Abstract
The transbilayer movement of phosphatidylserine from the inner to the outer leaflet of the membrane bilayer during platelet activation is associated with the release of procoagulant phosphatidylserine-rich small membrane vesicles called platelet-derived microvesicles. We tested the effect of lactadherin, which promotes the phagocytosis of phosphatidylserine-expressing lymphocytes and red blood cells, in the clearance of platelet microvesicles. Platelet-derived microvesicles were labeled with BODIPY-maleimide and incubated with THP-1–derived macrophages. The extent of phagocytosis was quantified by flow cytometry. Lactadherin promoted phagocytosis in a concentration-dependent manner with a half-maximal effect at approximately 5 ng/mL. Lactadherin-deficient mice had increased number of platelet-derived microvesicles in their plasma compared with their wild-type littermates (950 ± 165 vs 4760 ± 650; P = .02) and generated 2-fold more thrombin. In addition, splenic macrophages from lactadherin-deficient mice showed decreased capacity to phagocytose platelet-derived microvesicles. In an in vivo model of light/dye-induced endothelial injury/thrombosis in the cremasteric venules, lactadherin-deficient mice had significantly shorter time for occlusion compared with their wild-type littermate controls (5.93 ± 0.43 minutes vs 9.80 ± 1.14 minutes;P = .01). These studies show that lactadherin mediates the clearance of phosphatidylserine-expressing platelet-derived microvesicles from the circulation and that a defective clearance can induce a hypercoagulable state.
Introduction
In platelets, as in most mammalian cells, anionic phospholipids such as phosphatidylserine are present only in the inner leaflet of the membrane bilayer.1 During platelet activation, phosphatidylserine moves from the inner to the outer leaflet of the membrane bilayer.2 The transbilayer movement of phosphatidylserine is responsible for platelet procoagulant activity by providing high-affinity binding sites for the assembly of the prothrombinase and tenase complex.3,4 Externalization of anionic phospholipids in platelets is accompanied by the release of phosphatidylserine-rich microvesicles.5,6 These microvesicles are procoagulant and account for the clot-promoting activity of the serum.7 More recently, in addition to their hemostatic role, platelet-derived microvesicles were shown to stimulate hematopoietic cells8 and to transfer platelet-specific receptors to the surface of other cells.9
Lactadherin, also known as milk fat globule–epidermal growth factor 8 (EGF-8), is a 45-kDa glycoprotein secreted by macrophages.10,11 Lactadherin contains EGF-like domains at the amino terminus and 2 C-domains at the carboxy terminus that share homology to the phosphatidylserine-binding domains of blood coagulation factors V and VIII.12,13 Lactadherin binds to apoptotic cells, activated platelets, and phosphatidylserine-expressing red blood cells via the C-domains and anchors them to macrophage integrins via its RGD sequence in the EGF domain.14-17 We have examined the role of lactadherin in the clearance of phosphatidylserine-rich platelet-derived microvesicles.
Methods
Reagents
Lactadherin was isolated from fresh unhomogenized milk and labeled with fluorescein isothiocyanate (FITC) as described previously.18 Annexin A5 was isolated as described previously.19 The carboxy-terminal fragment of human lactadherin (C1C2 fragment) was amplified from a lactadherin cDNA using primers 5′-TTGAATTCCAGTACGTGAGATTGTACCCCACG-3′ and 5′-TTTGCGGCCGCTAACAGCCCAGCAGCTCC-3′. The amplified fragments were digested with EcoR1 and Not1 and ligated to the bacterial expression vector pET-28. The recombinant fragment was isolated from Escherichia coli as described for bovine lactadherin fragment.20 Generation of monoclonal antibody to lactadherin was previously described.21 BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene)–maleimide was purchased from Invitrogen (Carlsbad, CA). Human thrombin and purified Russell viper venom were purchased from Haematologic Technologies (Essex Junction, VT). Collagen was purchased from Helena Laboratories (Beaumont, TX). Phycoerythrin (PE)–labeled monoclonal anti–human platelet glycoprotein Ib (anti-CD42b) was purchased from Beckman Coulter (Fullerton, CA). PE-labeled murine anti-CD42b antibody was obtained from eBioscience (San Diego, CA).
Mice
All animal protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The generation of lactadherin-deficient mice was described previously.22 The lactadherin−/− mice were rederived at Baylor College of Medicine in C57BL/6 mice and backcrossed 6 times to C57BL/6 background.
Flow cytometric analysis of lactadherin binding to platelets and platelet-derived microvesicles
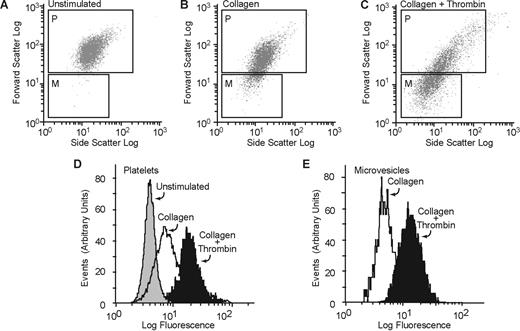
Washed platelets were prepared from healthy volunteers after informed consent was obtained in accordance with the Declaration of Helsinki and approved by the Committee for Protection of Human Subjects at Baylor College of Medicine, as described before.18 Platelets were resuspended in a modified Tyrode buffer18 containing 1% bovine serum albumin and 1 mM CaCl2. They were activated with collagen (50 μg/mL) or a combination of thrombin (0.1 U/mL) and collagen (50 μg/mL) for 20 minutes at room temperature. FITC-lactadherin (5 μg/mL) and a PE-labeled anti-CD42b (2.5 μg/mL) were added and incubated for 30 minutes at room temperature. Aliquots were then analyzed on a flow cytometer (Coulter FCC 500; Beckman Coulter) using the CXP software. The gates for microvesicles and intact platelets were set with the use of isolated microvesicles and unstimulated platelets, respectively. To differentiate platelets and platelet-derived microvesicles from background scatter, CD42b+ events were gated and analyzed for forward and side scattering. The light scatter and fluorescence channels were set at a logarithmic gain (Figure 1). Ten thousand events per sample were acquired to ensure adequate mean fluorescence levels.
Lactadherin binding to platelets and platelet-derived microvesicles. Washed human platelets were treated with (A) buffer only, (B) collagen (50 μg/mL), or (C) a combination of thrombin (0.1 U/mL) and collagen (50 μg/mL), and FITC-lactadherin (5 μg/mL) and a PE-labeled anti-CD42b (2.5 μg/mL) were added. The generation of microvesicles was analyzed by flow cytometry as described before.6 To resolve the platelets and platelet-derived microparticles from background scatter, only CD42b+ events were analyzed for forward and side scattering. The gates for microvesicles (gate M) and intact platelets (gate P) were set with the use of isolated microvesicles and unstimulated platelets, respectively. Platelets and microvesicles were analyzed separately for the expression of phosphatidylserine by 5 μg/mL FITC-lactadherin (D,E).
Lactadherin binding to platelets and platelet-derived microvesicles. Washed human platelets were treated with (A) buffer only, (B) collagen (50 μg/mL), or (C) a combination of thrombin (0.1 U/mL) and collagen (50 μg/mL), and FITC-lactadherin (5 μg/mL) and a PE-labeled anti-CD42b (2.5 μg/mL) were added. The generation of microvesicles was analyzed by flow cytometry as described before.6 To resolve the platelets and platelet-derived microparticles from background scatter, only CD42b+ events were analyzed for forward and side scattering. The gates for microvesicles (gate M) and intact platelets (gate P) were set with the use of isolated microvesicles and unstimulated platelets, respectively. Platelets and microvesicles were analyzed separately for the expression of phosphatidylserine by 5 μg/mL FITC-lactadherin (D,E).
Detection of platelet microvesicle-associated lactadherin in normal human plasma
Blood was drawn through 19-gauge needles into polypropylene syringes containing a one-tenth volume of 3.8% trisodium citrate, pH 6.5. The blood was immediately transferred to polypropylene tubes, and the platelet-rich plasma (PRP) was obtained by centrifugation at 1000g for 3 minutes at room temperature. The PRP was centrifuged at 5000g for 15 minutes at 4°C to obtain platelet-poor plasma (PPP). The PPP was further centrifuged at 20 000g for 20 minutes to sediment the microvesicles. The pellet containing microvesicles was suspended in the modified Tyrode buffer for further analysis. The microvesicles were incubated with a PE-labeled anti-CD42b (2.5 μg/mL) and FITC-anti–lactadherin antibody, L688 (5 μg/mL) for 30 minutes at room temperature for flow cytometry.
Detection of microvesicle-bound lactadherin
An aliquot (100 μg in 100 μL) of rabbit polyclonal anti-IIb/IIIa antibody23 or irrelevant rabbit control antibody was incubated over-night at 4°C with 100 μL Protein A+G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) and washed 3 times (10 000g for 2 minutes) in HEPES-buffered saline (HBS). The washed beads were incubated overnight at 4°C with 5 mL human PPP, centrifuged, and washed 3 times in HBS. Bound proteins were eluted in 50 μL 1% SDS and centrifuged and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10%), transferred to a PVDF membrane. The blot was incubated with a monoclonal antibody to lactadherin, L688,17 and developed with a peroxidase-labeled goat anti–mouse antibody (Thermo Scientific, Rockford, IL) using 4-chloro-1-naphthol (0.3 mg/mL) and H2O2 (0.03%).
Isolation and labeling of microvesicles
Washed human platelets were incubated with a combination of thrombin (0.1 U/mL) and collagen (50 μg/mL) for 30 minutes and centrifuged at 2000g for 15 minutes. The supernatant plasma, which is platelet-free and microvesicle-rich (as determined by flow cytometry), was further centrifuged at 20 000g for 20 minutes. The pellet containing microvesicles was resuspended in HBS and incubated with BODIPY-maleimide (2.5 μM) for 20 minutes and washed twice by centrifugation at 20 000g for 20 minutes in HBS.
Peripheral blood mononuclear cells were isolated as described previously,24 and an aliquot of mononuclear cells (108/mL) was stimulated with calcium ionophore A23187 (10 μM) for 30 minutes at 37°C with gentle stirring. The cell suspension was centrifuged at 5000g for 15 minutes, the pellet was discarded, and the microvesicles were isolated by centrifugation at 20 000g for 20 minutes and labeled with BODIPY-maleimide as described for platelet-derived microvesicles. Human umbilical vein endothelial cells were detached with trypsin-EDTA, washed in serum-free tissue- culture medium (RPMI 1640), and stimulated with calcium ionophore A23187 (10 μM) for 30 minutes at 37°C to release microvesicles. The microvesicles were isolated by centrifugation and labeled as described earlier for human platelet-derived microvesicles.
To isolate microvesicles from mouse platelets, blood was drawn in EDTA (5 mM final concentration) from 4-month-old mice under isoflurane anesthesia from the inferior vena cava and diluted with an equal volume of HBS. PRP was obtained by centrifugation at 260g for 10 minutes. Prostaglandin E1 (1 μM) was added, and platelets were sedimented by centrifugation at 1000g for 10 minutes and washed twice in modified Tyrode buffer as described for human platelets. Platelet suspension was stimulated with calcium ionophore A23187 (10 μM) containing 1.5 mM CaCl2 at 37°C for 1 hour. The suspension was centrifuged at 5000g for 10 minutes, and the pellet was discarded. The microvesicles were labeled with BODIPY-maleimide as described earlier. Splenic macrophages were isolated as described before.24
Quantitation of microvesicles from mouse blood
Microvesicles in mouse plasma were quantified as described before with some modification.25 Briefly, mouse PRP was centrifuged at 5000g for 10 minutes at room temperature, and the PPP (250 μL) was diluted to 1 mL by adding 750 μL HBS and incubated with PE-labeled anti–mouse CD42b and analyzed by flow cytometry. The microvesicles were gated as described before for human microvesicles. The flow rate was kept at 10 μL/minute and was run for exactly 3 minutes. On the basis of the dilution, rate, and the time of flow, we calculated the number of microvesicles per microliter of plasma.
Phagocytosis assay
Phagocytosis of platelet-derived microvesicles was quantified by the method described by Hoffmeister et al26 with some modifications. THP-1 cells (ATCC, Manassas, VA) were grown in tissue culture medium (RPMI 1640 containing 10% fetal bovine serum). The cells were washed in serum-free medium, and phorbol 12-myristate 13-acetate (150 ng/mL) was added and plated on 12-well tissue-culture dish (106 cells/well) coated with 2% poly 2-hydroxyethyl methacrylate and incubated for 15 minutes at 37°C. BODIPY-labeled microvesicles (∼80 μg) were added to the cells and incubated at 37°C for 30 minutes in the presence of various concentrations of lactadherin. THP-1 cells were incubated with 0.05% trypsin-EDTA to promote detachment and to remove any surface-bound platelet-derived microvesicles. The trypsin was neutralized with fetal calf serum, and the cells were washed twice in serum-free medium and incubated with a PE-labeled anti-CD11b monoclonal antibody (5 μg/mL). The THP-1 cells were gated based on PE fluorescence and forward light scatter and were analyzed for BODIPY fluorescence (green). The results were presented as the percentage of green (BODIPY) fluorescence-positive macrophages. The basal level of phagocytosis, in absence of lactadherin, varied from 10% to 25%.
Thrombin generation assay
Blood samples were collected from mice after anesthesia with a “clean” puncture of the inferior vena cava one-tenth volume of 3.8% trisodium citrate, pH 6.5. Blood samples from 5 mice from each group (lactadherin−/− and littermate controls [lactadherin+/+]) were pooled, and PRP was obtained by centrifugation at 260g for 10 minutes. PPP was obtained from PRP by centrifuging the blood at 5000g for 10 minutes. Microvesicle-free plasma was obtained by further centrifuging the plasma at 20 000g for 20 minutes. Thrombin generation in plasma was measured as described before.27 Briefly, each plasma sample (80 μL) was combined with trigger solution (40 μL) consisting of purified Russell viper venom (3 ng), fluorogenic thrombin substrate Z-Gly-Gly-Arg-AMC (2.5 mM), and CaCl2 (10 mM). The generation of thrombin was measured as a function of fluorescence measured on a fluorimeter over 30 minutes. Thrombin was then quantified by the method described by Hemker et al27 with the use of an α2-macroglobulin thrombin complex as standard.
Splenectomy in mice
Mice (4 months old) were anesthetized with an inhalation of isoflurane for the duration of the surgery. After a midline laparotomy, the small bowel was moved to the right of the abdominal cavity and covered with sterile saline. The spleen was approached by dissection, and the splenic blood vessels were tied with Vicryl sutures, and the spleen was removed. The laparotomy was closed in 2 layers with sutures. Four hours later, blood sample was obtained from the inferior vena cava as described earlier.
Mouse model of thrombosis
The in vivo thrombosis was assessed in a mouse model of light/dye-induced endothelial injury as described previously.28 Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg), with additional doses (12.5 mg/kg) as needed. The mice were then placed on a custom Plexiglas tray and maintained at 37°C with a homeothermic blanket and a rectal temperature probe (FHC, Bowdoinham, ME). A tracheotomy was performed to facilitate breathing, and an internal jugular vein and common carotid artery were cannulated for intravenous drug administration and blood pressure/heart rate monitoring, respectively. The cremaster microvascular bed was prepared as described28 and was superfused continuously with a pH-equilibrated (pH range, 7.35-7.45) bicarbonate-buffered saline solution at 35°C. The preparation was then transferred to the stage of an upright intravital videomicroscope (BX-50; Olympus, Tokyo, Japan) and allowed to equilibrate for 30 minutes. A 4× objective (NA 0.13) lens was used to survey the vascular bed, and a 40× water-immersion objective (NA 0.8) was used to monitor thrombosis kinetics. After a 30-minute equilibration period on the microscope stage, 10 mL/kg 5% FITC-labeled dextran (150 kD) was injected intravenously through the jugular vein. A suitable venule with brisk flow and minimal leukocyte adhesion was selected. After measuring the diameter and blood flow velocity of the vessel (Doppler velocimeter; Microcirculation Research Institute, College Station, TX), a photochemical injury was started by exposing approximately 100 μm of the vessel to filtered excitation light at 0.6 W/cm2 (from a 175W xenon lamp; Sutter Instrument, Novato, CA; and an HQ-FITC filter cube; Chroma Technology, Brattleboro, VT).28,29 Epi-illumination was applied continuously, and the time of onset of platelet aggregates (thrombus onset) and the time of flow cessation (for at least 60 seconds) were recorded. Typically, thrombi were induced in 2 venules per animal, and the results for each animal were averaged.
Statistical analysis
All data are expressed as means and standard deviations of triplicate measurements except when indicated otherwise. Comparisons between individual groups were performed with the use of the Student t test with paired and unpaired samples. A probability value (P) of .05 or less was considered statistically significant.
Results
Phosphatidylserine expression on platelet microvesicles
Resting washed platelet suspensions had few microparticles (Figure 1A). After activation with collagen, or with a combination of collagen and thrombin, a distinct subpopulation of microvesicles was seen with different light-scattering characteristics (Figure 1A-C) as described before.6 The expression of phosphatidylserine was measured by FITC-lactadherin binding. FITC-lactadherin binding was increased both in platelets and in microparticle fractions after activation (Figure 1D,E).
Lactadherin is bound to circulating platelet-microvesicles
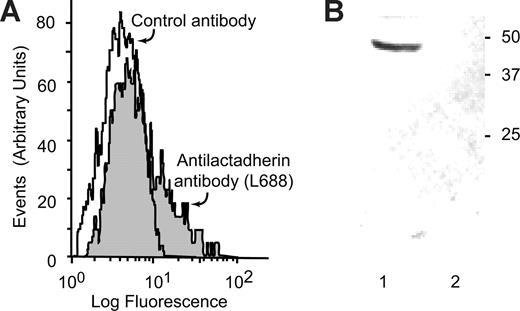
In normal plasma, platelet-derived microvesicles constitute a major fraction of circulating microvesicles. Because lactadherin is also present in normal plasma,30 the circulating platelet-derived microvesicles were examined for the presence of lactadherin. Lactadherin is present on the outer surface of platelet-derived microvesicles, as determined by flow cytometry, using a monoclonal antibody to lactadherin L688 (Figure 2A). Lactadherin was also shown in immunoblots of platelet microvesicles, isolated by immunoabsorption with agarose-bound anti–glycoprotein IIb/IIIa antibodies (Figure 2B).
Lactadherin is present in circulating platelet-derived microvesicles in normal human plasma. (A) Microvesicles, isolated from normal human plasma by centrifugation, were incubated with PE-labeled anti-CD42b (2.5 μg/mL) and FITC-antilactadherin antibody L688 (5 μg/mL) or an FITC-labeled irrelevant control antibody. The CD42b-expressing particles were gated and analyzed for FITC fluorescence. (B) Immunoblot of microvesicle-associated lactadherin. Protein A + G agarose beads (100 μL) were incubated overnight at 4°C with 100 μg rabbit polyclonal anti-IIb/IIIa antibody or an irrelevant rabbit control antibody. The beads were washed and incubated with 5 mL platelet-poor plasma and washed. Bound proteins were eluted in 50 μL 1% SDS, subjected to SDS-PAGE, transferred to PVDF membrane, and probed with the monoclonal antibody to lactadherin L68829 and developed by a peroxidase-labeled goat anti–mouse antibody (1/2000 dilution) and chloronaphthol (0.3 mg/mL) and H2O2 (0.03%). (Lane 1) Anti-IIb/IIIa anti-body and (lane 2) irrelevant control antibody. (Lane 1) Immunoprecipitation with anti-IIb/IIIa and (lane 2) irrelevant control antibody.
Lactadherin is present in circulating platelet-derived microvesicles in normal human plasma. (A) Microvesicles, isolated from normal human plasma by centrifugation, were incubated with PE-labeled anti-CD42b (2.5 μg/mL) and FITC-antilactadherin antibody L688 (5 μg/mL) or an FITC-labeled irrelevant control antibody. The CD42b-expressing particles were gated and analyzed for FITC fluorescence. (B) Immunoblot of microvesicle-associated lactadherin. Protein A + G agarose beads (100 μL) were incubated overnight at 4°C with 100 μg rabbit polyclonal anti-IIb/IIIa antibody or an irrelevant rabbit control antibody. The beads were washed and incubated with 5 mL platelet-poor plasma and washed. Bound proteins were eluted in 50 μL 1% SDS, subjected to SDS-PAGE, transferred to PVDF membrane, and probed with the monoclonal antibody to lactadherin L68829 and developed by a peroxidase-labeled goat anti–mouse antibody (1/2000 dilution) and chloronaphthol (0.3 mg/mL) and H2O2 (0.03%). (Lane 1) Anti-IIb/IIIa anti-body and (lane 2) irrelevant control antibody. (Lane 1) Immunoprecipitation with anti-IIb/IIIa and (lane 2) irrelevant control antibody.
Lactadherin promotes the phagocytosis of microvesicles by macrophages
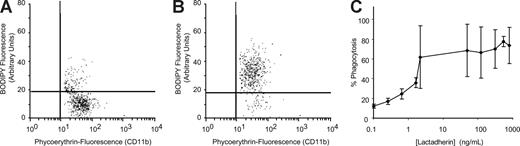
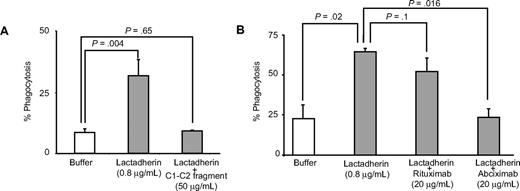
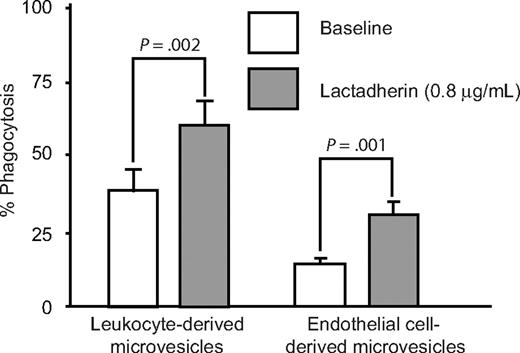
Differentiated human monocytic THP-1 cells have been used as an in vitro model for phagocytic clearance.26 When THP-1–derived macrophages were incubated with BODIPY-labeled platelet-derived microvesicles, approximately 10% to 25% of macrophages had phagocytosed platelet-derived microvesicles (Figure 3A). Lactadherin promoted phagocytosis in a concentration-dependent manner with half-maximal effect at approximately 5 ng/mL (Figure 3B,C). The carboxy-terminal fragment of lactadherin, which has only the phosphatidylserine binding domain (but not the integrin binding domain), inhibited lactadherin-dependent phagocytosis of platelet-derived microvesicles (Figure 4A). Because lactadherin-dependent phagocytosis is mediated by integrins,10 we examined the effect of abciximab, a human-mouse hybrid Fab fragment that reacts with integrin β3. Abciximab inhibited lactadherin-dependent phagocytosis to approximately 50%, whereas under similar conditions an irrelevant control antibody had no effect (Figure 4B). The stimulatory effect of lactadherin was also seen for the phagocytosis of leukocyte and endothelial cell–derived microvesicles (Figure 5).
Phagocytosis of platelet-derived microvesicles by macrophages. BODIPY-maleimide–labeled human platelet-derived microvesicles (80 μg) were incubated with THP-1–derived macrophages (106 cells/well) for 30 minutes. The nonadherent and the surface-bound microvesicles were detached with trypsin-EDTA solution. The macrophages were washed and analyzed by flow cytometry. The macrophages were identified by PE-labeled CD11b and gated. Phagocytosis was quantified by measuring the percentage of BODIPY (green) fluorescence-positive macrophages. (A) Phagocytosis in the absence of lactadherin. (B) Phagocytosis in the presence of lactadherin (0.8 μg/mL). (C) Lactadherin concentration-dependent phagocytosis of microvesicles. Each point represents the mean and SD of 3 or more separate experiments.
Phagocytosis of platelet-derived microvesicles by macrophages. BODIPY-maleimide–labeled human platelet-derived microvesicles (80 μg) were incubated with THP-1–derived macrophages (106 cells/well) for 30 minutes. The nonadherent and the surface-bound microvesicles were detached with trypsin-EDTA solution. The macrophages were washed and analyzed by flow cytometry. The macrophages were identified by PE-labeled CD11b and gated. Phagocytosis was quantified by measuring the percentage of BODIPY (green) fluorescence-positive macrophages. (A) Phagocytosis in the absence of lactadherin. (B) Phagocytosis in the presence of lactadherin (0.8 μg/mL). (C) Lactadherin concentration-dependent phagocytosis of microvesicles. Each point represents the mean and SD of 3 or more separate experiments.
Inhibition of lactadherin-dependent phagocytosis of platelet-derived microvesicles. (A) THP-1–derived macrophages were incubated with BODIPY-labeled human platelet-derived microvesicles and lactadherin in the presence of C1C2 fragment (A) or abciximab (B). The extent of phagocytosis was measured as described in Figure 3. The results are the means and SDs of triplicate measurements.
Inhibition of lactadherin-dependent phagocytosis of platelet-derived microvesicles. (A) THP-1–derived macrophages were incubated with BODIPY-labeled human platelet-derived microvesicles and lactadherin in the presence of C1C2 fragment (A) or abciximab (B). The extent of phagocytosis was measured as described in Figure 3. The results are the means and SDs of triplicate measurements.
Effect of lactadherin on the phagocytosis of leukocytes and endothelial cell–derived microvesicles. BODIPY-maleimide–labeled microvesicles from peripheral blood leukocytes or human umbilical vein endothelial cells were incubated with THP-1–derived macrophages (106 cells/well) for 30 minutes with or without lactadherin (0.8 μg/mL), and the extent of phagocytosis was quantified by flow cytometry by measuring the percentage of green fluorescence (BODIPY)–positive macrophages as in Figure 3.
Effect of lactadherin on the phagocytosis of leukocytes and endothelial cell–derived microvesicles. BODIPY-maleimide–labeled microvesicles from peripheral blood leukocytes or human umbilical vein endothelial cells were incubated with THP-1–derived macrophages (106 cells/well) for 30 minutes with or without lactadherin (0.8 μg/mL), and the extent of phagocytosis was quantified by flow cytometry by measuring the percentage of green fluorescence (BODIPY)–positive macrophages as in Figure 3.
Lactadherin-deficient mice have increased microvesicles in the circulation
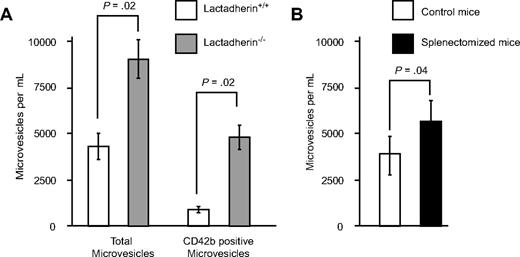
To determine whether the in vitro findings have an in vivo significance, we quantified the microvesicles in lactadherin-deficient mice and the wild-type littermates by flow cytometry. Littermate control mice had on, an average, 4257 (± 700) microvesicles/μL of blood (Figure 6). In contrast, lactadherin-deficient mice had 8940 (± 1035) microvesicles/μL (P = .02; Figure 6). The platelet-derived microvesicles (as determined by CD42b expression) were increased 5-fold in the lactadherin-deficient mice compared with littermate controls (950 ± 165 vs 4760 ± 650; P = .02). We posit that the difference is due to the impaired clearance of phosphatidylserine-expressing microvesicles.
Circulating microvesicles in mouse blood. (A) Plasma was collected from lactadherin-deficient mice and their littermate controls (N = 3 for each group). The microvesicles were quantified by flow cytometry, based on the light scatter and surface expression of CD42b (n = 3). (B) Wild-type mice were subjected to splenectomy, and the circulating microvesicles were measured 4 hours later. The results are the means and SDs (N = 3).
Circulating microvesicles in mouse blood. (A) Plasma was collected from lactadherin-deficient mice and their littermate controls (N = 3 for each group). The microvesicles were quantified by flow cytometry, based on the light scatter and surface expression of CD42b (n = 3). (B) Wild-type mice were subjected to splenectomy, and the circulating microvesicles were measured 4 hours later. The results are the means and SDs (N = 3).
Lactadherin-deficient mice have defective phagocytosis of phosphatidylserine-expressing microvesicles
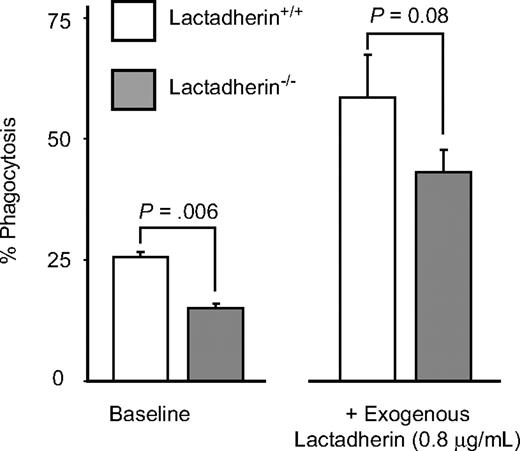
We further studied the phagocytosis of platelet-derived microvesicles by the splenic macrophages isolated from lactadherin-deficient mice and their littermate controls. The splenic macrophages from lactadherin-deficient mice had decreased phagocytosis of phosphatidylserine-expressing microvesicles compared with the wild-type littermate control macrophages (Figure 7; P = .006; n = 3). Addition of exogenous lactadherin to lactadherin-deficient macrophages corrected the defect in phagocytosis (Figure 7).
Phagocytosis of platelet-derived microvesicles by splenic macrophages in mice. Splenic macrophages were isolated from lactadherin-deficient mice and their wild-type littermate controls and incubated with BODIPY-labeled mouse platelet-derived microvesicles in the absence or presence of exogenous lactadherin. Phagocytosis of microvesicles was quantified as described before. The results are the means and SDs of triplicate measurements.
Phagocytosis of platelet-derived microvesicles by splenic macrophages in mice. Splenic macrophages were isolated from lactadherin-deficient mice and their wild-type littermate controls and incubated with BODIPY-labeled mouse platelet-derived microvesicles in the absence or presence of exogenous lactadherin. Phagocytosis of microvesicles was quantified as described before. The results are the means and SDs of triplicate measurements.
Lactadherin-deficient mice generate significantly more thrombin compared with wild-type littermate controls
To determine whether the increased presence of microvesicles in the plasma of lactadherin-deficient mice is associated with increased procoagulant activity, we measured thrombin generation in their plasma. Plasma from lactadherin-deficient mice generated twice as much thrombin as did their wild-type littermate controls (41.5 nM vs 20.4 nM in 30 min) under similar conditions (Figure 8). The difference between the lactadherin-deficient mice and their wild-type littermates was not present in the supernatant plasma, from which microvesicles had been removed by ultracentrifugation.
Thrombin generation in the plasma of lactadherin-deficient mice and control littermates. The reaction mixture consisted of plasma (80 μL) and the trigger solution (3 ng purified Russell viper venom in 40 μL), fluorogenic thrombin substrate Z-Gly-Gly-Arg-AMC (2.5 mM), and CaCl2 (10 mM). The generation of thrombin was measured as a function of fluorescence in a fluorimeter over a period of 30 minutes. Thrombin was then calculated using an α2-macroglobulin thrombin complex standard as described by Hemker et al.27 Representative data are presented. Blood samples from 5 mice from each group (lactadherin−/− and littermate controls [lactadherin+/+]) were pooled for each experiment.
Thrombin generation in the plasma of lactadherin-deficient mice and control littermates. The reaction mixture consisted of plasma (80 μL) and the trigger solution (3 ng purified Russell viper venom in 40 μL), fluorogenic thrombin substrate Z-Gly-Gly-Arg-AMC (2.5 mM), and CaCl2 (10 mM). The generation of thrombin was measured as a function of fluorescence in a fluorimeter over a period of 30 minutes. Thrombin was then calculated using an α2-macroglobulin thrombin complex standard as described by Hemker et al.27 Representative data are presented. Blood samples from 5 mice from each group (lactadherin−/− and littermate controls [lactadherin+/+]) were pooled for each experiment.
Hypercoagulable state in lactadherin-deficient mice
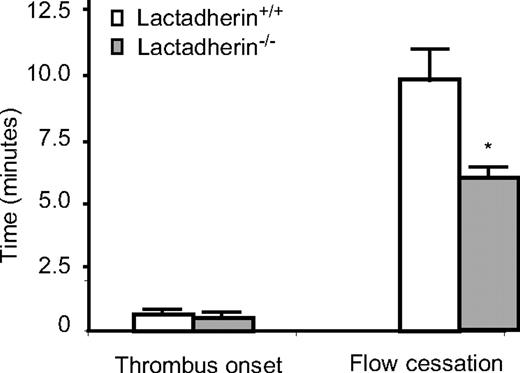
Because microvesicles are increased in lactadherin-deficient mice, and plasma from lactadherin-deficient mice generates more thrombin, we tested in vivo thrombus formation in a light/dye-induced endothelial injury/thrombosis model in cremasteric venules. No significant difference was observed in the initiation time for thrombus formation (0.53 ± 0.07 min vs 0.65 ± 0.17 min; P = .46; n = 9 in each group) between lactadherin-deficient mice and their wild-type littermate controls (lactadherin+/+). However, in lactadherin-deficient mice the time for occlusion was significantly shorter (5.93 ± 0.43 min vs 9.80 ± 1.14 min; P = .01; n = 9 in each group; Figure 9). Microvascular diameters and wall shear rates did not differ between lactadherin-deficient mice and littermate controls (44.3 ± 1.0 μm vs 44.8 ± 1.3 μm, and 513.7 ± 29.8 sec−1 vs 550.6 ± 54.0 sec−1, respectively). Similarly, heart rate and blood pressure did not differ sta-tistically between lactadherin-deficient mice and controls (440.6 ± 24.4 beats/min vs 453.3 ± 15.1 beats/min, and 76.8 ± 4.0 mm Hg vs 68.2 ± 2.2 mm Hg, respectively).
Thrombus formation in lactadherin-deficient mice. Endothelial injury was induced by light/dye in vivo in the cremasteric venules. Thrombus onset and flow cessation were monitored by intravital microscopy in lactadherin-deficient mice ( ) and the littermate controls (□). *P = .01; n = 9.
) and the littermate controls (□). *P = .01; n = 9.
Thrombus formation in lactadherin-deficient mice. Endothelial injury was induced by light/dye in vivo in the cremasteric venules. Thrombus onset and flow cessation were monitored by intravital microscopy in lactadherin-deficient mice ( ) and the littermate controls (□). *P = .01; n = 9.
) and the littermate controls (□). *P = .01; n = 9.
Discussion
The clearance of apoptotic cells by phagocytes is an extremely efficient process. However, even in tissues that are known to contain a large fraction of cells undergoing apoptosis (such as the bone marrow), it is difficult to detect apoptosis by traditional methods, because the apoptotic cells are engulfed rapidly by macrophages.31 The exposure of phosphatidylserine that occurs during apoptosis is the best-studied macrophage recognition signal, and several receptors have been identified in macrophages that mediate apoptotic cell clearance by binding to phosphatidylserine on apoptotic cells directly or indirectly.32
Platelets lack the machinery to undergo the type of programmed cell death of nucleated cells. However, they appear to have a similar pathway of clearance when activated, involving exposure of phosphatidylserine. Phosphatidylserine is normally located on the cytoplasmic face of the resting platelet membrane. However, after platelet activation with certain agonists that induce sustained increase in intraplatelet calcium such as collagen and shear stress, phosphatidylserine appears on the plasma-oriented surface.2,4 Externalization of phosphatidylserine in platelet is accompanied by the release of phosphatidylserine-rich microvesicles that are derived from the surface blebs of activated platelets.6 These microvesicles, initially described as “platelet dusts,” are responsible for the clot-promoting activity of plasma.7 In addition to providing an efficient catalytic surface for prothrombin and factor X activation, microvesicles may have additional hemostatic effects. Microvesicles localize to the subendothelium after vessel wall injury,33 providing sites for adhesion of platelets and neutrophils.34 They also improve hemostasis in hemophilic mice.35 Microvesiculation is defective in Scott syndrome, an inherited bleeding disorder with impaired platelet procoagulant activity resulting from the failure to externalize phosphatidylserine after platelet activation.36
Our results show that lactadherin, a macrophage opsonin that mediates the clearance of apoptotic lymphocytes, is one of the mediators of the clearance of phosphatidylserine-expressing procoagulant platelet-derived microvesicles. Platelet-derived microvesicles have a short half-life of less than 5 minutes37 and must be continuously formed. In an enzyme-linked immunoabsorbent assay assay, we detected approximately 19 plus or minus 1 ng/mL in normal plasma. After centrifugation at 20 000g for 20 minutes, the level decreased to 10 plus or minus 4 ng/mL, suggesting at least 25% of the plasma lactadherin is bound to microvesicles. Lactadherin-deficient mice have increased concentration of microvesicles at steady state, presumably because of their defective clearance, leading to a procoagulant state. The defective phagocytosis by the splenic macrophages from the lactadherin-deficient mice is corrected by exogenous lactadherin. Furthermore, complement-dependent phagocytosis of zymosan-coated fluorescent particles is normal in lactadherin-deficient mice,24 indicating no global defect in macrophage function.
Lactadherin competes efficiently with factor V and factor VIII for binding sites on phosphatidylserine with a half-maximal effect at 1 to 4 nM.38 The procoagulant state seen in lactadherin deficiency could also be due to the absence of natural anticoagulant function of lactadherin. However, increased thrombin is not apparent in microvesicle-poor plasma, suggesting that microvesicles are responsible for the increased thrombin generation. The clearance of phosphatidylserine-containing apoptotic cell surfaces is a fundamental process in development and tissue repair. Therefore, it is not surprising that multiple redundant receptors are involved in their clearance.31 These receptors are also probably involved in the clearance of platelet-derived microvesicles.
Plasma levels of platelet-derived microvesicles are increased in cancer-associated deep vein thrombosis,39 antiphospholipid antibody syndrome,40 disseminated intravascular coagulation, heparin-induced thrombocytopenia,41 and thrombotic thrombocytopenic purpura,42 all conditions associated with either arterial and venous thrombosis. The procoagulant microvesicles may have a role in the hypercoagulable state in these conditions. Furthermore, defective clearance of apoptotic cell surfaces is a prominent pathogenetic mechanism in human and murine lupus, and the defective clearance of these vesicles may also play a role in the hypercoagulable state seen in these disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Veterans Affairs Research Service (Washington, DC), the Gulf Coast Regional Blood Center (Houston, TX), and the National Institutes of Health (Bethesda, MD; HL-079368).
National Institutes of Health
Authorship
Contribution: R.E.R. and P.T. designed the experiments; P.N. performed the thrombin generation assay; A.L. cultured the cells and generated the antibodies; R.V.B. and K.L. performed the mouse thrombosis assay; S.N. provided the lactadherin-deficient mice; and S.K.D. and H.A.-M. performed the protein isolation and phagocytosis assay.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Perumal Thiagarajan, Michael E. DeBakey VA Medical Center, Mail Stop #113, 2002 Holcombe Boulevard, Houston, TX 77030; e-mail perumalt@bcm.tmc.edu.
References
Author notes
*S.K.D. and H.A.-M. contributed equally to this study.

![Figure 8. Thrombin generation in the plasma of lactadherin-deficient mice and control littermates. The reaction mixture consisted of plasma (80 μL) and the trigger solution (3 ng purified Russell viper venom in 40 μL), fluorogenic thrombin substrate Z-Gly-Gly-Arg-AMC (2.5 mM), and CaCl2 (10 mM). The generation of thrombin was measured as a function of fluorescence in a fluorimeter over a period of 30 minutes. Thrombin was then calculated using an α2-macroglobulin thrombin complex standard as described by Hemker et al.27 Representative data are presented. Blood samples from 5 mice from each group (lactadherin−/− and littermate controls [lactadherin+/+]) were pooled for each experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/6/10.1182_blood-2008-07-167148/4/m_zh80060930840008.jpeg?Expires=1767793222&Signature=eL-04fRLdFGtdrcimf7NF8s-VS1IgUmg23WZujcFmPnveGtq2Dt5bM3qodSbcgQBkhj6ehf-8MoxNpLLswb6L-mpVlc6N0je7aYh9igcEoP0Y99yXsQDXiXwpLOhbPYn2vQTk3heRF1YMmpa-Qh4KGXgJ3UwAIiHLYBXCz86Rayt3uy0yrCkdauPETOtfoE0KAaze4Y4xk79n-ypwBcwezszARMk49rNw2bT4daVVblhAKRJg1Tz9ZxzoQBAtGgoVOqLlEer-GltHgHWwIw5POowbq~BfiJLBjv2MZpqX9JrJ4UlVwiEysd-nIcmgXaqYWehGwh1MK7-zpFqoK7E0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal