Abstract
Whether the eradication of Helicobacter pylori infection can increase the platelet count in patients with immune thrombocytopenic purpura (ITP) is still a controversial issue. To provide evidence-based guidance, we performed a systematic review of the literature published in English, selecting articles reporting 15 or more total patients. We identified 25 studies including 1555 patients, of whom 696 were evaluable for the effects of H pylori eradication on platelet count. The weighted mean complete response (platelet count ≥ 100 × 109/L) and overall response (platelet count ≥ 30 × 109/L and at least doubling of the basal count) were 42.7% (95% confidence interval [CI], 31.8%-53.9%) and 50.3% (95% CI, 41.6%-59.0%), respectively. In 222 patients with a baseline platelet count less than 30 × 109/L, the complete response rate was 20.1% (95% CI, 13.5%-26.7%) and the overall response rate was 35.2% (95% CI, 28.0%-42.4%). The response rate tended to be higher in countries with a high background prevalence of H pylori infection and in patients with milder degrees of thrombocytopenia. These findings suggest that the detection and eradication of H pylori infection should be considered in the work-up of patients with seemingly typical ITP.
Introduction
In addition to its well-demonstrated role in gastroduodenal diseases, Helicobacter pylori infection has been associated with a diverse spectrum of nondigestive system diseases. According to the Maastricht III consensus conference, immune thrombocytopenic purpura (ITP) is 1 of the 2 extraintestinal diseases for which H pylori infection detection and eradication is indicated (the other is unexplained iron deficiency anemia).1 However, as this recommendation was supported by neither the results of randomized clinical trials nor a systematic review of cohort studies and case series, it should be considered opinion-based rather than evidence-based.
The relationship between H pylori infection and ITP was initially described in 1998, when an Italian group reported a significant increase of the platelet count in 8 of the 11 ITP patients in whom the bacterium was eradicated.2 However, subsequent reports have produced discrepant results. Most of these studies involved a relatively small number of patients, the median observation period after eradication was often less than 1 year, the responses to prior therapies were unclear, and there was only one controlled trial comparing eradication to no eradication in infected patients with ITP. In addition, studies often included patients with mild thrombocytopenia who would not ordinarily have been treated. Therefore, the role of H pylori eradication in the management of patients with ITP requires clarification.
To understand and interpret the evidence for the many different reported outcomes, we performed a systematic review of published studies describing the effects of bacterial eradication on the platelet count of adult patients with ITP.
Methods
Literature search
Articles were identified by a computer-assisted search of the literature published in English. The review was conducted independently by 2 of the authors (M.L.E. and R.S.) in April 2008 using the search engines of the National Library of Medicine PubMed database, EMBASE,3 Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Cochrane Database of Abstracts of Reviews of Effects. The initial PubMed search was performed by combining the medical subject headings (MeSH) term “Helicobacter pylori” (no restrictions, 21 504 hits) with either the MeSH term “thrombocytopenia” (no restrictions, 31 989 hits) or the MeSH term “purpura, thrombocytopenic, idiopathic” (no restrictions, 3020 hits). The combination “Helicobacter pylori” (MeSH) and “thrombocytopenia” (MeSH) produced 116 hits, whereas the combination “Helicobacter pylori” (MeSH) and “purpura, thrombocytopenic, idiopathic” (MeSH) produced 104 hits. Articles were subsequently identified by review of titles, abstracts (when available), or text (when the abstract was not available and the article was in English). A similar strategy was adopted for the EMBASE search, whereas the Cochrane databases were searched identifying all titles that contained either “Helicobacter pylori” or “thrombocytopenia.” The bibliographies of all retrieved articles were searched by hand for additional relevant citations.
Criteria for article selection
Articles were included if they reported on randomized, cohort, case-control, or retrospective studies evaluating the effects of H pylori eradication on the platelet count in adult patients with ITP. Reports published only in abstract form were not considered eligible, nor were articles reporting fewer than 15 patients. Articles reporting 15 or more total patients were reviewed to determine whether they described patients with ITP according to the American Society of Hematology guidelines.4 If no reference to such guidelines was made, articles were selected only if secondary causes of thrombocytopenia had been clearly ruled out, including hepatitis B or C virus infection, HIV infection, lupus, antiphospholipid antibody syndrome, bone marrow failure syndromes, drug-induced thrombocytopenia, and malignancies such as chronic lymphocytic leukemia and malignant lymphoma. H pylori infection had to be documented by the 13C-urea breath test (UBT) or other tests indicating active infection such as the stool antigen test or the histology of gastric biopsies. Serologic tests were not considered adequate to diagnose active infection because of their lower sensitivity and specificity compared with other diagnostic methods.5 Finally, studies had to be evaluable for the effects of H pylori eradication on platelet count over time.
Initially, titles and abstracts of all articles were assessed according to the above selection criteria using a standard checklist performed independently by the 2 reviewers. Full-text articles were retrieved when they were judged by either reviewer to possibly contain relevant original data. Final article selection was carried out independently by both reviewers, and disagreements were resolved by consensus in all cases. When a study had generated multiple publications, the latest or most informative version was retained. Duplicate publications were used to provide information on baseline characteristics or methodology where necessary.
Data extraction
Data extraction of the content from each article was carried out independently by the 2 reviewers. Our research strategy was to obtain individual-level patient data from case series. If patient-level data were not reported, we used the group-level data. For the purposes of this review, the following data were collected by each reviewer: (1) study design and use of controls; (2) patient demographics; (3) previous and concomitant treatment for ITP; (4) baseline platelet count; (5) duration of ITP before eradication treatment; (6) diagnostic methods for H pylori detection; (7) type and duration of the eradication regimen administered; (8) toxicities associated with the eradication regimen; (9) proportion of H pylori eradication; (10) criteria for platelet count response (including time to response assessment); (11) proportion of patients with platelet count responses; (12) time to platelet count responses if different from established criteria for response assessment; (13) follow-up and duration of platelet count responses; (14) effects of H pylori eradication therapy on uninfected patients; and (15) predictors of platelet response. Discrepancies in the data extracted by the 2 reviewers were resolved by joint reassessment of the original publication. For publications with missing or incomplete information, attempts were made to obtain additional data from study authors.
Assessment of validity
The patient population and trial methods were systematically examined. Methodologic quality of the cohort studies was assessed using the Newcastle-Ottawa Scale,6 which assigns a number of stars to 3 quality items of the studies: selection bias, comparability of cohorts on the basis of the design or analysis, and outcome assessment. An overall quality score is generated by adding up the scores of each item (highest score: 9 stars).
The quality of the randomized-controlled trial was assessed with a scale developed by Jadad et al.7 With this instrument, 0 to 2 points are assigned for randomization, 0 to 2 points for double blinding, and 0 to 1 points for the description of withdrawals and dropouts. This gives a score ranging from 0 to 5 with a higher score indicative of more rigorous methodology.
Assessment of publication bias
Susceptibility of the systematic review to publication bias, that is, the tendency of studies with positive findings to be preferentially published, was formally assessed with the Egger test. This is a test for a Y intercept equal to 0 from a linear regression of normalized effect estimate (estimate divided by its standard error) against precision (reciprocal of the standard error of the estimate). The assessment of the Egger test was coupled with an informal visual inspection of the funnel plot.8 Heterogeneity among studies was assessed using the Cochran Q test9 and the I-square (I2) test of inconsistency.10
Quantitative data synthesis
To determine estimates of platelet count response for individual studies, we used the criteria recently adopted by the International Working Group on ITP11 irrespective of the criteria reported in each article. ITP was defined in the presence of a platelet count less than 100 × 109/L. A complete response was defined as a platelet count of at least 100 × 109/L, and overall response as any platelet count of at least 30 × 109/L and at least doubling the baseline count. Accordingly, in some instances, the results presented in our tables differ from those shown in the original articles. For some parameters, such as time to response, for which we could not combine data, we have described the results in narrative form.
When possible, we calculated the mean platelet counts with the related standard deviations at baseline and at the time of response assessment. Individual-level data were used to perform sensitivity analyses in patients with baseline platelet counts greater than or less than 30 × 109/L.
As considerable heterogeneity was anticipated based upon the findings of previous reviews,12,13 the DerSimonian-Laird random-effects method was used to pool data for summary estimates.14 The random-effects model recognizes that the studies are a sample of all potential studies and incorporates an additional between-study component to the estimate of variability. Thus, larger studies with smaller variances have relatively more impact on the final estimate. In fact, this impact is also present in the fixed effect model and is relatively greater because the variability between studies is not included in the estimated standard error of the estimate. To stabilize variance, eradication and treatment-response proportions were subject to a Freeman-Tukey arcsine square root transformation and back-transformed after quantitative data synthesis.15 Proportions of 0 and 1 were adjusted as follows to enable their inclusion in study weighting: 0 = 1/(4 × sample size); 1 = (number of successes−0.25)/(sample size). Results are presented as the weighted mean with 95% confidence intervals (CIs).
The linear regression procedures were used for correlation analyses. Unweighted chance-corrected κ values were used to assess agreement between reviewers for study selection.16 Assessment of publication bias and tests of heterogeneity were performed with Stata 8.2 (Stata Corp, College Station, TX). All other statistical tests were performed with the NCSS 2007 software package (NCSS, Kaysville, UT).
Role of the funding source
This systematic review had no external source of funding. The organizations that funded the individual authors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Results
Selection of studies
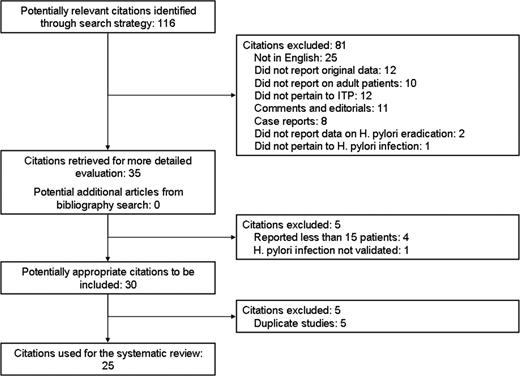
Figure 1 summarizes the results of our literature searches. We reviewed a total of 116 titles. After exclusion of 81 publications on the basis of the aforementioned selection criteria (Figure 1), we retrieved 35 articles for full-text evaluation and found no additional titles from our bibliographic search. No study described both children and adults. Of the 25 publications excluded because they were not in English, 8 were case reports, 14 were narrative reviews, 1 was a survey of current treatment strategies in Japan, and only 2 were case series (1 with 11 ITP patients and the other with 20 ITP patients).
The overall proportion of agreement for initial study inclusion was 93%, with a κ of 0.84. We excluded 5 redundant or duplicate publications, and 4 studies that reported fewer than 15 patients. One cohort study investigated H pylori infection only by serologic tests (H pylori antibodies) and was also excluded. Twenty-five studies (1555 patients) enrolled at least 15 patients each and were included in our efficacy analysis. The quality of included cohort studies according to the Newcastle-Ottawa scale was considered acceptable, as all studies had a score of at least 5. The Jadad score for the only randomized study was 2; although the authors of the study reported that patients were randomly assigned by concealed allocation, no information on the method to generate the sequence of randomization was provided.
Study design and sources of funding
Study designs are detailed in Table 1. There was just 1 randomized-controlled trial,17 22 prospective case series,2,18-38 and 2 retrospective studies.39,40 Two of the observational cohort studies had an internal control.29,30 In the Italy–United Kingdom study, eradication therapy was administered to H pylori–positive patients only if they had a platelet count under 50 × 109/L or had symptoms of dyspepsia.30 In one study from Japan, all patients with ITP received eradication therapy irrespective of the H pylori status.29
Design of studies included in the systematic review
| Study . | Country . | Study design . | Type of data reported for H pylori–positive patients . | No. of ITP patients . | Diagnostic tests for H pylori infection . |
|---|---|---|---|---|---|
| Gasbarrini et al (1998)2 | Italy | Observational, case series, single center | Group | 18 | UBT |
| Jarque et al (2001)18 | Spain | Observational, case series, single center | Group | 56 | UBT |
| Kohda et al (2002)19 | Japan | Observational, case series, single center | Group | 40 | UBT/RUT/His/Ab |
| Hino et al (2003)20 | Japan | Observational, case series, single center | Individual | 30 | UBT/Ab |
| Hashino et al (2003)21 | Japan | Observational, case series, single center | Individual | 22 | UBT/RUT/His/Cul |
| Ando et al (2003)22 | Japan | Observational, case series, single center | Individual | 61 | UBT |
| Michel et al (2004)23 | US | Observational, case series, single center | Group and individual | 74 | UBT |
| Takahashi et al (2004)24 | Japan | Observational, case series, single center | Individual | 20 | UBT |
| Sato et al (2004)25 | Japan | Observational, case series, single center | Group | 53 | UBT |
| Ando et al (2004)26 | Japan | Observational, case series, single center | Group | 20 | UBT/Ab |
| Nomura et al (2004)27 | Japan | Observational, case series, single center | Group | 42 | UBT |
| Veneri et al (2005)28 | Italy | Observational, case series, single center | Group | 52 | UBT |
| Inaba et al (2005)29 | Japan | Observational, case series, multicenter | Group | 35 | UBT/Ab |
| Stasi et al (2005)30 | Italy-United Kingdom | Observational, case series, multicenter | Group and individual | 137 | UBT |
| Fujimura et al (2005)39 | Japan | Retrospective, case series, multicenter | Group | 435 | UBT |
| Suzuki et al (2005)17 | Japan | Randomized, phase 3, single center | Group | 36 | UBT/His |
| Suvajdzić et al (2006)31 | Serbia | Observational, case series, single center | Group | 54 | UBT |
| Ahn et al (2006)32 | US | Observational, case series, single center | Individual | 15 | UBT |
| Sayan et al (2006)33 | Turkey | Observational, case series, single center | Individual | 34 | UBT |
| Asahi et al (2006)34 | Japan | Observational, case series, single center | Group | 37 | UBT ± Ab/SAT |
| Kodama et al (2007)35 | Japan | Observational, case series, single center | Group | 116 | UBT |
| Campuzano-Maya (2007)40 | Colombia | Retrospective, case series, single center | Group | 32 | UBT |
| Estrada-Gómez et al (2007)36 | Mexico | Observational, case series, single center | Group | 23 | SAT |
| Satake et al (2007)37 | Japan | Observational, case series, single center | Group | 38 | UBT or RUT |
| Emilia et al (2007)38 | Italy | Observational, case series, single center | Individual | 75 | UBT ± His |
| Study . | Country . | Study design . | Type of data reported for H pylori–positive patients . | No. of ITP patients . | Diagnostic tests for H pylori infection . |
|---|---|---|---|---|---|
| Gasbarrini et al (1998)2 | Italy | Observational, case series, single center | Group | 18 | UBT |
| Jarque et al (2001)18 | Spain | Observational, case series, single center | Group | 56 | UBT |
| Kohda et al (2002)19 | Japan | Observational, case series, single center | Group | 40 | UBT/RUT/His/Ab |
| Hino et al (2003)20 | Japan | Observational, case series, single center | Individual | 30 | UBT/Ab |
| Hashino et al (2003)21 | Japan | Observational, case series, single center | Individual | 22 | UBT/RUT/His/Cul |
| Ando et al (2003)22 | Japan | Observational, case series, single center | Individual | 61 | UBT |
| Michel et al (2004)23 | US | Observational, case series, single center | Group and individual | 74 | UBT |
| Takahashi et al (2004)24 | Japan | Observational, case series, single center | Individual | 20 | UBT |
| Sato et al (2004)25 | Japan | Observational, case series, single center | Group | 53 | UBT |
| Ando et al (2004)26 | Japan | Observational, case series, single center | Group | 20 | UBT/Ab |
| Nomura et al (2004)27 | Japan | Observational, case series, single center | Group | 42 | UBT |
| Veneri et al (2005)28 | Italy | Observational, case series, single center | Group | 52 | UBT |
| Inaba et al (2005)29 | Japan | Observational, case series, multicenter | Group | 35 | UBT/Ab |
| Stasi et al (2005)30 | Italy-United Kingdom | Observational, case series, multicenter | Group and individual | 137 | UBT |
| Fujimura et al (2005)39 | Japan | Retrospective, case series, multicenter | Group | 435 | UBT |
| Suzuki et al (2005)17 | Japan | Randomized, phase 3, single center | Group | 36 | UBT/His |
| Suvajdzić et al (2006)31 | Serbia | Observational, case series, single center | Group | 54 | UBT |
| Ahn et al (2006)32 | US | Observational, case series, single center | Individual | 15 | UBT |
| Sayan et al (2006)33 | Turkey | Observational, case series, single center | Individual | 34 | UBT |
| Asahi et al (2006)34 | Japan | Observational, case series, single center | Group | 37 | UBT ± Ab/SAT |
| Kodama et al (2007)35 | Japan | Observational, case series, single center | Group | 116 | UBT |
| Campuzano-Maya (2007)40 | Colombia | Retrospective, case series, single center | Group | 32 | UBT |
| Estrada-Gómez et al (2007)36 | Mexico | Observational, case series, single center | Group | 23 | SAT |
| Satake et al (2007)37 | Japan | Observational, case series, single center | Group | 38 | UBT or RUT |
| Emilia et al (2007)38 | Italy | Observational, case series, single center | Individual | 75 | UBT ± His |
The H pylorieradication regimen consisted of a proton pump inhibitor, clarithromycin, and amoxicillin in all of the studies; however, bismuth subsalicylate was added to this combination in the study by Estrada-Gomez et al36 performed in Mexico.
UBT indicates 13C-urea breath test; RUT, rapid urease test; His, histopatological examination; Ab, serum antibodies; Cul, culture; and SAT, stool antigen test.
Publication bias
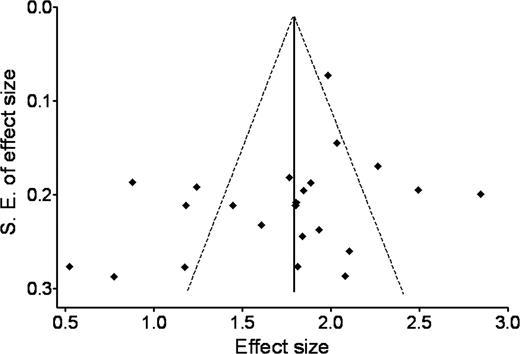
The Egger test indicated no evidence of publication bias (P = .167). A plot of precision versus response proportion failed to exhibit a funnel shape, while still showing an approximate symmetric spread around the mean response (Figure 2). Estimates of the effectiveness of eradication therapy in elevating platelet count in H pylori–infected ITP patients were noted to diverge among studies possessing lower standard errors, supporting the existence of nonrandom differences in success. As expected, we found a high degree of variance among the studies (heterogeneity χ2 = 115.600, P < .001; I2 = 86.3%).
Begg funnel plot with pseudo 95%, fixed-effect confidence limits. This plot compares the inverse-variance weighted linear regression of the effect size of each study (Freeman-Tukey arcsine square root transformed proportion of the response) against a measure of precision (its standard error). The logic behind funnel plots is that those studies with a smaller sample size or precision will have larger random error, thus a larger spread when graphed. Hence, when publication bias is absent, as it was in this review, the effects from smaller studies will have a larger, but symmetric, spread around the mean effect.
Begg funnel plot with pseudo 95%, fixed-effect confidence limits. This plot compares the inverse-variance weighted linear regression of the effect size of each study (Freeman-Tukey arcsine square root transformed proportion of the response) against a measure of precision (its standard error). The logic behind funnel plots is that those studies with a smaller sample size or precision will have larger random error, thus a larger spread when graphed. Hence, when publication bias is absent, as it was in this review, the effects from smaller studies will have a larger, but symmetric, spread around the mean effect.
Patient demographics
Table 2 details the characteristics of the 1555 ITP patients included in the 25 reports and investigated for H pylori infection. There were 985 (63.3%) patients in the 14 studies from Japan and 570 (36.7%) patients in the 11 studies from other countries. The pooled prevalence of H pylori infection was 65.0% (95% CI, 62.8%-67.3%), with no difference between men (63.4%) and women (66.5%). The figures of H pylori infection were much lower in the United States (21.6%) than in other countries (range 46.7%-90.6%). The pooled estimate of age in 1132 patients from 13 studies2,19-24,30,31,33,35,38,39 was 57.5 years (95% CI, 56.4–58.5 years) for H pylori-positive patients (n = 680), and 45.3 years (95% CI, 43.7–46.8 years) for those (n = 452) without H pylori infection. The H pylori status was not associated with sex distribution, ITP duration, baseline platelet count, and number of previous treatments (including splenectomy). A significant association between H pylori infection and the presence of symptoms of dyspepsia has been reported in an American study23 but not in a European study.30
Demographic features of adult ITP patients
| Study . | No. of patients . | Male/female . | No. of infected patients (%) . | Age of infected patients, y . | Age of uninfected patients, y . | Platelet count of infected patients, ×109/L . | Platelet count of uninfected patients, ×109/L . | ITP duration, mo . | Concomitant therapy . | Untreated/splenectomized patients . |
|---|---|---|---|---|---|---|---|---|---|---|
| Gasbarrini et al (1998)2 | 18 | 5/13 | 11 (61) | 43 ± 14 | 49 ± 12 | 95 ± 39 | 103 ± 24 | NR | NR | <NR |
| Jarque et al (2001)18 | 56 | 18/38 | 40 (71) | 54 (17–80)* | 57 ± 22† | 58 ± 23 | 32 (2–50) | 0/56 | 39/4 | |
| Kohda et al (2002)19 | 40 | 12/28 | 25 (62) | 54 ± 14 | 48 ± 13 | 67 ± 54 | 60 ± 41 | 41 ± 38 | 19/40 | 13/NR |
| Hino et al (2003)20 | 30 | 8/22 | 21 (70) | 55 ± 15 | 51 ± 17 | 38 ± 20 | 22 ± 12 | NR | 7/30 | 15/1 |
| Hashino et al (2003)21 | 22 | 9/13 | 14 (64) | 53.2 ± 12.9 | 41.8 ± 18.6 | 61 ± 26 | 63 ± 20 | 110 ± 81 | 8/22 | 8/2 |
| Ando et al (2003)22 | 61 | 12/49 | 50 (82) | 58 ± 11 | 40 ± 16 | 56 ± 24 | 42 ± 24 | 78 ± 65 | NR | 38/NR |
| Michel et al (2004)23 | 74 | 21/53 | 16 (22) | 52.5 ± 15.9 | 38.5 ± 18.3 | 32 ± 15 | 26 ± 17‡ | 10.2 y | 9/25 | 0/NR |
| Takahashi et al (2004)24 | 20 | 5/15 | 15 (75) | 54 ± 13 | 46 ± 18 | 40 ± 27 | 39 ± 22 | 51 ± 15 | NR | 7/13 |
| Sato et al (2004)25 | 53 | 16/37 | 39 (74) | 62 (37–87)§ | 52 (39–77)§ | 54 ± 17 | 59 ± 22 | 59 (6–624)§ | 27/53 | NR |
| Ando et al (2004)26 | 20 | 5/15 | 17 (85) | 62 (38–83)‖ | 48 (4–86) | 41 (12–82) | NR | NR | NR | |
| Nomura et al (2004)27 | 42 | 15/27 | 28 (66) | NR | NR | 29 ± 6 | 31 ± 5 | NR | NR | 21/21 |
| Veneri et al (2005)28 | 52 | 23/29 | 34 (65) | 57 (24–72)¶ | NR | 57 ± 23 | NR | NR | NR | 34/6 |
| Inaba et al (2005)29 | 35 | 11/24 | 25 (71) | 57 (25–82)* | 52 ± 26 | 40 | 0/35 | 8/27 | ||
| Stasi et al (2005)30 | 137 | 57/80 | 64 (47) | 58 ± 13 | 42 ± 16 | 42 ± 25 | 46 ± 23 | 25 ± 19 | 16/137 | 67/10 |
| Fujimura et al (2005)39 | 435 | 120/315 | 300 (69) | 59 ± 14 | 47 ± 16 | NA | NA | 8.2 ± 6.8 y | NR | 133/70 |
| Suzuki et al (2005)17 | 36 | NR | 25 (69) | NR | NR | 55 ± 27 | NR | NR | NR | NR |
| Suvajdzić et al (2006)31 | 54 | 12/42 | 39 (72) | 54 ± 13 | 42 ± 16 | 68 ± 32 | 78 ± 32 | 6 (1–30) y | 0/54 | NR |
| Ahn et al (2006)32 | 15 | 5/10 | 15 (100)# | 56.8 ± 18.5 | NA | 72 ± 45 | NA | 104.4 ± 79.2 | 11/15 | 0/5 |
| Sayan et al (2006)33 | 34 | 22/12 | 20 (59) | 50.8 ± 16.2 | 53.8 ± 17.6 | 37 ± 16 | 39 ± 16 | 19 ± 15 | NR | NR |
| Asahi et al (2006)34 | 37 | 14/23 | 26 (70) | NR | NR | NR | NR | NR | NR | NR |
| Kodama et al (2007)35 | 116 | 32/74 | 67 (58) | 57.9 ± 14.3 | 47.8 ± 17.2 | 39 ± 29 | 30 ± 24 | NA | NA | NA |
| Campuzano-Maya (2007)40 | 32 | 7/25 | 29 (91) | NR | NR | 57 ± 38 | NR | NR | NR | NR |
| Estrada-Gómez et al (2007)36 | 23 | NR | 14 (61) | NR | NR | NR | NR | NR | NR | NR |
| Satake et al (2007)37 | 38 | 9/29 | 12 (68) | NR | NR | NR | NR | NR | NR | 19/8 |
| Emilia et al (2007)38 | 75 | 36/39 | 38 (51) | 58 ± 19 | 49.9 ± 20.7 | 41 ± 24 | 28 ± 18 | 24.4 ± 30.1 | 24/75 | 36/4 |
| Study . | No. of patients . | Male/female . | No. of infected patients (%) . | Age of infected patients, y . | Age of uninfected patients, y . | Platelet count of infected patients, ×109/L . | Platelet count of uninfected patients, ×109/L . | ITP duration, mo . | Concomitant therapy . | Untreated/splenectomized patients . |
|---|---|---|---|---|---|---|---|---|---|---|
| Gasbarrini et al (1998)2 | 18 | 5/13 | 11 (61) | 43 ± 14 | 49 ± 12 | 95 ± 39 | 103 ± 24 | NR | NR | <NR |
| Jarque et al (2001)18 | 56 | 18/38 | 40 (71) | 54 (17–80)* | 57 ± 22† | 58 ± 23 | 32 (2–50) | 0/56 | 39/4 | |
| Kohda et al (2002)19 | 40 | 12/28 | 25 (62) | 54 ± 14 | 48 ± 13 | 67 ± 54 | 60 ± 41 | 41 ± 38 | 19/40 | 13/NR |
| Hino et al (2003)20 | 30 | 8/22 | 21 (70) | 55 ± 15 | 51 ± 17 | 38 ± 20 | 22 ± 12 | NR | 7/30 | 15/1 |
| Hashino et al (2003)21 | 22 | 9/13 | 14 (64) | 53.2 ± 12.9 | 41.8 ± 18.6 | 61 ± 26 | 63 ± 20 | 110 ± 81 | 8/22 | 8/2 |
| Ando et al (2003)22 | 61 | 12/49 | 50 (82) | 58 ± 11 | 40 ± 16 | 56 ± 24 | 42 ± 24 | 78 ± 65 | NR | 38/NR |
| Michel et al (2004)23 | 74 | 21/53 | 16 (22) | 52.5 ± 15.9 | 38.5 ± 18.3 | 32 ± 15 | 26 ± 17‡ | 10.2 y | 9/25 | 0/NR |
| Takahashi et al (2004)24 | 20 | 5/15 | 15 (75) | 54 ± 13 | 46 ± 18 | 40 ± 27 | 39 ± 22 | 51 ± 15 | NR | 7/13 |
| Sato et al (2004)25 | 53 | 16/37 | 39 (74) | 62 (37–87)§ | 52 (39–77)§ | 54 ± 17 | 59 ± 22 | 59 (6–624)§ | 27/53 | NR |
| Ando et al (2004)26 | 20 | 5/15 | 17 (85) | 62 (38–83)‖ | 48 (4–86) | 41 (12–82) | NR | NR | NR | |
| Nomura et al (2004)27 | 42 | 15/27 | 28 (66) | NR | NR | 29 ± 6 | 31 ± 5 | NR | NR | 21/21 |
| Veneri et al (2005)28 | 52 | 23/29 | 34 (65) | 57 (24–72)¶ | NR | 57 ± 23 | NR | NR | NR | 34/6 |
| Inaba et al (2005)29 | 35 | 11/24 | 25 (71) | 57 (25–82)* | 52 ± 26 | 40 | 0/35 | 8/27 | ||
| Stasi et al (2005)30 | 137 | 57/80 | 64 (47) | 58 ± 13 | 42 ± 16 | 42 ± 25 | 46 ± 23 | 25 ± 19 | 16/137 | 67/10 |
| Fujimura et al (2005)39 | 435 | 120/315 | 300 (69) | 59 ± 14 | 47 ± 16 | NA | NA | 8.2 ± 6.8 y | NR | 133/70 |
| Suzuki et al (2005)17 | 36 | NR | 25 (69) | NR | NR | 55 ± 27 | NR | NR | NR | NR |
| Suvajdzić et al (2006)31 | 54 | 12/42 | 39 (72) | 54 ± 13 | 42 ± 16 | 68 ± 32 | 78 ± 32 | 6 (1–30) y | 0/54 | NR |
| Ahn et al (2006)32 | 15 | 5/10 | 15 (100)# | 56.8 ± 18.5 | NA | 72 ± 45 | NA | 104.4 ± 79.2 | 11/15 | 0/5 |
| Sayan et al (2006)33 | 34 | 22/12 | 20 (59) | 50.8 ± 16.2 | 53.8 ± 17.6 | 37 ± 16 | 39 ± 16 | 19 ± 15 | NR | NR |
| Asahi et al (2006)34 | 37 | 14/23 | 26 (70) | NR | NR | NR | NR | NR | NR | NR |
| Kodama et al (2007)35 | 116 | 32/74 | 67 (58) | 57.9 ± 14.3 | 47.8 ± 17.2 | 39 ± 29 | 30 ± 24 | NA | NA | NA |
| Campuzano-Maya (2007)40 | 32 | 7/25 | 29 (91) | NR | NR | 57 ± 38 | NR | NR | NR | NR |
| Estrada-Gómez et al (2007)36 | 23 | NR | 14 (61) | NR | NR | NR | NR | NR | NR | NR |
| Satake et al (2007)37 | 38 | 9/29 | 12 (68) | NR | NR | NR | NR | NR | NR | 19/8 |
| Emilia et al (2007)38 | 75 | 36/39 | 38 (51) | 58 ± 19 | 49.9 ± 20.7 | 41 ± 24 | 28 ± 18 | 24.4 ± 30.1 | 24/75 | 36/4 |
Results are given as mean plus or minus standard deviation, or as median (range), unless otherwise noted.
NR indicates not reported; and NA, not assessable.
Age of all patients.
Values of 23 patients who underwent eradication therapy.
H pylori–negative patients who underwent eradication therapy.
Mean (range).
Platelet count of all patients.
Median values (range).
This series included only infected patients.
Of 584 H pylori–positive patients in 11 studies with detailed medication history,19-24,30,32,37-39 227 (38.9%) had not received any treatment before eradication and 341 (58.4%) had received corticosteroids. Conventional ITP treatments such as corticosteroids, azathioprine or danazole, were given concomitantly with the eradication therapy in 121 (21%) patients. Of 699 H pylori–positive patients in 15 studies with available information,19-21,23-25,27-32,37-39 116 (16.6%) underwent splenectomy.
Eradication of H pylori infection
The effects of eradication therapy could be assessed in 825 (83%) of the 998 infected patients. Eradication of the infection was noted in 699 (84%) of these 825 patients. The random effects eradication proportion was 89.7% (95% CI, 85.1%-93.5%). With the exception of one study from Japan,27 with an unusually low eradication success rate (42.9%), all studies reported an eradication rate in excess of 70%.
In all but one study eradication therapy consisted of the so-called triple therapy, a combination of amoxicillin, clarithromycin, and a proton pump inhibitor usually given for 1 or 2 weeks. In a single report from Mexico, a 4-drug regimen was used, which consisted of the same 3 medications plus bismuth subsalicylate.36
Response of platelet count to eradication therapy
In most reports, complete and partial responses were defined according to the achievement of predefined platelet count thresholds; however, these thresholds varied. According to the criteria adopted for this systematic review, complete and overall responses were demonstrated in 42.7% (95% CI, 31.8%-53.9%) and 50.3% (95% CI, 41.6%-59.0%) of 696 patients, respectively. Restricting the analysis to patients with a base-line platelet count of no more than 30 × 109/L (15 studies, 222 patients with H pylorieradicated), the complete response rate was 20.1% (95% CI, 13.5%-26.7%) and the overall response rate was 35.2% (95% CI, 28.0%–42.4%).
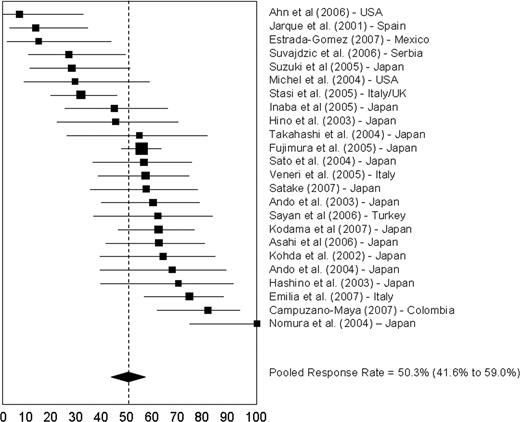
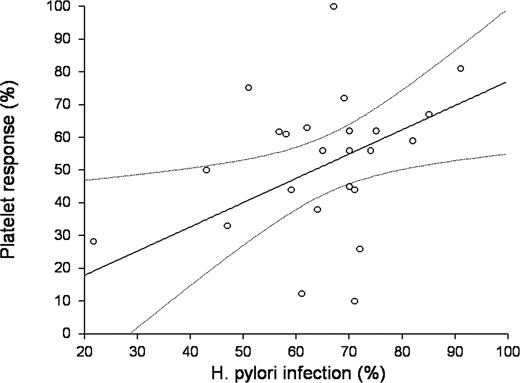
Responses ranged from 7% in an American series32 to 100% in a study from Japan27 (Figure 3). A subset analysis in studies from Japan revealed a complete response rate of 43.5% (95% CI, 31.1%-56.3%) and an overall response rate of 57.9% (95% CI, 50.1%-65.4%). The complete and overall response rates for patients from other countries were 27.3% (95% CI, 8.9%-51.2%) and 38.3% (95% CI, 50.1%-65.4%), respectively. Regression analysis indicated a significant positive correlation between the prevalence of H pylori infection in the ITP patients in the study and platelet response to eradication therapy (r = 0.351, P = .018; Figure 4).
Response rates to H pylori eradication. Solid boxes indicate the response rates and their dimensions are proportional to the weight of the study. Horizontal lines indicate 95% CIs. The dashed vertical line indicates the weighted mean response rate for all studies (56.5%). Of 11 studies with response rates in excess of 56.5%, 8 were from Japan; of 13 studies with response rates less than 56.5%, 6 were from Japan. One study from Gasbarrini et al (Italy)2 did not report the response rate and was excluded from the analysis of the pooled estimate.
Response rates to H pylori eradication. Solid boxes indicate the response rates and their dimensions are proportional to the weight of the study. Horizontal lines indicate 95% CIs. The dashed vertical line indicates the weighted mean response rate for all studies (56.5%). Of 11 studies with response rates in excess of 56.5%, 8 were from Japan; of 13 studies with response rates less than 56.5%, 6 were from Japan. One study from Gasbarrini et al (Italy)2 did not report the response rate and was excluded from the analysis of the pooled estimate.
Correlation between prevalence of infection and response rate. There was a significant positive correlation between the 2 variables (simple Pearson coefficient r = 0.3510, P = .018). The equation of the line relating response rate (y) and prevalence of infection (x) is estimated as: y = (7.2148) + (0.6744) x. The slope (r correlation coefficient), that is, the estimated change in response rate per unit change in the prevalence of infection, is 0.6744 with a standard error of 0.3058. The value of R2, the proportion of the variation in the response rate that can be accounted for by variation in the prevalence of infection, is 0.2038. The lower limit of the 95% CI for the slope is 0.0343 and the upper limit is 1.3144. The estimated intercept is 7.2148. The lower limit of the 95% CI for the intercept is −36.2226 and the upper limit is 50.6521 (curved dotted lines).
Correlation between prevalence of infection and response rate. There was a significant positive correlation between the 2 variables (simple Pearson coefficient r = 0.3510, P = .018). The equation of the line relating response rate (y) and prevalence of infection (x) is estimated as: y = (7.2148) + (0.6744) x. The slope (r correlation coefficient), that is, the estimated change in response rate per unit change in the prevalence of infection, is 0.6744 with a standard error of 0.3058. The value of R2, the proportion of the variation in the response rate that can be accounted for by variation in the prevalence of infection, is 0.2038. The lower limit of the 95% CI for the slope is 0.0343 and the upper limit is 1.3144. The estimated intercept is 7.2148. The lower limit of the 95% CI for the intercept is −36.2226 and the upper limit is 50.6521 (curved dotted lines).
Significant responses in patients in whom eradication of H pylori infection was not successful were described only in a large retrospective Japanese survey.39 In that study, 15 of 46 patients (33%) in whom eradication therapy failed nonetheless developed a platelet response. On the other hand, none of the 41 uninfected patients in 6 studies20,23,24,26,29,34 who received eradication therapy showed any significant platelet recovery. The details for the platelet count responses of individual studies are reported in Table 3.
Results of H pylori eradication
| Study . | Bacterial eradication (%)* . | Platelet response (%)† . | Platelet count before eradication, ×109/L‡ . | Platelet count after eradication, ×109/L‡ . | Follow-up duration, mo§ . | No. of relapsed patients . |
|---|---|---|---|---|---|---|
| Gasbarrini et al (1998)2 | 8/11 (73) | NA | 95 ± 39 | 140 ± 34 | 4 | NR |
| Jarque et al (2001)18 | 23/32 (72) | 3/23 (13) | 58 ± 24 | 65 ± 32 | 21 (18–24) | 2 |
| Kohda et al (2002)19 | 19/19 (100) | 12/19 (63) | 67 ± 54 | 120 ± 50 | 14.8 (9–39) | 0 |
| Hino et al (2003)20 | 18/21 (86) | 8/18 (44) | 37 ± 21 | 67 ± 54 | 37.8 | NR |
| Hashino et al (2003)21 | 13/14 (93) | 9/13 (69) | 58 ± 30 | 99 ± 56 | 15 | 1 |
| Ando et al. (2003)22 | 27/29 (93) | 16/27 (59) | 56 ± 24 | 93 ± 49 | 11 (4–15) | 1 |
| Michel et al (2004)23 | 14/15 (93) | 4/14 (29) | 32 ± 15 | 66 ± 98 | 11.5 (3–18) | 1 |
| Takahashi et al (2004)24 | 13/15 (87) | 7/13 (54) | 40 ± 27 | 101 ± 86 | 4 | NR |
| Sato et al (2004)25 | 27/32 (84) | 15/27 (56) | 54 ± 17 | 110 ± 21 | 12 | 0 |
| Ando et al (2004)26 | 15/17 (88) | 10/15 (67) | 49 ± 26 | 168 ± 43 | 24 | 0 |
| Nomura et al (2004)27 | 12/28 (43) | 12/12 (100) | 29 ± 6 | 78 ± 11 | NR | NR |
| Veneri et al (2005)28 | 32/34 (94) | 18/32 (56) | 57 ± 23 | 122 ± 33 | 24.2 (3–62) | 1 |
| Inaba et al (2005)29 | 25/25 (100) | 11/25 (44) | 52 ± 26 | NR | NR | 0 |
| Stasi et al (2005)30 | 52/52 (100) | 16/52 (31) | 42 ± 25 | 129 ± 61 | 25 (7–42) | 6 |
| Fujimura et al (2005)39 | 161/207 (78) | 88/161 (55) | NR | NR | 12 (3–12) | NR |
| Suzuki et al (2005)17 | 22/25 (88) | 6/22 (28) | 55 ± 27 | 114 ± 90 | 6 | NR |
| Suvajdzić et al (2006)31 | 23/30 (77) | 6/23 (26) | 68 ± 33 | 84 ± 45 | 18 (14–32) | 0 |
| Ahn et al (2006)32 | 15/15 (100) | 1/15 (7) | 72 ± 45 | 69 ± 65 | (6–24) | 1 |
| Sayan et al (2006)33 | 18/20 (90) | 11/18 (61) | 39 ± 16 | 100 ± 63 | 11 (4–24) | 0 |
| Asahi et al (2006)34 | 26/26 (100) | 16/26 (61) | 35 ± 13 | 114 ± 61 | >12 | 0 |
| Kodama et al (2007)35 | 44/52 (85) | 27/44 (61) | 40 ± 29 | NR | NR | NR |
| Campuzano-Maya (2007)40 | 26/29 (90) | 21/26 (81) | 57 ± 38 | 198 ± 98 | 12.2 | NR |
| Estrada-Gómez et al (2007)36 | 14/14 (100) | 2/14 (14) | NR | NR | 5 (2–7) | 1 |
| Satake et al (2007)37 | 23/25 (92) | 13/23 (57) | NR | NR | 25.4‖ (6–48) | 0 |
| Emilia et al (2007)38 | 34/38 (89) | 25/34 (74) | 41 ± 24 | 134 ± 96 | 43.5 (18–90) | 1 |
| Study . | Bacterial eradication (%)* . | Platelet response (%)† . | Platelet count before eradication, ×109/L‡ . | Platelet count after eradication, ×109/L‡ . | Follow-up duration, mo§ . | No. of relapsed patients . |
|---|---|---|---|---|---|---|
| Gasbarrini et al (1998)2 | 8/11 (73) | NA | 95 ± 39 | 140 ± 34 | 4 | NR |
| Jarque et al (2001)18 | 23/32 (72) | 3/23 (13) | 58 ± 24 | 65 ± 32 | 21 (18–24) | 2 |
| Kohda et al (2002)19 | 19/19 (100) | 12/19 (63) | 67 ± 54 | 120 ± 50 | 14.8 (9–39) | 0 |
| Hino et al (2003)20 | 18/21 (86) | 8/18 (44) | 37 ± 21 | 67 ± 54 | 37.8 | NR |
| Hashino et al (2003)21 | 13/14 (93) | 9/13 (69) | 58 ± 30 | 99 ± 56 | 15 | 1 |
| Ando et al. (2003)22 | 27/29 (93) | 16/27 (59) | 56 ± 24 | 93 ± 49 | 11 (4–15) | 1 |
| Michel et al (2004)23 | 14/15 (93) | 4/14 (29) | 32 ± 15 | 66 ± 98 | 11.5 (3–18) | 1 |
| Takahashi et al (2004)24 | 13/15 (87) | 7/13 (54) | 40 ± 27 | 101 ± 86 | 4 | NR |
| Sato et al (2004)25 | 27/32 (84) | 15/27 (56) | 54 ± 17 | 110 ± 21 | 12 | 0 |
| Ando et al (2004)26 | 15/17 (88) | 10/15 (67) | 49 ± 26 | 168 ± 43 | 24 | 0 |
| Nomura et al (2004)27 | 12/28 (43) | 12/12 (100) | 29 ± 6 | 78 ± 11 | NR | NR |
| Veneri et al (2005)28 | 32/34 (94) | 18/32 (56) | 57 ± 23 | 122 ± 33 | 24.2 (3–62) | 1 |
| Inaba et al (2005)29 | 25/25 (100) | 11/25 (44) | 52 ± 26 | NR | NR | 0 |
| Stasi et al (2005)30 | 52/52 (100) | 16/52 (31) | 42 ± 25 | 129 ± 61 | 25 (7–42) | 6 |
| Fujimura et al (2005)39 | 161/207 (78) | 88/161 (55) | NR | NR | 12 (3–12) | NR |
| Suzuki et al (2005)17 | 22/25 (88) | 6/22 (28) | 55 ± 27 | 114 ± 90 | 6 | NR |
| Suvajdzić et al (2006)31 | 23/30 (77) | 6/23 (26) | 68 ± 33 | 84 ± 45 | 18 (14–32) | 0 |
| Ahn et al (2006)32 | 15/15 (100) | 1/15 (7) | 72 ± 45 | 69 ± 65 | (6–24) | 1 |
| Sayan et al (2006)33 | 18/20 (90) | 11/18 (61) | 39 ± 16 | 100 ± 63 | 11 (4–24) | 0 |
| Asahi et al (2006)34 | 26/26 (100) | 16/26 (61) | 35 ± 13 | 114 ± 61 | >12 | 0 |
| Kodama et al (2007)35 | 44/52 (85) | 27/44 (61) | 40 ± 29 | NR | NR | NR |
| Campuzano-Maya (2007)40 | 26/29 (90) | 21/26 (81) | 57 ± 38 | 198 ± 98 | 12.2 | NR |
| Estrada-Gómez et al (2007)36 | 14/14 (100) | 2/14 (14) | NR | NR | 5 (2–7) | 1 |
| Satake et al (2007)37 | 23/25 (92) | 13/23 (57) | NR | NR | 25.4‖ (6–48) | 0 |
| Emilia et al (2007)38 | 34/38 (89) | 25/34 (74) | 41 ± 24 | 134 ± 96 | 43.5 (18–90) | 1 |
NR indicates not reported; NA, not assessable.
Results are expressed as the total number of patients with bacterial eradication among the total number of treated patients.
Complete or partial response among patients with successful eradication.
Including noneradicated patients.
Median, with range in parentheses.
Mean value.
Time to response and response duration
Time of assessment for platelet response after eradication therapy was predefined, but variable from study to study. Most study designs involved the first assessment of the platelet count 1 month after eradication therapy, although for some studies the final assessment was at 6 months.21,25,34,41 However, platelet recovery in one report was observed as early as 3 days after eradication,20 and in another there was a rapid platelet increase by 1 week in roughly half of the responders.34 In an Italian study, the initial platelet count improvement was reported to occur most commonly 2 weeks after completion of eradication therapy.38 Responses have also been observed several weeks after eradication.21,34 The number of platelet counts needed to assess response was not explicitly mentioned in any study and, when data were available, a single platelet count for individual patients was reported. The minimum duration of the platelet increase that defined a response was 1 month in 1 study,22 3 months in 2 studies,32,38 and not stated in the other studies.
The duration of response was not reported in 4 studies.27,29,35,40 In the remaining studies, median duration of follow-up for responders ranged from 4 to 43.5 months. Responses were ongoing at the time of the report for more than 90% of responders (Table 3). In the Italian study with the longest follow-up (median 43.5 months, range 18–90 months), only 1 of 23 patients experienced an ITP recurrence 7 or more months after eradication.38 Relapses of ITP have anecdotally been associated with H pylori reinfection, and may respond to a second eradication therapy.30
Predictors of a response to H pylori eradication therapy
Clinical characteristics were analyzed for their association with platelet responses in 13 studies, including a total of 511 cases where the bacteria were affectively eradicated (Table 4). Predictors of response appeared to be as heterogeneous from study to study as the response rates. The most consistently reported characteristic associated with better chances of response was a shorter ITP duration, which was found significant in 3 studies with a total of 257 cases of H pylori infection.30,35,39 In particular, disease duration was 89.1 (± 71.2) months in responders versus 181.00 (± 124.6) months in nonresponders (P < .001) in the study by Kodama et al35 ; 14 (± 7) months versus 34 (± 23) months (P = .035) in the study by Stasi et al30 ; and 78.24 (± 56.04) months versus 118.2 (± 93.24) months (P < .001) in the report by Fujimura et al39 There are conflicting reports about the predictive value of some characteristics such as age and baseline platelet count. A genetic predisposition to response has been suggested by an Italian study, in which the presence of the HLA-DQB1*03 allele was associated with higher response rates.28 In one study, a complete response was associated with no prior prednisone therapy,22 and in another concomitant corticosteroid therapy was reported to be slightly more common in nonresponders than in responders.25 Finally, 5 studies, including a total of 130 patients, reported no association between background characteristics and platelet response.17,29,31,34,38
Predictors of response to eradication therapy
| Study . | No. of bacterial eradication cases . | Characteristics predicting response . | Characteristics not predicting response . |
|---|---|---|---|
| Ando et al (2003)22 | 27 | No prior corticosteroid therapy | Sex, age, ITP duration, baseline platelet count, pretreatment of H pylori antibody levels and PAIgG levels |
| Sato et al (2004)25 | 27 | No concomitant corticosteroid therapy | Sex, age, ITP duration, baseline platelet count, previous splenectomy |
| Ando et al (2004)26 | 15 | Age ≥ 60 y at the time of ITP diagnosis | None |
| Veneri et al (2005)28 | 32 | HLA-DQB1*03 allele | None |
| Inaba et al (2005)29 | 25 | None | Sex, age, ITP duration, baseline platelet count, PAIgG levels, previous drug therapy, previous splenectomy |
| Stasi et al (2005)30 | 52 | Age < 65 y, higher baseline platelet count, shorter ITP duration, no prior therapy for ITP | Sex, PAIgG levels |
| Fujimura et al (2005)39 | 161 | Shorter ITP duration | Sex, age, baseline platelet count, ITP treatment before eradication |
| Suzuki et al (2005)17 | 22 | None | Sex, age, ITP duration, previous corticosteroid therapy |
| Suvajdzić et al (2006)31 | 23 | None | Previous corticosteroid therapy, response to corticosteroids |
| Asahi et al (2006)34 | 26 | None | Antinuclear antibodies |
| Kodama et al (2007)35 | 44 | Shorter ITP duration | Sex, age, baseline platelet count, drug treatment before eradication, splenectomy, serum levels of gastrin, pepsinogen and anti-CagA antibodies |
| Satake et al (2007)37 | 23 | Lower baseline platelet count | Sex, age, ITP treatment before eradication, history of peptic ulcer |
| Emilia et al (2007)38 | 34 | None | Sex, age, ITP duration, baseline platelet count, ITP treatment before eradication |
| Study . | No. of bacterial eradication cases . | Characteristics predicting response . | Characteristics not predicting response . |
|---|---|---|---|
| Ando et al (2003)22 | 27 | No prior corticosteroid therapy | Sex, age, ITP duration, baseline platelet count, pretreatment of H pylori antibody levels and PAIgG levels |
| Sato et al (2004)25 | 27 | No concomitant corticosteroid therapy | Sex, age, ITP duration, baseline platelet count, previous splenectomy |
| Ando et al (2004)26 | 15 | Age ≥ 60 y at the time of ITP diagnosis | None |
| Veneri et al (2005)28 | 32 | HLA-DQB1*03 allele | None |
| Inaba et al (2005)29 | 25 | None | Sex, age, ITP duration, baseline platelet count, PAIgG levels, previous drug therapy, previous splenectomy |
| Stasi et al (2005)30 | 52 | Age < 65 y, higher baseline platelet count, shorter ITP duration, no prior therapy for ITP | Sex, PAIgG levels |
| Fujimura et al (2005)39 | 161 | Shorter ITP duration | Sex, age, baseline platelet count, ITP treatment before eradication |
| Suzuki et al (2005)17 | 22 | None | Sex, age, ITP duration, previous corticosteroid therapy |
| Suvajdzić et al (2006)31 | 23 | None | Previous corticosteroid therapy, response to corticosteroids |
| Asahi et al (2006)34 | 26 | None | Antinuclear antibodies |
| Kodama et al (2007)35 | 44 | Shorter ITP duration | Sex, age, baseline platelet count, drug treatment before eradication, splenectomy, serum levels of gastrin, pepsinogen and anti-CagA antibodies |
| Satake et al (2007)37 | 23 | Lower baseline platelet count | Sex, age, ITP treatment before eradication, history of peptic ulcer |
| Emilia et al (2007)38 | 34 | None | Sex, age, ITP duration, baseline platelet count, ITP treatment before eradication |
PAIgG indicates platelet-associated IgG.
In addition to individual associations, the response rates were higher in areas where H pylori is more prevalent (Figure 4).
Toxicities
In the 10 reports (482 patients) that described toxicities,20-23,25,29,30,37-39 56 patients (12.4%) experienced mild or moderate diarrhea with or without abdominal pain, and 1 patient also reported vomiting that resulted in discontinuation of the eradication regimen. In an additional patient medication was discontinued due to skin rash on the fourth day of treatment.
Discussion
This article summarizes the effects of the eradication of H pylori infection in patients with a diagnosis of ITP. The presence of just one controlled trial and the predominant use of observational data from retrospective series admittedly subjected the review to possible bias. Despite the relevant heterogeneity of treatment outcomes, which would usually argue against the use of pooled estimates, we decided to use meta-analytical methods for the following reasons: (1) the lack of publication bias; (2) the comparable number of studies with similar weight at both extremes of the response-rate spectrum; and (3) the availability of individual-level data for many of the case series included in our review that could be tabulated and analyzed rigorously according to predefined assessment criteria.
Patient demographics in patients with ITP reflect what has been found in the general population.42 H pylori infection in patients with ITP varies greatly from country to country, being higher in developing countries and lower in industrialized countries, and the prevalence increases with age with no difference between men and women.
The primary efficacy analysis addressed whether eradication of the bacterium results in a sustained platelet response and found that it did in roughly 50% of adults with ITP. There was sizeable variation in the response rate among the various series and the predefined secondary analyses explored which factors might underlie this difference.
There were no specific control groups, and very few uninfected patients received eradication therapy. However, in the one study in which the response rate is given for patients where eradication therapy failed, 15 of 46 (36%) responded.39 Also in 6 studies, in which just 41 uninfected patients received eradication therapy, none showed a platelet response.20,23,24,26,29,34 This finding would imply that the platelet response may be influenced by the eradication of H pylori, rather than the eradication therapy.
When looking at the results of the pooled analysis, it is important to remark that most of the studies were from Japan and that Japanese patients accounted for almost two-thirds of the ITP population analyzed in this review. The preponderance of reports from one country where infection rates and response to eradication therapy are consistently higher than in other countries adds another potential element of bias to our analysis and suggests caution in the generalizability of results.
Response rates appeared to be lower in those patients with more severe thrombocytopenia, but this finding may not be accurate. In fact, our analysis was hampered by the lack of data for individual patients in some of the Japanese studies, in which high response rates were seen even in cohorts with low mean platelet counts. These findings contradict the generally held impression that H pylori eradication is not effective in markedly thrombocytopenic patients with ITP.
For many of the studies, the follow-up period was relatively short, but the minimum time was 4 months. While not optimal, this may be a clinically meaningful outcome for a patient with chronic ITP. Nevertheless, the real impact of H pylori eradication on the natural history of ITP, specifically the effects over years after eradication, remains to be fully demonstrated. Furthermore, a substantial proportion of patients had been previously treated or was receiving immunosuppressive treatment when eradication therapy was started. This factor could potentially confound the platelet response after bacterial eradication either by preventing eradication of the bacterium or by combining therapeutic effects.
Two other systematic reviews on the effects of H pylori eradication on the platelet count have been published.12,13 While using different methods and assessing considerably fewer studies and patients than the present review, these other studies drew similar conclusions. In the first review, the overall response rate was 52% (arithmetic mean) in 193 subjects in whom H pylori was eradicated, with cohorts from Japan and Italy reporting higher response rates.12 The second review involved data from 788 ITP patients in 17 studies.13 There was a statistically significant difference in the increase in platelet count in patients in whom eradication was successful compared with control groups. In a metaregression model, the success of H pylori eradication was highly significant as an explanatory variable for platelet count increase.
Because of the very small number of patients with relapses in the published reports, the impact of H pylori retesting has not been elucidated. Our own limited experience suggests that patients with relapses can be reinfected and benefit from repeat eradication therapy.30
The response rate reported here is roughly equivalent to that found to rituximab,43 a similarity that may not be coincidental. Several studies have shown evidence of abnormalities of T-cell subsets in ITP, and 2 studies have demonstrated that failure to normalize these abnormalities in response to either splenectomy44 or rituximab45 is associated with a lower rate of response to these therapies. This may be relevant to the response to H pylori eradication where a proportion of patients are known to have complicated T-cell abnormalities and may have less response to immunomodulatory therapy. This hypothesis needs to be prospectively investigated.
This review also demonstrates a correlation between the H pylori infection rate in the countries studied and the response rate to eradication therapy. Interestingly, platelet response rates were higher in the series with a higher prevalence of H pylori infection. One mechanism for this association may relate to the CagA-positive strains of H pylori. This strain has been identified as a pathogenic candidate for ITP in 2 molecular studies demonstrating molecular mimicry of CagA to platelet antigens and associations between eradication of H pylori and both disappearance of anti-CagA antibodies and an increase in the platelet count.24,46
The CagA positivity of H pylori varies depending upon geographic location. For example, in Japan, where both infection rates and response rates to eradication therapy are high, most H pylori strains express CagA, whereas the proportion of CagA-positive strains in Western countries is much lower.47,48 Furthermore, the amino acid sequence of H pylori CagA protein is known to show significant diversity among the East Asian and Western strains.49 The immunologic response to H pylori and especially to eradication therapy has not yet been fully assessed and therefore the understanding of the mechanisms behind the heterogeneous responses to the bacterium and its eradication remain limited. Expression of CagA is almost always associated with increased inflammation and more severe disease,50 and contributes to shifting the Th1/Th2 balance in favor of Th1 by a variety of mechanisms involving induction of lymphocyte cell-cycle arrest.51 In this regard, it is noteworthy that chronic ITP is also associated with a polarized Th1-type.45,52 Recent data also indicate that CagA causes changes in epithelial cells, including an increase in expression of plasminogen activator inhibitor-1, which may contribute to a lack of tolerance in addition to molecular mimicry.53 Accordingly, it may be speculated that CagA-positive strains create an immunologic environment that facilitates the onset and/or persistence of ITP.
Interestingly, our review shows that clinical responses may occur as early as 1 week from initiation of eradication therapy, before antibody synthesis by plasma cells is affected. Two recent studies have provided clues that can account for this observation. In the first study, Semple and colleagues demonstrated that in the presence of antiplatelet antibodies, the lipopolysaccharide of Gram-negative bacteria can significantly enhance Fc-dependent platelet phagocytosis.54 In the second study, Asahi et al demonstrated that improvements in autoimmune and platelet kinetic parameters 1 week after starting the H pylori eradication regimen were associated decreased phagocytic capacity and modulation of the inhibitory Fcγ receptor IIB (FcγRIIB) in peripheral blood monocytes.55
Given the findings of this meta-analysis, should patients with ITP be routinely screened for H pylori? Because of the statistical limitations of the analysis we have performed, we were not able to identify a target population that might most benefit from H pylori screening and eradication. The identification of this subpopulation represents the challenge of the near future. Consideration should be given to the low costs, the noninvasiveness of diagnostic methods, and the favorable toxicity profile of eradication therapy compared with standard ITP therapy. H pylori screening appears certainly worthwhile in Japan, a country with a high background prevalence of the infection, where significant response rates have been consistently reported. For countries, such as the United States, in which both the prevalence of infection and the response rates to eradication therapy are low, the assessment of the cost-benefit ratio needs to take into account several factors. First, the prevalence of ITP is low, and screening a small population of ITP patients for H pylori infection would not have a significant economical impact on the health care system's resources (the cost for the 13C-UBT and the H pylori stool test does not exceed US $100-$200 each). Second, even if only 1 in 100 patients with chronic ITP would benefit from H pylori eradication, the savings in terms of cost of drugs for that single patient would probably cover the cost of the whole screening program for 100 patients with ITP. The cost of intravenous immunoglobulin, often a second- and third-line agent, is around US $2500 for a 70-g dose, without considering costs related to administration. The cost of rituximab for the standard 4-week course at 375 mg/m2 is approximately US $12 000 without considering costs related to administration. Finally, the opportunity to treat ITP medically with less risk of both acute and chronic drug-related toxicity would be welcomed by patients and health care providers.
The recommendation for H pylori screening would include use of the UBT and stool antigen tests but not antibody testing because of its high rate of both false positive and false negative tests and especially its inability to determine whether infection is active or occurred in the past. The lack of correlation of duration of ITP with response to H pylori in many studies suggests that this screening, if undertaken, could occur at any point in time and not only at diagnosis. Because this approach has not thus far been examined, ideally its institution should be within the context of randomized controlled studies enrolling a large number of patients from various ethnic backgrounds, to determine a more precise response rate in association with predictors of response and to monitor the duration of response. Such randomized controlled trials, in addition to assessing the efficacy of this therapy, would also allow further immunologic investigation of why H pylori causes ITP and what mechanism is behind the response to eradication therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Thomas A. Trikalinos, from the Center for Clinical Evidence Synthesis, Institute for Clinical Research and Health Policy Studies, Tufts-New England Medical Center, Boston, MA, for helpful suggestions.
A.S. is a PhD candidate at the University of Cambridge, and this work is submitted in partial fulfillment of the requirement for the PhD.
Authorship
Contribution: R.S. and M.L.E. performed research and extracted data; J.O., A.S., and R.S. performed statistical analysis; J.B.S. and J.B.B. reviewed study design and methods; and R.S., A.S., N.C., D.P., A.N., S.A., and J.B.B. contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Roberto Stasi, Department of Medical Sciences, Regina Apostolorum. Hospital, Via S Francesco, 50, 00041 Albano Laziale, Italy; e-mail: roberto.stasi@uniroma2.it.