nervous system involvement) may have a reasonable chance of cure and may be offered an allo-SCT with either a related or unrelated donor upon achieving second CR.
Very recently, the MRC/ECOG study group presented a beneficial effect of allo-SCT in standard-risk ALL in first CR.20 According to a strict intention-to-treat principle, the MRC/ECOG results were analyzed and presented as from diagnosis, thereby clearly showing the advantage of an early-on strategy of tissue typing and strong commitment for eventual allo-SCT. The present study is presented as from remission evaluation prior to consolidation by autologous SCT or allo-SCT to come as close as possible to a real randomized study, according to the (intention-to-treat) policy applied in acute myeloid leukemia (AML).21 Obviously, HOVON and MRC/ECOG results cannot be compared directly due to time difference of analysis. To facilitate a meaningful comparison, the HOVON data were reanalyzed according to donor availability from diagnosis. Again, a statistical significant benefit of donor availability was demonstrated comparing 106 patients with a donor versus 189 patients without a donor (Figure S1). OS estimated 60% at 5 years in standard-risk patients with a donor, which compares well to the 62% OS in standard-risk patients with a donor in the MRC/ECOG study.20 Collectively, their results and those of the present study firmly show that patients with a standard-risk profile may benefit from allo-SCT in first CR, provided the counterbalancing NRM does not exceed approximately 20% to 25%. Therefore, adult patients with ALL in first CR with a relapse risk exceeding approximately 50% or more (after continuing chemotherapy or autologous SCT) and an NRM risk of less than 20% to 25% may be expected to benefit from allo-SCT, which should then preferably be performed in first CR. Thus, apart from assessing the risk of relapse, it is imperative that the risk of NRM should be taken into account in decision making. Both the European Group for Blood and Marrow Transplantation (EBMT) risk score developed by Gratwohl et al,26 which now also applies in acute leukemia,27 and the Seattle comorbidity score,28 are risk scores that have been validated in independent cohorts of patients and can be applied more generally in patients with acute leukemia.29
Standard-risk ALL represents the major subset of adult ALL, it comprised approximately 55% of all patients in the present study. While survival in these standard-risk CR1 patients with a sibling donor estimated an appreciable 69% at 5 years, those without a donor but with standard-risk features showed a 5-year survival of 49% (Table 3). These results may compare well to a recent larger analysis reported by Dhedin et al,30 which may suggest that a yet unknown subcategory of standard-risk patients may be cured by chemotherapy and/or autologous SCT alone. The prognostic variables at diagnosis, however, are insufficient to accurately identify those standard-risk patients. Therefore, better estimates are needed to identify those patients. Currently, the most promising approach may be offered by the serial monitoring of minimal residual disease (MRD) by using clonal immunoglobulin and T-cell receptor gene rearrangements.31-37 Using those techniques in pediatric patients, it has been shown that high MRD at any time point after consolidation is associated with a high risk of relapse, while an early and rapid clearance of MRD may identify a subgroup of patients with a very low risk of relapse and may even be designated as “good-risk” patients. However, mature results of MRD monitoring using current treatment strategies for adult patients are still scarce, but eagerly awaited.
In conclusion, adult patients with ALL in first CR may experience favorable outcome after sibling donor allo-SCT, due to a strong reduction of relapse and a modest NRM. These results emphasize the strong antileukemic activity of the allogeneic graft-versus-leukemia effect in acute lymphoblastic leukemia in first CR and underline the application of allo-SCT also in standard-risk patients, especially those for whom an acceptable risk of NRM can be estimated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Wim L. J. van Putten is acknowledged for statistical comments and Janine Vrij is gratefully acknowledged for secretarial assistance.
This work was supported by the Dutch Cancer Society (KWF-Kankerbestrijding) which provided support for data management.
The authors (for affiliations, see title page) are all members of The Dutch-Belgian HOVON Cooperative Group; for HOVON studies and members: see www.HOVON.nl.
Authorship
Contribution: J.J.C., B.v.d.H., G.O., G.E.G.V., M.B.v.V., L.F.V., B.L., and A.W.D. designed the study; J.J.C., G.E.G.V., M.B.v.V., M.H.J.v.O., H.C.S., G.O., P.S., J.M., M.v.M.K., M.R.S., P.W.W., D.H.B., S.W., P.J.V., J.W.B., P.Z., L.F.V., and A.W.D. performed the study and included patients; J.J.C., B.v.d.H., D.H.B., S.W., P.J.V., J.W.B., P.Z., L.F.V., and A.W.D. analyzed the data; J.J.C., B.v.d.H., L.F.V., B.L., and A.W.D. wrote the paper; and M.B.v.V., M.H.J.v.O., H.C.S., G.O., P.S., J.M., M.v.M.K., M.R.S., P.W.W., D.H.B., S.W., P.J.V., J.W.B., and P.Z. critically provided comments on the (final) draft.
Conflict-of-interest disclosure: G.E.G.V. served as a consultant to Roche, Bristol-Myers-Squib, and Novartis. G.O., P.S., and J.J.C. all served as consultants to Novartis Oncology in Holland. The remaining authors declare no competing financial interests.
Correspondence: Jan J. Cornelissen, Department of Hematology, Erasmus University Medical Center, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands; e-mail: j.cornelissen@erasmusmc.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal