Abstract
Professional antigen-presenting cells (APCs) are sentinel cells of the immune system that present antigen to T lymphocytes and mediate an appropriate immune response. It is therefore surprising that knowledge of the professional APCs in human lymph nodes is limited. Using 3-color immunohistochemistry, we have identified APCs in human lymph nodes, excluding plasmacytoid APCs, that fall into 2 nonoverlapping classes: (1) CD209+ APCs, coexpressing combinations of CD206, CD14, and CD68, that occupied the medullary cords, lined the capsule and trabeculae and were also scattered throughout the diffuse T-lymphocyte areas of the paracortex; and (2) APCs expressing combinations of CD1a, CD207, and CD208, that were always restricted to the paracortex. Surprisingly, this second class of APCs was almost entirely absent from many lymph nodes. Our data suggest that most CD208+ cells, often referred to as “interdigitating cells,” derive from migratory APCs, and that the major APC subset consistently resident in the paracortex of human lymph nodes is the CD209+ subset. All APC subsets were demonstrated to be in close contact with the fibroreticular network. The identification of 2 distinct APC populations in the paracortex of human lymph nodes has important implications for understanding T-lymphocyte responses and optimizing vaccine design.
Introduction
Lymph nodes provide an interface between the blood and lymphatic systems, enabling antigen-presenting cells (APCs) that reside in the lymph node or have migrated there from peripheral tissues to present antigen to blood-derived T lymphocytes and initiate an immune response. It is therefore surprising that knowledge of APCs in the human lymph node, in particular those in the T lymphocyte–rich paracortex, is limited.1 Identifying the APC populations in human lymph nodes will provide a better understanding of how immune responses are initiated and may also help improve vaccine design and delivery strategies
Studies using murine models have demonstrated that several populations of APCs are present in lymph nodes draining skin, including migratory dermal APCs and Langerhans cells, and residential APCs.2-4 In murine models, the precise role of Langerhans cells remains a matter of debate4-10 ; in contrast, APCs that have migrated from the murine dermis have been shown to be essential for the initiation of certain T-lymphocyte responses.2,11 We recently identified a human dermal APC subset capable of migrating in response to lymph node–homing chemokines and stimulating naive CD4 T lymphocytes12 ; furthermore, a recent study has shown that they also stimulate CD8 T lymphocytes.13 Given the pivotal role of dermal APCs for the generation of immune responses in mice, we sought to confirm that their putative human counterparts migrate to lymph nodes draining human skin.
We used 3-color immunohistochemistry to study frozen sections of human lymph nodes, with the initial aim of confirming whether CD1a+CD207− dermal-derived APCs colonized the paracortical T-lymphocyte zones. In the process of characterizing the APCs in the paracortex, we analyzed a range of APC markers, including pattern recognition receptors (CD14, CD206, CD207, CD209, and BDCA-2) and antigen presentation machinery (CD1a and CD1b) together with the lysosome-associated proteins CD68 and CD208.14,15 This approach was more comprehensive than previous studies based on nondiscriminatory adhesion molecules or maturation markers, such as costimulatory molecules,16,17 and immediately revealed striking differences in the distribution of APC subsets, both between different types of APCs and between lymph nodes taken from different sites. We also combined our APC markers with stains to visualize the fibroreticular network, to establish which APC subsets were intimately associated with this network. The fibroreticular system in murine lymph nodes has been of intense interest to APC researchers because afferent lymph flowing from subcapsular sinuses into the conduit network may be sampled by APCs using dendrites inserted into the conduit lumen.18,19 It has also been proposed that the fibroreticular network provides a structure on which T lymphocytes can crawl and encounter APCs that are sessile and in intimate contact with the conduit ducts.20 However, data are lacking concerning the association of APCs with fibroreticular structures in human lymph nodes.
Methods
Two-color immunofluorescence staining
We obtained lymph nodes that were as close as possible to normal lymph nodes from 3 living donors undergoing surgery and 3 donors postmortem (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Histology on all lymph nodes was reported as no abnormality detected, although the lymph nodes from patients undergoing surgery (for lymphadenopathy) showed mild reactive changes as described in Table S1. Lymph nodes were obtained from several different node fields, including the axillary (5 samples) and inguinal fields (1 sample), which commonly drain large areas of skin,21 as well as cervical (3 samples) and hilar (3 samples) fields. Written informed consent from patients or next of kin was obtained in accordance with the Declaration of Helsinki, under protocols approved by the Austin Health Human Research Ethics Committee, Heidelberg. Fresh skin samples were obtained from healthy patients undergoing plastic or reconstructive surgery, who gave written informed consent, under a protocol approved by the New Zealand Northern Regional Ethics Committee and the Clinical Board of the Counties-Manukau District Health Board.
Lymph nodes and skin were embedded in TissueTek OCT compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), snap-frozen in liquid nitrogen, and sectioned using a cryostat. Sections 5-μm thick were fixed with ice-cold acetone and blocked with serum-free protein block (Dako Denmark, Glostrup, Denmark). Fixed sections were probed with the following mouse monoclonal antibodies: podoplanin (clone18H5; Abcam, Cambridge, United Kingdom); CD1b (4.A7.6), CD207 (DCGM4), and CD208 (104.G4) (Beckman Coulter, Fullerton, CA); CD68 (Y1/82A), CD206 (19.2), CD209 (DCN46), and fibronectin (2B6-D4) (BD Biosciences, San Jose, CA); CD1a (NA1/34), CD14 (MEM18), CD14 (UCHM1), and CD21 (LB21) (Serotec, Oxford, United Kingdom); BDCA-2 (AC144; Miltenyi Biotec, Auburn, CA); LYVE-1 (264712; R&D Systems, Minneapolis, MN); rabbit polyclonal antibodies CD3 (Invitrogen, Carlsbad, CA) and collagen (Serotec); and goat polyclonal CD207-biotin (R&D Systems). The primary antibodies were detected with the corresponding isotype specific goat antimouse, goat antirabbit or goat anti–fluorescein isothiocyanate (FITC) secondary antibodies (Southern Biotechnology Associates, Birmingham, AL; Invitrogen), or ExtrAvidin (Sigma-Aldrich, St Louis, MO) conjugated to a fluorochrome (Alexa 488 or 555, FITC or tetramethylrhodamine isothiocyanate). The specificity of each secondary antibody was confirmed using an isotype or species-mismatched primary antibody. When 2 primary antibodies of the same isotype were applied to the same section, they were applied sequentially; after application of the first primary antibody and detecting isotype-specific secondary antibody, the section was blocked with 1% mouse IgG, and the second FITC-conjugated primary antibody was then applied and detected using anti–FITC-Alexa 488. 4,6-Diamidino-2-phenylindole (DAPI) was included at 0.0005% wt/vol with secondary antibody.
The slides were mounted using Vectashield (Vector Laboratories, Burlingame, CA). Sections were visualized with a Leica DMRE Fluorescent microscope equipped with the following epifluorescent filters: UV, 470 to 490 μm and 515 to 560 μm (Leica, Wetzlar, Germany). Images were acquired at room temperature using 5×/0.15 numerical aperture (NA), 10×/0.30 NA, 20×/0.50 NA, 40×/0.7 NA, 63×/1.4 NA, and 100×/1.3 NA Leica objectives, a Leica DC500 digital camera and analySIS FIVE software (Olympus, Tokyo, Japan). Images were processed using Portia image manipulation software (CytoCode, Auckland, New Zealand, www.cytocode.com) and figures were generated using Photoshop (Adobe Systems, Mountain View, CA).
Results
APCs expressing different phenotypic markers colonize distinct areas of human axillary lymph nodes
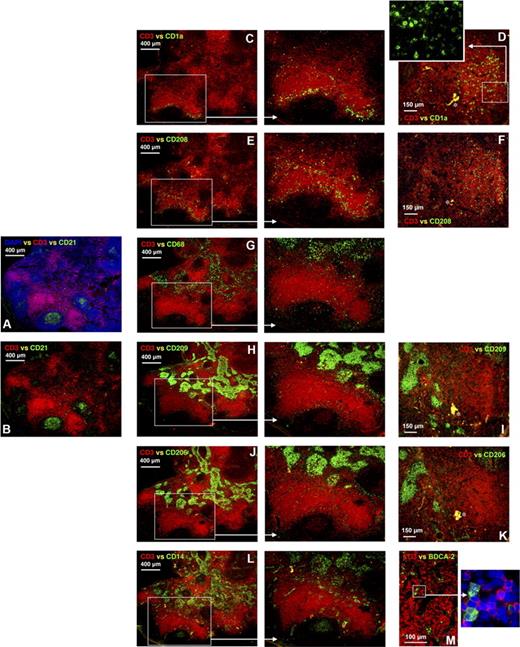
Five human axillary lymph nodes were stained with anti-CD3 to detect the T lymphocytes in the paracortex and anti-CD21 to locate the follicular APCs and mature B lymphocytes in the follicles (Figure 1A,B). The lymph node paracortex consists of 2 areas: one where T lymphocytes are densely packed and surrounding areas where they are more diffuse (Figure 1A–L). APCs expressing CD1a were exclusively located in the paracortex, and in all lymph nodes were found in both the dense and diffuse T lymphocytes areas. In one lymph node, a high proportion of the CD1a+ APCs was concentrated in the diffuse T-lymphocyte areas (Figure 1C), whereas in other lymph nodes the majority of CD1a+ APCs were found in the dense T-lymphocyte regions (Figure 1D). This variability in the location of CD1a+ APCs within the paracortex is summarized in Table S1 and did not correlate with clinical features. APCs expressing CD208 were detected in the same regions of the paracortex as CD1a+ APCs (Figure 1E,F). In contrast, most cells expressing CD68, CD209, CD206, and CD14 were located in the medullary cords encapsulating the medullary sinuses (Figure 1G–L). As described later in “Results,” most of this staining pattern could be attributed to APCs expressing all 4 markers.
APCs expressing different markers colonize distinct areas of the human axillary lymph node. Frozen lymph node sections were probed with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively, thus providing anatomical definition (A,B). Within the paracortex, T lymphocytes were densely packed in some regions and more diffuse in others (A,B). Immunohistochemistry illustrated that cells expressing CD1a were either concentrated in diffuse T-lymphocyte zones surrounding the dense T-lymphocyte regions (C) or evenly distributed throughout areas densely packed with T lymphocytes (D). Cells expressing low and high levels of CD1a were detected (D, inset). CD208+ cells colonized similar areas to cells expressing CD1a (E,F). Dense bodies of cells expressing CD68, CD209, CD206, and CD14 were located in the medullary cords (G-L). Less frequent cells expressing CD68, CD206, and CD209 were also detected in the diffuse T-lymphocyte regions surrounding the medullary cords and follicles (G-K). Cells expressing CD68 or CD14 were also detected in the follicles (G,L). Rare BDCA-2+ plasmacytoid APCs were detected in the paracortex (M). Panels A to C, E, G, H, J, and L were acquired from the same area on sequential sections as were panels D, F, I, and K. Blue represents DAPI staining of cell nuclei (A,M, inset). *Background autofluorescence. Data are representative of 5 independent experiments. BDCA-2+ APC data are representative of 3 independent experiments.
APCs expressing different markers colonize distinct areas of the human axillary lymph node. Frozen lymph node sections were probed with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively, thus providing anatomical definition (A,B). Within the paracortex, T lymphocytes were densely packed in some regions and more diffuse in others (A,B). Immunohistochemistry illustrated that cells expressing CD1a were either concentrated in diffuse T-lymphocyte zones surrounding the dense T-lymphocyte regions (C) or evenly distributed throughout areas densely packed with T lymphocytes (D). Cells expressing low and high levels of CD1a were detected (D, inset). CD208+ cells colonized similar areas to cells expressing CD1a (E,F). Dense bodies of cells expressing CD68, CD209, CD206, and CD14 were located in the medullary cords (G-L). Less frequent cells expressing CD68, CD206, and CD209 were also detected in the diffuse T-lymphocyte regions surrounding the medullary cords and follicles (G-K). Cells expressing CD68 or CD14 were also detected in the follicles (G,L). Rare BDCA-2+ plasmacytoid APCs were detected in the paracortex (M). Panels A to C, E, G, H, J, and L were acquired from the same area on sequential sections as were panels D, F, I, and K. Blue represents DAPI staining of cell nuclei (A,M, inset). *Background autofluorescence. Data are representative of 5 independent experiments. BDCA-2+ APC data are representative of 3 independent experiments.
Cells expressing CD14 were also detected in the germinal center of follicles (Figure 1L), consistent with earlier observations that follicular APCs express CD14.22 CD14 was also expressed in ring-like clusters primarily in the diffuse T-lymphocyte areas (Figure 1L), consistent with vascular structures. Cells expressing CD68, CD209, and CD206 also colonized the diffuse T-lymphocyte areas (Figure 1G–K), as described in more detail later in “Results.” A few CD68+ cells were also detected in the follicles (Figure 1G). Rare BDCA-2+ plasmacytoid APCs were detected in the paracortex (Figure 1M).
In summary, the distribution of single APC markers within human axillary lymph nodes immediately revealed a striking spatial separation between APC populations. APCs expressing CD1a or CD208 were restricted to the paracortex, whereas the other markers studied (CD14, CD68, CD206, CD209) were consistently expressed by APCs in the medulla and variably expressed by APCs scattered through the diffuse T-lymphocyte areas of the paracortex. CD14 was also expressed by vascular-like structures in the paracortex and also by cells in the B-lymphocyte follicles, probably follicular APCs. BDCA-2+ cells were rare and restricted to the paracortex.
APCs expressing combinations of CD1a, CD207, and CD208 are restricted to the paracortex of human axillary lymph nodes
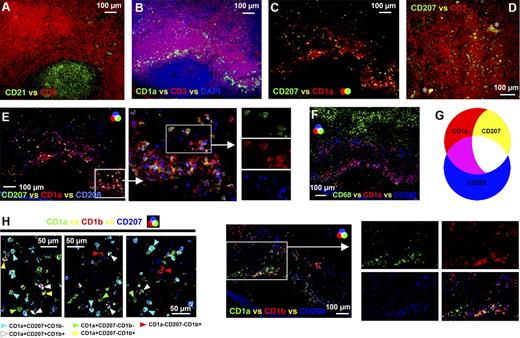
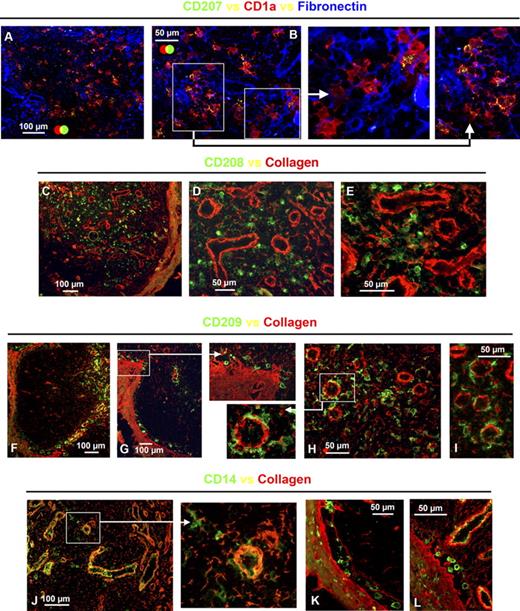
APCs expressing CD1a alone or together with CD207 (langerin) were detected in the paracortex of all axillary lymph nodes (Figure 2B–D). These cells have the same molecular phenotype as CD1a+CD207− dermal APCs and CD1a+CD207+ Langerhans cells, respectively.12 No CD1a−CD207+ cells were observed (Figure 2C). Across the 5 axillary lymph nodes examined, approximately 20% to 50% of CD1a+ cells expressed CD207. CD1a+CD207− APCs colocalized with and were often in intimate contact with CD1a+CD207+ APCs (Figure 2C,E). Depending on the region of the lymph node examined, these APCs were either clustered in the diffuse T-lymphocyte area (Figure 2B,C) or distributed throughout the dense T-lymphocyte area of the paracortex (Figures 1D, 2D). Consistent with earlier observations, the distribution of these APCs within the paracortex of each lymph node varied, in terms of the proportions of APCs colonizing the dense T-lymphocyte regions as opposed to the diffuse; again, these minor differences did not correlate with clinical features (Table S1). A proportion of both of these subsets expressed the maturation marker CD208/DC-LAMP (Figure 2E); approximately 40% to 80% of CD1a+CD207− APCs and 50% to 65% of CD1a+CD207+ APCs expressed CD208. In addition, a CD208+ subset lacking expression of CD1a and CD207 was detected in the same areas as APCs expressing CD1a or CD207 (Figure 2E). Approximately 30% to 50% of the CD208+ APCs lacked CD1a. None of the APC populations expressing CD1a or CD208 coexpressed CD68 (Figure 2F), and no CD1a+ cells coexpressed BDCA-2 (data not shown); hence, the CD68+ and few BDCA-2+ cells in the paracortex represent different cell types. Expression of CD1a, CD207, and CD208 can therefore be used to distinguish between 3 APC populations in the paracortex of the human axillary lymph node: CD1a+CD207−CD208+/−, CD1a+CD207+CD208+/−, and CD208+CD1a−CD207− APCs (Figure 2G; Table 1). Additional studies revealed that subsets of CD1a+CD207− and CD1a+CD207+ APCs (Figure 2H) and a large proportion of CD1a−CD208+ APCs (Figure 2I) expressed CD1b. Cells expressing CD1b but no CD208 or CD1a were rare (Figure 2I). Interestingly, although CD1a+CD207+ Langerhans cells in the human epidermis lacked CD1b, both CD1a+CD207− and the rare CD1a+CD207+ APCs in the dermis expressed CD1b (Figure S1A–D). Furthermore, a number of dermal CD207+ APCs were in intimate contact with LYVE-1+ or podoplanin+ lymphatic vessels (Figure S1F–H).
APC populations expressing a similar phenotype to cutaneous migratory APCs were detected in the paracortex of the human axillary lymph node. Frozen lymph node sections were probed with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively (A). Immunohistochemistry illustrated that a subset of CD1a+ APCs in the paracortex (B) coexpressed CD207 or Langarin (C). CD207+ APCs were either concentrated in the diffuse T-lymphocyte zones surrounding dense T-lymphocyte regions (B,C) or distributed throughout areas densely packed with T lymphocytes (D). Proportions of both CD1a+CD207− and CD1a+CD207+ APCs coexpressed CD208/DC-LAMP; however, not all CD208+ cells expressed CD1a or CD207 (E). APCs expressing CD1a or CD208 did not coexpress CD68 (F). Differing expression patterns of CD1a, CD207, and CD208 can therefore be used to distinguish between 3 APC populations in the paracortex: CD1a+CD207−CD208+/−, CD1a+CD207+CD208+/−, and CD208+CD1a−CD207− APCs (G). Subsets of CD1a+CD207− and CD1a+CD207+ APCs (H) and the majority of CD1a−CD208+ APCs (I) expressed the lipid presentation molecule CD1b. Cells expressing CD1b but no CD208 or CD1a were rare (I). Panels A to C, E, F, and I were acquired from the same area on sequential sections. The images shown in panel H were acquired from different fields. *Background autofluorescence. Data are representative of 5 independent experiments.
APC populations expressing a similar phenotype to cutaneous migratory APCs were detected in the paracortex of the human axillary lymph node. Frozen lymph node sections were probed with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively (A). Immunohistochemistry illustrated that a subset of CD1a+ APCs in the paracortex (B) coexpressed CD207 or Langarin (C). CD207+ APCs were either concentrated in the diffuse T-lymphocyte zones surrounding dense T-lymphocyte regions (B,C) or distributed throughout areas densely packed with T lymphocytes (D). Proportions of both CD1a+CD207− and CD1a+CD207+ APCs coexpressed CD208/DC-LAMP; however, not all CD208+ cells expressed CD1a or CD207 (E). APCs expressing CD1a or CD208 did not coexpress CD68 (F). Differing expression patterns of CD1a, CD207, and CD208 can therefore be used to distinguish between 3 APC populations in the paracortex: CD1a+CD207−CD208+/−, CD1a+CD207+CD208+/−, and CD208+CD1a−CD207− APCs (G). Subsets of CD1a+CD207− and CD1a+CD207+ APCs (H) and the majority of CD1a−CD208+ APCs (I) expressed the lipid presentation molecule CD1b. Cells expressing CD1b but no CD208 or CD1a were rare (I). Panels A to C, E, F, and I were acquired from the same area on sequential sections. The images shown in panel H were acquired from different fields. *Background autofluorescence. Data are representative of 5 independent experiments.
Comparison of the APC populations in the human axillary lymph nodes with the APC in human skin
| APC population . | CD1a . | CD1b . | CD14 . | CD68 . | CD206 . | CD207 . | CD208 . | CD209 . | BDCA2 . | References . |
|---|---|---|---|---|---|---|---|---|---|---|
| Lymph node APC populations | ||||||||||
| Migratory CD1a+CD207+ Langerhans cells | ++ | +/− | − | − | − | ++ | +/− | − | − | |
| Migratory CD1a+CD207− dermal APCs | ++ | +/− | − | − | − | − | +/− | − | − | |
| CD208+ interdigitating APCs | − | +/− | − | − | − | − | ++ | − | − | |
| CD209+ APCs | ||||||||||
| In diffuse T-lymphocyte zones of paracortex | − | − | +/− | ++ | +/− | − | − | + | − | |
| In medullary cords | − | − | ++ | ++ | ++ | − | − | ++ | − | |
| Subcapsular and trabecular | − | − | ++ | ++ | +/− | − | − | ++ | − | |
| Plasmacytoid APCs | − | − | − | − | − | − | − | − | ++ | |
| Cutaneous APC populations | ||||||||||
| Epidermal Langerhans cells | ++ | − | − | − | − | ++ | − | − | − | 12, 24 |
| Dermal CD207+ APCs | ++ | ++ | − | − | − | ++ | ++ | − | − | 26 |
| CD1a+ dermal APCs | ++ | ++ | − | − | − | − | − | − | − | 12, 26 |
| CD14+ dermal APCs | − | + | ++ | ++ | ++ | − | − | ++ | − | 12, 26 |
| APC population . | CD1a . | CD1b . | CD14 . | CD68 . | CD206 . | CD207 . | CD208 . | CD209 . | BDCA2 . | References . |
|---|---|---|---|---|---|---|---|---|---|---|
| Lymph node APC populations | ||||||||||
| Migratory CD1a+CD207+ Langerhans cells | ++ | +/− | − | − | − | ++ | +/− | − | − | |
| Migratory CD1a+CD207− dermal APCs | ++ | +/− | − | − | − | − | +/− | − | − | |
| CD208+ interdigitating APCs | − | +/− | − | − | − | − | ++ | − | − | |
| CD209+ APCs | ||||||||||
| In diffuse T-lymphocyte zones of paracortex | − | − | +/− | ++ | +/− | − | − | + | − | |
| In medullary cords | − | − | ++ | ++ | ++ | − | − | ++ | − | |
| Subcapsular and trabecular | − | − | ++ | ++ | +/− | − | − | ++ | − | |
| Plasmacytoid APCs | − | − | − | − | − | − | − | − | ++ | |
| Cutaneous APC populations | ||||||||||
| Epidermal Langerhans cells | ++ | − | − | − | − | ++ | − | − | − | 12, 24 |
| Dermal CD207+ APCs | ++ | ++ | − | − | − | ++ | ++ | − | − | 26 |
| CD1a+ dermal APCs | ++ | ++ | − | − | − | − | − | − | − | 12, 26 |
| CD14+ dermal APCs | − | + | ++ | ++ | ++ | − | − | ++ | − | 12, 26 |
++ indicates strong expression on all cells; +, weak expression on all cells; −, no expression on any cells; and +/−, expressed by some cells but not others.
CD1a+ APCs are not present in all human lymph nodes
To establish whether the APCs in the axillary lymph node are present in lymph nodes from other sites, lymph nodes from inguinal, cervical, and hilar lymph node fields were examined (Figure S2). APCs expressing CD1a alone or together with CD207, analogous to CD1a+CD207− dermal APCs and CD1a+CD207+ Langerhans cells, respectively, were only detected in the inguinal and one of the 3 hilar lymph nodes (Figure S2A,D). The remaining hilar and all cervical lymph nodes lacked APC populations expressing these phenotypes (Figure S2B,C). Furthermore, earlier studies have also noted the absence of APCs expressing CD1a in mesenteric lymph nodes draining the gastrointestinal tract.23 Importantly, APCs expressing CD208 showed a similar distribution pattern; substantial numbers of CD208+ APCs were only detected in the inguinal and the same hilar lymph node that contained APCs expressing CD1a and CD207 (Figure S2E,I). Very few, if any, CD208+ APCs were detected in the remaining hilar and cervical lymph nodes (Figure S2F–H). In contrast, CD209+ APCs were detected in all lymph nodes screened (Figure S2E–I). Similar to the distribution pattern in axillary lymph nodes, dense clusters of CD209+ APCs were detected in the medullary regions and sparsely scattered throughout the lymph node (Figure S2E–I). Hence, looking across the lymph nodes from different sites, there was a strong correlation between the numbers of CD208+ APCs detected and the presence of CD1a+ or CD207+ APCs. This was not the result of differing numbers of APCs in general because the number of CD209+ APCs did not vary appreciably between lymph nodes from different sites, either in the medulla or the paracortex. Furthermore, because a large area of paracortex was assessed for each lymph node, we can conclude with confidence that the paracortex-restricted APCs expressing CD1a, CD207, and CD208 were rare or absent in 2 of the hilar and all the cervical lymph nodes screened. The striking lack of CD1a+ and CD208+ APCs in some lymph nodes was not a postmortem artifact because lymph nodes replete with both these APC subsets were found in other fields within the same donors (Table S1).
CD209+ APCs line the capsule and trabeculae and also colonize the diffuse T-lymphocyte regions of the paracortex
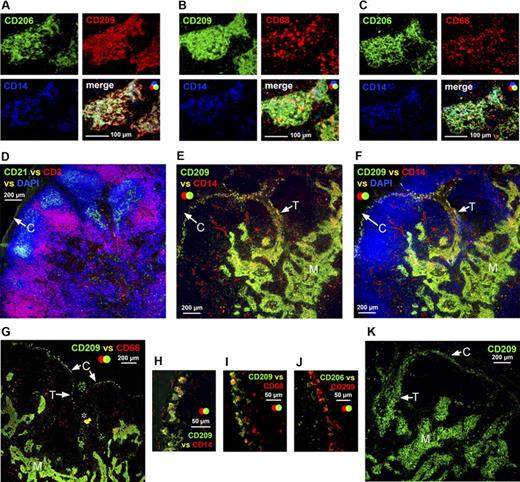
The majority of CD209+ APCs in the medullary cords expressed CD206, CD14, and perinuclear CD68 (Figure 3A–C). APCs expressing a similar phenotype also lined the capsule (Figure 3E–G); these APCs were located at the capsule's interface with both the follicles and diffuse T-lymphocyte interfollicular regions (Figure 3D–F). These subcapsular APCs also extended along trabeculae radiating into the lymph node (Figure 3E–G); in some samples, trabeculae appeared to merge with the medullary cords (Figure 3E,F). High-magnification images confirmed that subcapsular APCs expressed CD14 (Figure 3H), CD68 (Figure 3I), and CD209 (Figure 3H–J), although only a few expressed CD206 (Figure 4J). In one of the 5 axillary lymph node samples studied, a large number of CD209+ APCs were detected along the basement membrane of the capsule and trabeculae (Figure 3K).
APCs expressing a similar phenotype to the APCs in medullary cords lined the capsule and trabeculae in the human axillary lymph node. Immunohistochemistry demonstrated that the APCs packed into the medullary cords expressed CD209; the majority of these APCs also expressed CD206, CD14, and CD68 (A-C). Anatomical definition was obtained by staining lymph node sections with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively (D). A sequential section probed with antibodies detecting CD209 and CD14 demonstrated that a population expressing these markers was scattered along the basement membranes of the capsule and trabeculae (E,F). This subcapsular APC population was consistently observed in all axillary lymph nodes (H) and coexpressed CD68 (G,I); a few of these cells also expressed CD206 (J). In one axillary lymph node, an unusually large number of cells expressing CD209 were detected beneath the capsule and along the trabeculae (K). Panels A to C were acquired from the same area on sequential sections as were panels D to F. M indicates medullary cords; white arrows identify the capsule (C) and trabeculae (T) (D-G, K). *Background autofluorescence. Data are representative of 4 independent experiments.
APCs expressing a similar phenotype to the APCs in medullary cords lined the capsule and trabeculae in the human axillary lymph node. Immunohistochemistry demonstrated that the APCs packed into the medullary cords expressed CD209; the majority of these APCs also expressed CD206, CD14, and CD68 (A-C). Anatomical definition was obtained by staining lymph node sections with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively (D). A sequential section probed with antibodies detecting CD209 and CD14 demonstrated that a population expressing these markers was scattered along the basement membranes of the capsule and trabeculae (E,F). This subcapsular APC population was consistently observed in all axillary lymph nodes (H) and coexpressed CD68 (G,I); a few of these cells also expressed CD206 (J). In one axillary lymph node, an unusually large number of cells expressing CD209 were detected beneath the capsule and along the trabeculae (K). Panels A to C were acquired from the same area on sequential sections as were panels D to F. M indicates medullary cords; white arrows identify the capsule (C) and trabeculae (T) (D-G, K). *Background autofluorescence. Data are representative of 4 independent experiments.
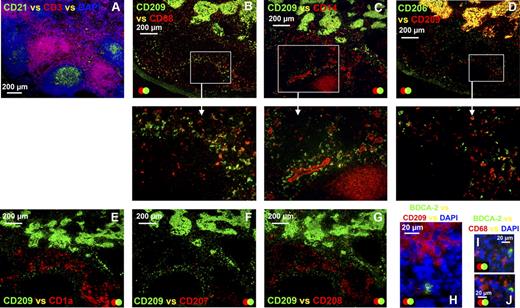
APCs expressing a similar phenotype to the APCs in medullary cords were also detected in the paracortex of the human axillary lymph node. Anatomical definition was obtained by staining frozen lymph node sections with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively (A). Sequential sections were probed with a panel of antibodies to defining APC markers (B-G). In addition to the CD209+ APCs in the medullary cords, cells expressing low-level CD209 colonized the diffuse T-lymphocyte areas surrounding the medullary cords and follicles (B-G). The majority of CD209lo APCs in the diffuse T-lymphocyte zones coexpressed CD68 (B), whereas CD14 (C) and CD206 (D) expression was variable. These CD209lo APCs did not express CD1a (E), CD207 (F), CD208 (G), or BDCA-2 (H). BDCA-2+ APCs also lacked CD68 expression (I,J). Panels A to G were acquired from the same area on sequential sections. Blue represents DAPI staining of cell nuclei (A,H-J). Data are representative of 4 independent experiments. BDCA-2+ APC data are representative of 3 independent experiments.
APCs expressing a similar phenotype to the APCs in medullary cords were also detected in the paracortex of the human axillary lymph node. Anatomical definition was obtained by staining frozen lymph node sections with antibodies detecting CD3 and CD21 to identify the T lymphocyte–rich paracortex and follicles, respectively (A). Sequential sections were probed with a panel of antibodies to defining APC markers (B-G). In addition to the CD209+ APCs in the medullary cords, cells expressing low-level CD209 colonized the diffuse T-lymphocyte areas surrounding the medullary cords and follicles (B-G). The majority of CD209lo APCs in the diffuse T-lymphocyte zones coexpressed CD68 (B), whereas CD14 (C) and CD206 (D) expression was variable. These CD209lo APCs did not express CD1a (E), CD207 (F), CD208 (G), or BDCA-2 (H). BDCA-2+ APCs also lacked CD68 expression (I,J). Panels A to G were acquired from the same area on sequential sections. Blue represents DAPI staining of cell nuclei (A,H-J). Data are representative of 4 independent experiments. BDCA-2+ APC data are representative of 3 independent experiments.
APCs expressing a low level of CD209 were scattered around the medullary cords and in the perifollicular zone of the paracortex (Figure 4B–G). The majority of CD209lo cells coexpressed CD68 (Figure 4B), whereas CD14 (Figure 4C) and CD206 (Figure 4D) were expressed at varying levels. These CD209lo cells did not coexpress CD1a (Figure 4E), CD207 (Figure 4F), or CD208 (Figure 4G) and therefore represent a distinct subset from the aforementioned APC populations in the paracortex. Interestingly, the CD209lo cells appeared to directly neighbor the follicle, whereas other APCs in this region expressing CD1a, CD207, and CD208 were located further into the paracortex closer to the dense T-lymphocyte area (Figure 4E–G). The few BDCA-2+ plasmacytoid APCs that were observed did not coexpress CD209 (Figure 4H) or CD68 (Figure 4I,J) and therefore represent an APC subset distinct from CD209lo APCs. Cells expressing CD68 but lacking CD209, possibly T or B lymphocytes or an additional APC subset, were also present in the diffuse T-lymphocyte areas of the paracortex (Figure 4B). Ring-like clusters of CD14+CD209− cells consistent with high endothelial venules were scattered throughout the diffuse T-lymphocyte regions of the paracortex (Figures 3E,F,4C).
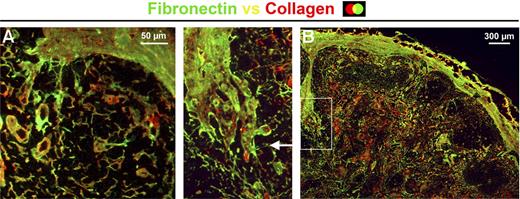
Conduits radiate from the capsule and trabeculae
A dense network of fibers expressing collagen and fibronectin spanned the lymph node parenchyma (Figure 5). A number of these fibers branched from the capsule and merged with the fibroreticular network inside the lymph node (Figure 5A). Trabeculae constructed of collagen fibers and expressing fibronectin also radiated from the outer capsule deep into the lymph node (Figure 5B). Interestingly, the trabeculae shown in Figure 5B also appear to branch and join the conduit network.
Conduits branch from the capsule and trabeculae and radiate into the human axillary lymph node. Immunohistochemistry illustrated that collagen fibers expressing fibronectin branched from the outer capsule of the lymph node and integrated with the fibroreticular cell network in the lymph node (A). Similarly, trabeculae that were constructed of collagen fibers and expressed fibronectin radiated deep into the lymph node, branched, and merged with the conduit network (B). Data are representative of 4 independent experiments.
Conduits branch from the capsule and trabeculae and radiate into the human axillary lymph node. Immunohistochemistry illustrated that collagen fibers expressing fibronectin branched from the outer capsule of the lymph node and integrated with the fibroreticular cell network in the lymph node (A). Similarly, trabeculae that were constructed of collagen fibers and expressed fibronectin radiated deep into the lymph node, branched, and merged with the conduit network (B). Data are representative of 4 independent experiments.
All APC subsets associate with the conduit network
APCs expressing CD1a alone or together with CD207 were in intimate contact with structures expressing fibronectin (Figure 6A,B). High magnification images also highlight that CD1a+CD207− and CD1a+CD207+ cells were often in intimate contact with one another (Figure 6B). Although these cells were sometimes in proximity to ring-like fibronectin-expressing structures resembling high endothelial venules, they were rarely in direct contact with them (Figure 6A,B). The majority of CD208+ APCs were anchored to collagen fibers (Figure 6C–E); these cells also rarely associated with structures resembling high endothelial venules (Figure 6C–E). In contrast, APCs expressing CD209 and CD14 were in intimate contact with collagen fibers and structures, likely high endothelial venules (Figure 6F–J). High magnification images of the capsule demonstrate that, although there appears to be a narrow void lacking a fibroreticular cell network, CD209+ and CD14+ subcapsular APCs remain tethered to the collagen fibers on either side, or to short fibers that radiate into this subcapsular space possibly sinus (Figure 6F,G,K,L).
APCs in the human axillary lymph node associate with the fibroreticular cell network. Frozen lymph node sections were probed with antibodies to either collagen or fibronectin to map the fibroreticular cell network. Multicolor immunohistochemistry illustrated that the majority of CD1a+CD207−, CD1a+CD207+ (A,B), and CD208+ APCs (C-E) were either in intimate contact with or tethered to fibroreticular cell structures. Most APCs expressing CD209 (F-I) and CD14 (J-L) were also in close contact with fibroreticular cell structures. Interestingly, CD209+ and CD14+ APCs tended to cluster around ring-like structures resembling high endothelial venules (F-J). Just beneath the outer capsule, there was a narrow void lacking collagen fibers; the CD209+ and CD14+ APCs distributed along the basement membrane of the capsule were tethered to the collagen fibers at either side of this subcapsular space, possibly sinus (F,G,K,L). Data are representative of 3 independent experiments.
APCs in the human axillary lymph node associate with the fibroreticular cell network. Frozen lymph node sections were probed with antibodies to either collagen or fibronectin to map the fibroreticular cell network. Multicolor immunohistochemistry illustrated that the majority of CD1a+CD207−, CD1a+CD207+ (A,B), and CD208+ APCs (C-E) were either in intimate contact with or tethered to fibroreticular cell structures. Most APCs expressing CD209 (F-I) and CD14 (J-L) were also in close contact with fibroreticular cell structures. Interestingly, CD209+ and CD14+ APCs tended to cluster around ring-like structures resembling high endothelial venules (F-J). Just beneath the outer capsule, there was a narrow void lacking collagen fibers; the CD209+ and CD14+ APCs distributed along the basement membrane of the capsule were tethered to the collagen fibers at either side of this subcapsular space, possibly sinus (F,G,K,L). Data are representative of 3 independent experiments.
Discussion
Although the APC cell subsets in murine lymph nodes have been well defined,2-4 little is known about their human equivalents.1 We have conducted detailed 3-color fluorescence immunohistochemistry on human lymph nodes with the initial aim of confirming the lymph node–homing capacity of CD1a+ dermal APCs.12 These data cast new light on the APC subsets in human lymph nodes.
To briefly summarize our key results, we have identified APCs in human lymph nodes, excluding plasmacytoid APCs, that fall into 2 nonoverlapping classes, namely, (1) APCs expressing combinations of CD1a, CD207, and CD208; and (2) CD209+ APCs expressing combinations of CD206, CD14, and CD68. CD1a+ dermal APCs, CD1a+CD207+ Langerhans cells, and CD208+CD1a−“interdigitating” cells colonized the same areas of the paracortex in each lymph node; these APCs were either distributed throughout the dense T-lymphocyte areas or clustered in the diffuse T-lymphocyte areas. Several lymph nodes lacked CD1a+ and CD207+ APCs, and there was a striking correlation between the lack of these cells and a paucity of CD208+CD1a− interdigitating cells, suggesting that CD208+ APCs derive from migratory APC subsets. CD209+ APCs were located in the medullary cords and lined the capsule and trabeculae. CD209+ APCs were also consistently found scattered throughout the paracortex of all lymph nodes examined, where they tended to wrap around high endothelial venules. Each of the APC subsets we identified was in intimate contact with the conduit network, which radiated from the trabeculae as well as the capsule.
Dermal APCs colonize the paracortex of the human lymph node
We demonstrate, for the first time, that a CD1a+CD207−CD1b+/− APC population expressing a similar phenotype to CD1a+ dermal APCs is present in the paracortex of human axillary lymph nodes that commonly drain skin.21 This confirms our earlier observation that CD1a+ dermal APCs have the capacity to migrate in response to lymph node-homing chemokines12 and supports other published data showing that CD1a+ cells lacking Birbeck granules typical of Langerhans cells24 could be detected in lymph nodes draining the skin of cancer patients17 and in lymph draining human skin.25 As these CD1a+CD207− APCs were detected in axillary lymph nodes exhibiting negligible or no reactivity, it appears that human CD1a+ dermal APCs continuously turn over and migrate to the sentinel lymph nodes under steady-state conditions. CD1a+CD207− dermal APCs were distributed throughout the diffuse or dense T-lymphocyte zones; and because earlier studies have shown that human CD1a+ dermal APCs are able to stimulate naive CD412 and CD813 T lymphocytes, it seems highly probable that these cells will be able to initiate T-lymphocyte responses. Similarly, the accumulation of dermal APCs in T-lymphocyte regions has been observed in murine lymph nodes.4 Furthermore, migration of murine dermal APCs to draining lymph nodes was essential for the initiation of a T-lymphocyte response to antigen encountered in the skin.2
A proportion of CD1a+CD207− dermal APCs in the lymph node expressed the maturation marker CD208. During our earlier studies, we did not detect CD208 expression by CD1a+ dermal APCs26 ; this suggests that CD1a+ dermal APCs only up-regulate CD208 once they enter the afferent lymphatics or the lymphoid tissue itself. A subset of CD1a+CD207− APCs also expressed CD1b, analogous to their dermal equivalent,12 providing further evidence that these cells derive from CD1a+ dermal APCs.
Langerhans cells colonize the paracortex of the human lymph node
For the first time, we show that human CD1a+CD207+ Langerhans cells are present in the paracortex of axillary lymph nodes in the absence of any pathologic skin conditions. This is consistent with earlier data where CD1a+ cells expressing CD207 were detected in lymphatic fluid draining healthy skin.25 CD207+ and CD1a+ cells have also been detected in the paracortex of lymph nodes excised from patients with chronic dermatopathic lymphadenitis27 and primary cutaneous melanoma.28 The fact that we detected Langerhans cells in all the axillary and inguinal lymph nodes we studied suggests that there is regular, perhaps continuous, trafficking of Langerhans cells to lymph nodes that directly drain healthy skin. It is important to note that many murine models have also demonstrated steady-state migration of Langerhans cells to the paracortex of skin-draining lymph nodes.3,29
In our studies, CD1a+CD207+ Langerhans cells colocalized with CD1a+CD207− dermal APCs in either the diffuse or dense T-lymphocyte regions. In contrast, in murine lymph nodes, Langerhans cells and dermal APCs migrated to separate compartments; Langerhans cells migrated into the inner cortex, whereas dermal APCs colonized the perifollicular region.4 The role of Langerhans cells in initiating immune responses is still controversial. A recent study has shown that human epidermal Langerhans cells can stimulate CD8+ T lymphocytes and the differentiation of CD4 T lymphocytes, which secrete Th2 cell type cytokines,13 whereas some murine studies question whether they are involved2,4,5,7,11 and others support the concept that they can initiate immune responses under restricted circumstances.8,10 Our observations that Langerhans cells always colocalize with dermal APCs suggest that these cells are either closely related or cooperate to initiate or regulate immune responses.
This colocalization is made even more intriguing by recent studies suggesting that not all CD207+ cells originate from the epidermis. A population of CD207+ APCs has been identified in the murine dermis with the capacity to migrate to skin-draining lymph nodes under steady-state conditions, although these cells are not deemed to be migrating epidermal Langerhans cells.30-32 It is therefore possible that a proportion of the CD207+ cells we observed in human lymph nodes represents a similar migratory dermal CD207+ population, especially as a small number of CD207+ are always present in the healthy human dermis.12,26,33
A subset of CD1a+CD207+ Langerhans cells in the lymph node expressed CD208 consistent with our earlier observation that possible migratory Langerhans cells in the dermis express CD208.26 This contrasts with an earlier report, where Langerhans cells in lymph nodes draining the skin of patients with chronic dermatopathic lymphadenitis lacked CD208 expression.27 One explanation for this slight discrepancy could be that the high rate of Langerhans cell turnover that occurs under inflammatory conditions34 may prevent these cells from fully differentiating and expressing CD208. A proportion of CD1a+CD207+ Langerhans cells also expressed CD1b, consistent with CD1b expression by CD1a+CD207+ APCs in the dermis, providing further evidence of their cutaneous origins.
Interdigitating APCs in the human lymph node
Expression of the maturation marker CD208 or DC-LAMP has long been used as a marker for lymph node interdigitating APCs. The precise role of this molecule remains unclear; however, it is thought to be involved in lysosomal function.15 Here we provide evidence indicating that a range of cells express CD208, including APCs that have probably migrated from the skin. In addition we detected a CD208 subset that lacked CD1a and CD207 but expressed CD1b. As these CD208+CD1a−CD207− APCs colocalized with CD1a+CD207− dermal APCs and CD1a+CD207+ Langerhans cells in the paracortex were restricted to the same lymph nodes as these APCs and expressed other markers in common (eg, CD208 and CD1b), it seems possible that these cells have differentiated from APCs migrating from the skin rather than representing an independent APC population. Alternatively, these APCs may have originated from the CD1b+ APC population in human blood.35
CD209+ APCs colonize the medullary cords and paracortex, and also line the capsule and trabeculae of the human lymph node
As expected, a large number of APCs were packed into the medullary cords. This population expressed CD209 and the macrophage marker CD206; however, these cells also expressed perinuclear CD68, which is typical of dendritic cells.14 This phenotype was consistent with earlier data,23,36,37 although in contrast to these studies, we also detected CD14 expression by these cells, perhaps resulting from use of different monoclonal antibodies. For the first time, we detected a similar APC subset dotted equidistantly along the basement membranes of the outer capsule and trabeculae. Interestingly, an earlier study detected CD209 expression by cells beneath the capsule in lymph nodes excised from patients with primary cutaneous melanoma. However, the authors proposed that the CD209 antibody was cross-reacting with its homolog L-SIGN, which is expressed by the endothelial cells of the sinus,28 rather than recognizing a distinct APC population as we have shown. APCs lining the outer capsule have recently been observed in murine lymph nodes; these cells were able to stimulate naive CD8 lymphocytes38 and B lymphocytes.39,40
APCs expressing low-level CD209 and CD68, with various levels of CD206 and CD14, also colonized the highly vascular diffuse T-lymphocyte regions surrounding the medulla and follicles; CD209, CD206, and CD68 expression by this paracortical population corresponds with earlier observations.28,36,37 The precise role of these APCs is open to speculation given the difficulties inferring functional properties from molecular phenotype; it remains a matter of debate whether CD209 is expressed by dendritic cells and not macrophages,41-43 whereas CD68 is acknowledged as being expressed by both cell types.14 However, as proportions of these APCs colocalize with T lymphocytes and directly neighbor B-lymphocyte follicles, they may be involved in the generation of both T- and B-lymphocyte responses. Interestingly, earlier studies have shown that, in reactive human lymph nodes, there is an increase in the number of CD209+ cells in the paracortex, suggesting an involvement in T-lymphocyte stimulation.36
We note with interest that the CD209+ APCs in the lymph node expressed a similar phenotype to CD14+ dermal APCs in the human dermis.12,26,44 Although our earlier data suggest that this population is less able than CD1a+ dermal APCs to migrate in response to lymph node–homing chemokines,12 afferent lymph draining the skin does contain some CD14+CD1a− APCs.25 So despite CD14+ dermal APCs lacking the capacity to actively migrate, they may still passively drain and constitute a proportion of the APCs in the lymph node. The contribution of such cells to the CD209+CD14+ APC population, in comparison to the migration of monocytic CD14+ APC precursors from the blood, remains to be determined.
Very few BDCA-2+ plasmacytoid APCs were detected in the human axillary lymph nodes, and the few cells that were present were located in the paracortex; this was consistent with earlier studies where BDCA-2+ plasmacytoid APCs that migrate from the blood35 were rarely detected in mesenteric lymph nodes23 and in the paracortex of inflamed tonsils.45
APCs intimately associate with the fibroreticular network spanning the human lymph node
By staining for collagen and fibronectin, we visualized the dense network of fibers that span the parenchyma of human lymph nodes. Although in general these structures resembled the conduit network produced by murine fibroreticular cells that radiates from the capsule,18,20 it is important to note that human lymph nodes have trabeculae, which murine lymph nodes lack. Trabeculae penetrate deep into the cortex, and sinuses within trabeculae are suspected of draining afferent lymph from subcapsular sinuses.46 We illustrate here that, as trabeculae radiate into the lymph node, they branch and join the fibro-reticular network, potentially providing an additional route for afferent lymph to perfuse both the medullary and para-cortical regions.
The majority of migratory CD1a+CD207− dermal APCs, CD1a+CD207+ Langerhans cells, and CD208+ APCs were in intimate contact with the conduit network. These observations differ from a murine study where mature APCs that had migrated from murine skin did not associate with the conduit network in the lymph node.18 Our data suggest that APCs expressing CD1a, CD207, and CD208 may be able to monitor afferent lymph for cytokine signals and possibly acquire antigen similar to resident APCs in the murine lymph node.18,19,47 The conduit network may also provide a support for the movement of APCs.
APCs expressing CD209 and CD14 were also in intimate contact with conduit ducts and ring-like structures resembling high endothelial venules. These findings corroborate an earlier study, which observed ring-like structures expressing CD209 in lymph nodes excised from patients with primary cutaneous melanoma28 ; in contrast, however, this study proposed that the high endothelial venules themselves expressed CD209 rather than the APCs that are in intimate contact with them. High endothelial venules were usually concentrated in the diffuse T-lymphocyte regions surrounding the medullary cords and follicles, possibly explaining why CD209lo APCs were detected in these areas. CD209lo APCs are therefore ideally located to encounter T lymphocytes as they enter the lymph node via high endothelial venules,48 as well as possibly remaining in contact with the afferent lymph flowing through the conduit network. In contrast, APCs expressing CD1a, CD207, and CD208 were rarely in intimate contact with high endothelial venules, consistent with earlier observations in murine lymph nodes where migratory APCs were remote from high endothelial venules.49 These observations highlight that APCs expressing combinations of CD1a, CD207, and CD208, and CD209+ APCs probably fulfill distinct functional roles.
Collectively, our observations suggest that APCs bearing CD1a, CD207, and CD208 relate to migratory APCs derived from skin or other epithelial surfaces. The striking loss of CD208+ APCs in the absence of CD1a+ or CD207+ APCs suggests that most CD208+ “interdigitating” cells are not resident APCs. In contrast, CD209+ APCs are found in all lymph nodes, suggesting that they are a true resident APC subset. In future studies, it will be interesting to establish whether the 2 dominant APC populations we describe represent the only APCs in the human lymph node or whether APCs expressing different phenotypes also exist. These findings suggest that, after cutaneous infection or vaccination, CD1a+ dermal APCs and Langerhans cells could deliver antigen to T lymphocytes in the paracortex of the human draining lymph node. Furthermore, resident CD209+ APCs that closely associate with the fibroreticular network may have the capacity to sample lymph draining sites of infection or vaccination. It will therefore be interesting to determine whether these APCs in human lymph nodes have similar functions to those reported for their equivalent populations in murine lymph nodes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Adrian Turner for technical assistance and advice, and the patients and staff of the Counties-Manukau District Health Board (Auckland, New Zealand) and the Austin Hospital (Melbourne, Australia) for donated clinical material.
This work was supported by the Wellcome Trust (WT066630), the Maurice and Phyllis Paykel Trust, and the Maurice Wilkins Center.
Wellcome Trust
Authorship
Contribution: C.E.A. designed research, analyzed and interpreted data, and wrote the manuscript; C.-J.J.C. performed research and collected data; O.C.H. and S.W. contributed analytical tools; T.J., J.B., D.M., and J.C. contributed and prepared human tissue; and P.R.D. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: P. Rod Dunbar, University of Auckland, School of Biological Sciences, Thomas Building, 3a Symonds Street, Auckland, 1142, New Zealand; e-mail: r.dunbar@auckland.ac.nz.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal