Abstract
Among the most common HIV-associated lymphomas are Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL) with immunoblastic-plasmacytoid differentiation (also involving the central nervous system). Lymphomas occurring specifically in HIV-positive patients include primary effusion lymphoma (PEL) and its solid variants, plasmablastic lymphoma of the oral cavity type and large B-cell lymphoma arising in Kaposi sarcoma herpesvirus (KSHV)–associated multicentric Castleman disease. These lymphomas together with BL and DLBCL with immunoblastic-plasmacytoid differentiation frequently carry EBV infection and display a phenotype related to plasma cells. EBV infection occurs at different rates in different lymphoma types, whereas KSHV is specifically associated with PEL, which usually occurs in the setting of profound immunosuppression. The current knowledge about HIV-associated lymphomas can be summarized in the following key points: (1) lymphomas specifically occurring in patients with HIV infection are closely linked to other viral diseases; (2) AIDS lymphomas fall in a spectrum of B-cell differentiation where those associated with EBV or KSHV commonly exhibit plasmablastic differentiation; and (3) prognosis for patients with lymphomas and concomitant HIV infection could be improved using better combined chemotherapy protocols in-corporating anticancer treatments and antiretroviral drugs.
Introduction
Infectious agents, mainly viruses, are among the few known causes of cancer and contribute to a variety of malignancies worldwide. The agents considered here, termed Epstein-Barr virus (EBV) and Kaposi sarcoma herpesvirus (KSHV–human herpesvirus 8 [HHV8]), are members of the gamma-herpesvirus subfamily.1
Since its discovery as the first human tumor virus, EBV has been implicated in the development of a wide range of B-cell lymphoproliferative disorders, including Burkitt lymphoma (BL), nasopharyngeal carcinoma, and Hodgkin and non-Hodgkin lymphomas (NHLs). KSHV, one of the most recently discovered human tumor viruses and the cause of Kaposi sarcoma (KS),2 also plays a role in the pathogenesis of primary effusion lymphoma [PEL], and multicentric Castleman disease [MCD]).3-6 Intriguingly, EBV and KSHV have been shown to associate with distinct lymphoproliferative diseases occurring most often in persons with HIV infection/AIDS7,8 or in association with other immunodeficiency conditions, such as iatrogenic immunodeficiency following solid organ transplantation.9,10
HIV-associated lymphoproliferative disorders are a heterogeneous group of diseases that arise in the presence of HIV-associated immunosuppression, a state that permits the unchecked proliferation of EBV- and KSHV-infected lymphocytes. Traditionally, these aggressive disorders mainly include both central nervous system and systemic lymphomas,11 whereas lymphomas specifically occurring in the setting of HIV infection include PEL and its solid variant, plasmablastic lymphoma (PBL) of the oral cavity type and large B-cell lymphoma arising in KSHV-associated MCD.12-18 Thus, HIV-related lymphomas are closely linked to EBV infection of the tumor clone or are associated with KSHV. PEL and its variants often involve EBV in addition to KSHV.19
We review here the current knowledge on these gamma-herpesvirus–associated lymphomas in the setting of HIV infection. The focus will be on pathology, diagnosis and classification, pathogenesis, and treatment of these lymphomas specifically occurring in HIV-induced immunodeficiency.
Gamma-herpesvirus–associated lymphomas in the setting of HIV infection
EBV-associated lymphomas
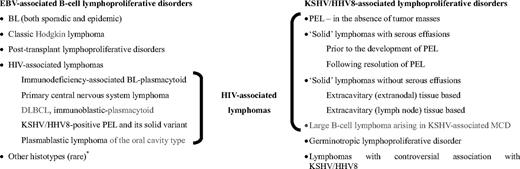
EBV has been implicated in the development of a wide range of B-cell lymphoproliferative disorders, including BL, classic Hodgkin lymphoma (HL), and lymphomas arising in immunocompromised individuals (posttransplantation and HIV-associated lymphoproliferative disorders; Figure 1).1,2 It is also associated with B-cell lymphomas in association with congenital immunodeficiencies, such as X-linked lymphoproliferative syndrome (XLP). T-cell lymphoproliferative disorders that have been reported to be EBV associated include a subset of peripheral T-cell lymphomas, angioimmunoblastic T-cell lymphoma, extranodal nasal type natural killer/T-cell lymphoma, and other rare histotypes.1,2
Relationship of HIV-associated lymphomas with EBV and KSHV/HHV8-associated lymphoproliferative disorders. *Other histotypes include lymphomatoid granulomatosis, DLBCL associated with chronic inflammation, EBV-positive DLBCL of the elderly. BL indicates Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; HHV8, human herpesvirus 8; KSHV, Kaposi sarcoma herpesvirus; MCD, multicentric Castleman disease; and PEL, primary effusion lymphoma.
Relationship of HIV-associated lymphomas with EBV and KSHV/HHV8-associated lymphoproliferative disorders. *Other histotypes include lymphomatoid granulomatosis, DLBCL associated with chronic inflammation, EBV-positive DLBCL of the elderly. BL indicates Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; HHV8, human herpesvirus 8; KSHV, Kaposi sarcoma herpesvirus; MCD, multicentric Castleman disease; and PEL, primary effusion lymphoma.
EBV-associated lymphomas in AIDS include BL, diffuse large B-cell lymphoma (DLBCL) with immunoblastic (IB) morphology, primary central nervous system lymphoma (PCNSL), KSHV+ PEL and its solid variant, and PBL of the oral cavity type.11-16,18 However, the percentage of cases within each histotypes with EBV viral infection is variable, ranging from 60% to 100%.
PEL, KSHV-associated solid lymphomas, and other KSHV-associated lymphomas
PEL and its extracavitary variant are by definition KSHV-associated lymphomas.11-14 KSHV-associated extracavitary lymphomas have been reported preceding the development of an effusion lymphoma or following resolution of PEL. Recently, the spectrum of KSHV-associated lymphoproliferative diseases in the HIV setting has been expanded by the identification of cases of KSHV-associated extracavitary lymphomas without serous effusions (Figure 1).13,14
In addition to PEL, KSHV is associated with another rare neoplastic lymphoproliferative disorder, namely large B-cell lymphoma arising in KSHV-associated MCD. KSHV-infected B cells in MCD have a pre–plasma cell phenotype and plasmacytic/plasmablastic morphology.17 A new KSHV-associated lymphoproliferative disorder has recently been described in HIV-seronegative persons.5 This disease, called germinotropic lymphoproliferative disorder, is characterized by plasmablasts that are coinfected by KSHV and EBV and preferentially involve the germinal centers of lymph nodes.
Relationship of HIV-associated lymphomas with EBV and KSHV infection
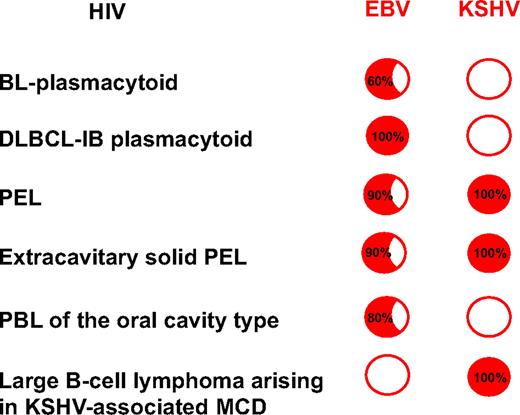
Figure 2 summarizes the relationship of HIV-associated lymphomas with EBV and KSHV infection. BL with plasmacytoid differentiation is often HIV associated and closely linked to EBV infection. When carrying EBV infection, the HIV-DLBCL-IB frequently displays a plasmacytoid differentiation. HIV-DLBCL-IB displays a phenotype mostly related to plasma cells while retaining features of the immunoblastic stage of B-cell development, suggesting that the normal cellular counterpart of AIDS-DLBCL-IB is a cell that might be defined as plasmablastic.20,21 PEL and its solid variant are universally linked to KSHV. Most HIV-associated PBLs of the oral cavity type are linked to EBV infection. Therefore, it follows that the spectrum of lymphomas occurring in HIV-infected patients is more clearly characterized thanks to the frequent plasma cell phenotype and the intriguing link to infection by gamma-herpesviruses. Although these lymphomas can occur in the general population, their presence in HIV-seropositive persons is predominant or almost exclusive.
The spectrum of HIV-associated lymphomas. Relationship with EBV and KSHV infection. The spectrum of lymphomas occurring in HIV-infected patients includes pathologic subtypes displaying specific association with distinct viruses. BL and DLBCL-IB with plasmacytoid differentiation are often HIV associated and closely linked to EBV infection. The HIV-associated DLBCL-IB is distinct from other large cell lymphomas occurring in both HIV-seropositive and -seronegative patients because HIV-associated DLBCL-IB lymphomas display a plasma cell–related phenotype; interestingly, the gene expression profile of PEL is plasmablastic. Therefore, most HIV-associated lymphoproliferative disorders, including primary central nervous system lymphoma, systemic DLBCL IB-plasmacytoid, KSHV + PEL and its solid variant, and PBLs of the oral cavity type, display a phenotype related to plasma cells and are linked to EBV infection. Red circle indicates positive infection (inside are the percentages); empty circle indicates no infection. DLBCL-IB indicates diffuse large B-cell lymphoma-immunoblastic; EBV, Epstein-Barr virus; KSHV, Kaposi sarcoma herpesvirus; MCD, multicentric Castleman disease; PBL, plasmablastic lymphoma; and PEL, primary effusion lymphoma.
The spectrum of HIV-associated lymphomas. Relationship with EBV and KSHV infection. The spectrum of lymphomas occurring in HIV-infected patients includes pathologic subtypes displaying specific association with distinct viruses. BL and DLBCL-IB with plasmacytoid differentiation are often HIV associated and closely linked to EBV infection. The HIV-associated DLBCL-IB is distinct from other large cell lymphomas occurring in both HIV-seropositive and -seronegative patients because HIV-associated DLBCL-IB lymphomas display a plasma cell–related phenotype; interestingly, the gene expression profile of PEL is plasmablastic. Therefore, most HIV-associated lymphoproliferative disorders, including primary central nervous system lymphoma, systemic DLBCL IB-plasmacytoid, KSHV + PEL and its solid variant, and PBLs of the oral cavity type, display a phenotype related to plasma cells and are linked to EBV infection. Red circle indicates positive infection (inside are the percentages); empty circle indicates no infection. DLBCL-IB indicates diffuse large B-cell lymphoma-immunoblastic; EBV, Epstein-Barr virus; KSHV, Kaposi sarcoma herpesvirus; MCD, multicentric Castleman disease; PBL, plasmablastic lymphoma; and PEL, primary effusion lymphoma.
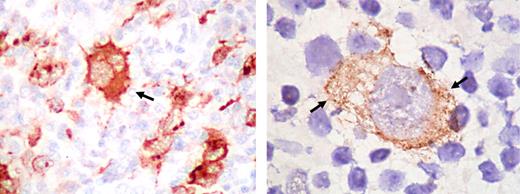
In contrast to other lymphomas, a high frequency of EBV association has been shown in HL (80%–100%) tissues from HIV-infected people and the EBV-transforming protein, EBV-encoded latent membrane protein-1 (LMP-1), is expressed in virtually all HIV-HL cases.22-28 On this basis, HL in HIV-infected persons appears to be an EBV-driven lymphoma (Figure 3).25
Expression of EBV LMP1 in the Reed-Sternberg cells of Hodgkin lymphoma. The immunostaining with LMP1 antibody is cytoplasmic (arrows). Original magnifications: ×400 (left), ×1000 (right). Images kindly provided by Dr L. Young, Birmingham. Images were assembled using Adobe Photoshop 6 (Adobe Systems, San Jose, CA).
Expression of EBV LMP1 in the Reed-Sternberg cells of Hodgkin lymphoma. The immunostaining with LMP1 antibody is cytoplasmic (arrows). Original magnifications: ×400 (left), ×1000 (right). Images kindly provided by Dr L. Young, Birmingham. Images were assembled using Adobe Photoshop 6 (Adobe Systems, San Jose, CA).
Pathologic features of lymphomas occurring specifically in HIV-induced immunodeficiency
PEL and its solid variant
PEL is a distinct type of B-cell non-Hodgkin lymphoma (NHL) that presents most frequently in body cavities as lymphomatous effusions without an associated tumor mass. In addition, some lymphomas occurring in HIV-infected individuals have very similar molecular and immunophenotypic characteristics, and yet do not involve body cavities. Thus, an extracavitary variant of PEL has been recognized. A defining property of PEL is its consistent association with KSHV infection. Most cases are also coinfected by EBV. It is believed that KSHV, rather than EBV, is a driving force in these tumors, as in PEL, at least 5 KSHV viral genes are expressed, which provide proliferative and antiapoptotic signals. In contrast, EBV has a restricted latency pattern of gene expression in PEL, where only EBNA1 and EBERs are expressed.
PELs have a distinctive set of morphologic and immunophenotypic properties, which prompted their description in the literature29,30 even before the discovery of KSHV. In cytospin preparations, the cells can have a range of appearances, from cells with anaplastic morphology to large immunoblastic or plasmablastic cells. Binucleated or multinucleated cells resembling Reed-Sternberg (RS) cells can be found. Nuclei are large with prominent nucleoli. The cytoplasm is usually abundant and is deeply basophilic. Some cases have cytoplasmic vacuoles and frequently a perinuclear hof consistent with plasmacytoid differentiation is seen. There is a high proliferation rate, as appreciated by numerous mitotic figures. The cells often appear more uniform in histologic sections than in cytospin preparations.31,32 Extracavitary PELs usually are immunoblastic in appearance, and have a high mitotic rate and variable amounts of apoptotic debris.13 However, they exhibit a spectrum of morphologic features, ranging from cells with moderate amounts of amphophilic to acidophilic cytoplasm with occasional perinuclear hofs and large nuclei containing a single prominent, centrally placed nucleolus, to cases with more variable and pleomorphic cells, some containing binucleated or multinucleated cells reminiscent of RS cells and variants. Some cases have a prominent “starry-sky” appearance.
PEL cells commonly express CD45, but lack pan-B-cell markers, including CD19, CD20, and CD79a as well as surface and cytoplasmic immunoglobulins.29,32 However, cases of extracavitary PEL express immunoglobulins somewhat more often than the classical effusion PEL.13 Expression of BCL6 is generally absent. Activation and plasma cell markers and miscellaneous non–lineage-associated antigens such as HLA-DR, CD30, CD38, Vs38c, CD138, and EMA are often expressed.11,12,18,20,21 PELs usually lack T/natural killer (NK)–cell antigens, although aberrant expression of T-cell markers may occur.33-35
Many cases are sent to cytopathology laboratories, where a cell block or smear shows the presence of neoplastic cells, but lymphoma may not be suspected. In conjunction with the aberrant phenotype these cases may be difficult to classify. A helpful procedure is immunohistochemistry for KSHV, which is best achieved with antibodies to the viral latency-associated nuclear antigen (LANA; ORF73). Positive staining shows characteristic nuclear dots. Extracavitary PELs are frequently classified as diffuse large cell, immunoblastic, or anaplastic large cell lymphomas in HIV+ individuals, and the diagnosis is made by immunohistochemistry for LANA, which allows demonstration of the presence of KSHV in practically all the lymphoma cells.13,36 Other KSHV proteins are also present in both cavitary and extracavitary PEL, in particular viral interleukin-6 (vIL-6) which is expressed by a variable proportion of neoplastic cells, so detection of this protein by immunohistochemistry can provide a confirmatory assay. In situ hybridization for EBV EBERs is also useful, as many cases contain both viral genomes. Further confirmation can be provided by molecular techniques, such as polymerase chain reaction documenting the presence of the viral genome.
PELs are of B-cell origin, which can be demonstrated by the presence of clonal immunoglobulin gene rearrangements. Evidence points toward a post–germinal center B-cell derivation, as most PELs contain somatic hypermutation of Ig genes as well as frequent somatic hypermutation of the noncoding region of the BCL6 gene.37,38 Consistent with this notion is the expression of plasma cell markers such as CD138/Syndecan-1. Recently, gene expression analysis of PEL showed features most similar to AIDS immunoblastic lymphoma and multiple myeloma, again indicating a pre–plasma cell or “plasmablastic” profile.21,39
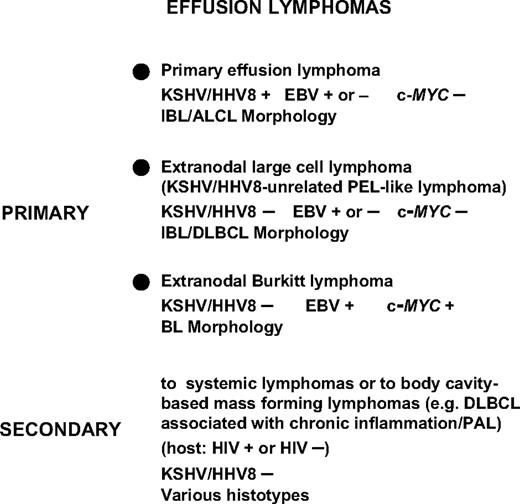
PEL and KSHV unrelated effusion lymphomas
PEL needs to be differentiated from those lymphomas occurring in patients in whom effusions complicate a tissue-based lymphoma, the so-called secondary lymphomatous effusion. However, secondary lymphomatous effusions closely mimic phenotypic and genotypic features of the corresponding tissue-based lymphoma and are consistently devoid of KSHV infection40-42 (Figure 4).
Classification and differential diagnosis of non-Hodgkin lymphomas involving the serous body cavities and presenting as effusion lymphomas. Lymphomas primarily involving the serous body cavities include a certain number of BLs, mainly occurring in the context of AIDS, which present as primary lymphomatous effusions without mass formation. The most specific biologic markers discriminating PEL from BL presenting as a primary lymphomatous effusion are represented by KSHV infection (assessed by ORF73/LNA-1 immunoreactivity), which clusters with PEL, and by translocation of the c-MYC proto-oncogene, which segregates with BL. KSHV-unrelated large B-cell lymphomas, also termed as KSHV-unrelated PEL-like lymphomas, can be differentiated from PEL because the neoplastic cells do not display evidence of KSHV infection, but display features related to large B-cell lymphoma. KSHV/HHV8 indicates Kaposi sarcoma herpesvirus/human herpesvirus 8; EBV, Epstein-Barr virus; IBL, immunoblastic lymphoma; ALCL, anaplastic large cell lymphoma; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; and PAL, pyothorax-associated lymphoma, now called DLBCL with chronic inflammation.
Classification and differential diagnosis of non-Hodgkin lymphomas involving the serous body cavities and presenting as effusion lymphomas. Lymphomas primarily involving the serous body cavities include a certain number of BLs, mainly occurring in the context of AIDS, which present as primary lymphomatous effusions without mass formation. The most specific biologic markers discriminating PEL from BL presenting as a primary lymphomatous effusion are represented by KSHV infection (assessed by ORF73/LNA-1 immunoreactivity), which clusters with PEL, and by translocation of the c-MYC proto-oncogene, which segregates with BL. KSHV-unrelated large B-cell lymphomas, also termed as KSHV-unrelated PEL-like lymphomas, can be differentiated from PEL because the neoplastic cells do not display evidence of KSHV infection, but display features related to large B-cell lymphoma. KSHV/HHV8 indicates Kaposi sarcoma herpesvirus/human herpesvirus 8; EBV, Epstein-Barr virus; IBL, immunoblastic lymphoma; ALCL, anaplastic large cell lymphoma; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; and PAL, pyothorax-associated lymphoma, now called DLBCL with chronic inflammation.
A more subtle diagnosis consists in differentiating PEL from other types of lymphomas primarily involving the serous body cavities that can present with a primary neoplastic effusion (Figure 4).41,43-47 Many of these cases are KSHV-unrelated large B-cell lymphomas, also termed KSHV-unrelated PEL-like lymphomas.45 KSHV-unrelated PEL-like lymphoma cases are associated with hepatitis C virus (HCV) (30%–40%). The most involved sites are peritoneum and pleura; lymphoma cells most commonly show large cell type morphology (80%) and B-cell immunophenotype (90%). In contrast, PEL cases are universally associated with KSHV and mostly occur in immunodeficiency states. They demonstrate distinctive morphology, and lack c-MYC gene rearrangement and B cell–associated antigen expression. It seems that PEL and KSHV-unrelated PEL-like lymphomas are different in terms of pathogenesis, morphologic-immunophenotypic features, clinical behavior, and prognosis.
Large B-cell lymphoma arising in KSHV-associated MCD
Our current knowledge indicates that Castleman disease48 (CD) actually represents several different clinicopathologic entities. Prior to the discovery of KSHV, 2 histopathologic types of CD had been described: (1) the hyaline vascular (HV) variant that is the most common form, affecting 90% of patients and usually involving a single lymph node in the mediastinum, and (2) the plasma cell (PC) variant, which is characterized by hyperplastic germinal centers, abundant plasma cells in the interfollicular areas, persistence of sinuses, and associated clinical and laboratory abnormalities.49 Two clinical entities were also described: (1) the localized form, which usually presents as lymph node hyperplasia in a single lymph node–bearing region (in most cases, the mediastinum) and that resolves with resection, and (2) MCD, which manifests as generalized lymphadenopathy with systemic symptoms and is characterized by a more aggressive clinical course and the potential for malignant transformation. MCD resembles the PC variant histopathologically, and it is frequently described as such in the literature. The PC variant can also be localized and the histologic appearance of MCD is somewhat different, so MCD should be classified separately. Besides primary MCD, cases associated with other diseases (secondary MCD) are common, and MCD represents one of the most ubiquitous associations in the literature. Secondary MCD is a large and heterogeneous group of clinical entities and is often referred to as interleukin-6 (IL-6) syndrome because of evidence that an overproduction of IL-6, probably in association with other cytokines, occurs in MCD-associated diseases as well as in MCD itself, suggesting a common underlying pathogenetic mechanism.
Understanding of the pathogenesis of MCD has greatly increased since the discovery of MCD's association with KSHV, which has been found in approximately half of the cases of MCD occurring in immunocompetent patients and in almost all those infected with HIV, suggesting a pathogenetic role in this disease.50 A plasmablastic variant of MCD characterized by the presence of medium-sized to large plasmablastic cells scattered in the mantle zones of the follicles has been described, most frequently in HIV-infected individuals. Whereas immunoglobulin M (IgM)–positive immunoblasts have been usually described in the interfollicular region in MCD,51 a unique population of cells with a similar morphology was found in the mantle zone of a subset of cases of MCD in association with KSHV infection.17,52-54 The cells harboring KSHV in MCD have been called plasmablasts, but they have been described as having classic immunoblastic features, including a moderate amount of amphophilic cytoplasm and a large vesicular nucleus containing 1 or 2 prominent nucleoli.17,52-54 These KSHV-positive immunoblasts are immature cells that express cytoplasmic IgMλ, have a blastic morphology, and are seen predominantly in the mantle zones. The consistent restricted expression of lambda light chain in the KSHV-positive plasmablasts is intriguing and could be involved in the mechanism of KSHV entry in the cells or selection for those cells. These cells may be scattered or found in small confluent clusters, sometimes coalesced to form foci of “microlymphomas” or in large sheets of cells thought to represent frank lymphomas.17,55 One study reported analysis of clonality and showed that despite monotypic expression of IgMλ, the scattered plasmablasts in MCD are polyclonal; 6 of the 8 cases with small clusters called microlymphomas were also polyclonal, whereas the investigated cases of large B-cell lymphomas arising in KSHV-associated MCD were monoclonal.56
Secondary MCD can be found in association with a variety of pathologic conditions, including HIV infection, plasma-cell dyscrasias (ie, POEMS syndrome), KS, B-cell lymphoma, and HL. In KSHV-positive cases, a common association is KS17,50 and a specific variant of NHL referred to as “plasmablastic lymphoma,”17 the so-called large B-cell lymphoma arising in KSHV-associated MCD. This lymphoma is specifically associated with KSHV and is considered KSHV-linked disease entity. The lymphoma cells show exactly the same phenotypic features as the plasmablasts described in MCD, including cytoplasmic IgMλ expression and lack of EBV infection,17,57 suggesting that the plasmablastic variant of MCD could precede the development of frank KSHV-positive lymphoma. PEL is different from large B-cell lymphoma arising in KSHV-associated MCD in that the tumor cells in PEL frequently lack expression of B-cell antigens and are frequently coinfected with EBV. In addition, in contrast to PEL, large B-cell lymphomas arising in KSHV-associated MCD lack somatic hypermutation of immunoglobulin genes and are therefore thought to derive from pre–plasma cells that bypassed the germinal center.55
PBLs of the oral cavity type
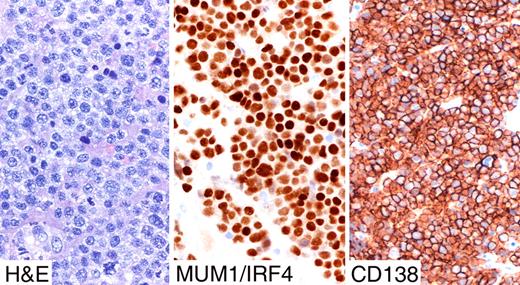
PBLs of the oral cavity type were first described as lymphomas occurring mostly in HIV+ individuals having an unusual immunophenotype (low or no CD45 and CD20), and frequent presence of EBV.15 This rare entity typically involves the jaw and oral cavity of HIV patients even if it has been documented in other sites than the oral cavity such as the anorectum, nasal and paranasal regions, skin, testes, bones, and lymph nodes. PBLs of the oral cavity type are composed of large neoplastic cells with a very high proliferation rate displaying a marked degree of plasma cell differentiation.15,16,58 Since lymphomas associated with MCD, which contain KSHV, have also been called plasmablastic lymphomas, these 2 diseases have been confused. However, they represent distinct entities, and most studies have found that PBLs of the oral cavity type do not contain KSHV.58 In PBLs of the oral cavity type, features of CD are absent. The neoplastic cells have round nuclei, moderately clumped chromatin, a single prominent nucleolus, and moderate to abundant basophilic cytoplasm with an excentric nucleus.58 The mitotic rate is very high, and there are frequent apoptotic cells and single-cell necrosis. The cytoplasm is usually deep basophilic with a paranuclear hof, and binucleation and multinucleation are common. Cells with features of maturing plasma cells can be seen and there is usually a spectrum of differentiation than can be appreciated morphologically. Phenotypically, PBLs of the oral cavity type display an unusual profile characterized by weak or absent expression of B-cell antigens (eg, CD20 and PAX5) coupled to strong immunostaining with the plasma cell markers CD138/syndecan-1, MUM1/IRF4, and VS38c (Figures 5,6). CD45 is expressed in most cases, but can be weak or negative. A recent study reported that only 5 of 11 cases express cytoplasmic Ig, which were IgGκ or IgGλ.58 EBV can be detected by EBER ISH, but LMP-1 and LMP2 are not expressed, consistent with a restricted latency, which is in contrast to AIDS-related IB lymphomas that usually express LMP-1.2
Strong immunostaining with the plasma cell markers CD138/syndecan1 and MUM1/IRF4 in plasmablastic lymphoma. Images were taken using a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan) with a pan fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2 (Nikon). Images were assembled using Adobe Photoshop 6 (Adobe Systems).
Strong immunostaining with the plasma cell markers CD138/syndecan1 and MUM1/IRF4 in plasmablastic lymphoma. Images were taken using a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan) with a pan fluor 40×/0.75 objective and Nikon digital sight DS-Fi1 camera equipped with control unit-DS-L2 (Nikon). Images were assembled using Adobe Photoshop 6 (Adobe Systems).
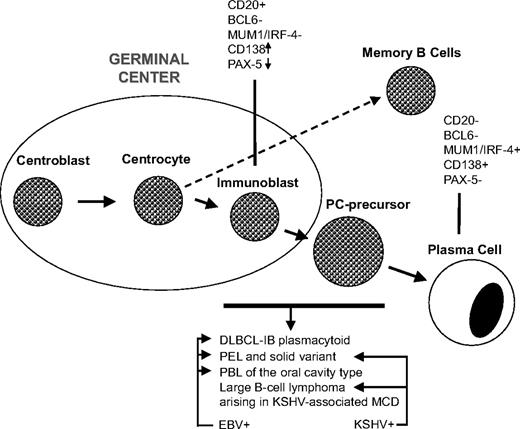
Lymphomas specifically arising in HIV-induced immunosuppression. Stage of differentiation and the putative cell of origin. Lymphoma histotypes occurring specifically in HIV-infected patients exhibit a common normal cellular counterpart that might be defined as plasmablast. The figure defines the following main subgroups of lymphoma with plasmablastic differentiation: (1) HIV-associated lymphoma showing IB morphology with plasmacytoid differentiation; these lymphomas can be either systemic or primary central nervous system lymphomas; (2) tumors classified as PELs and their extracavitary variant exhibiting infection by both KSHV and EBV; (3) tumors classified as PBL of the oral cavity type, showing a monomorphic population of immunoblasts with no or minimal plasmacytic differentiation; most patients are HIV infected and tumor cells are EBV positive but KSHV negative; most cases present in the oral mucosa, whereas a significant number of cases present in other extranodal or nodal site; and (4) large B-cell lymphoma arising in KSHV-associated multicentric Castleman disease (MCD) consisting of KSHV-infected plasmablasts, which show evidence of light chain restriction and may represent a monotypic cell population, found in small clusters surrounding or replacing follicles, in MCD. Therefore, important features to subclassify these neoplasms include the stage of differentiation of the putative cell of origin and association with viruses. Lymphomas with plasmablastic differentiation are a heterogeneous group of neoplasms with different clinicopathological characteristics and different associations with specific viruses. DLBCL-IB indicates diffuse large B-cell lymphoma-immunoblastic; EBV, Epstein-Barr virus; KSHV, Kaposi sarcomaherpesvirus; MCD, multicentric Castleman disease; PBL, plasmablastic lymphoma; PC, plasma cell; and PEL, primary effusion lymphoma.
Lymphomas specifically arising in HIV-induced immunosuppression. Stage of differentiation and the putative cell of origin. Lymphoma histotypes occurring specifically in HIV-infected patients exhibit a common normal cellular counterpart that might be defined as plasmablast. The figure defines the following main subgroups of lymphoma with plasmablastic differentiation: (1) HIV-associated lymphoma showing IB morphology with plasmacytoid differentiation; these lymphomas can be either systemic or primary central nervous system lymphomas; (2) tumors classified as PELs and their extracavitary variant exhibiting infection by both KSHV and EBV; (3) tumors classified as PBL of the oral cavity type, showing a monomorphic population of immunoblasts with no or minimal plasmacytic differentiation; most patients are HIV infected and tumor cells are EBV positive but KSHV negative; most cases present in the oral mucosa, whereas a significant number of cases present in other extranodal or nodal site; and (4) large B-cell lymphoma arising in KSHV-associated multicentric Castleman disease (MCD) consisting of KSHV-infected plasmablasts, which show evidence of light chain restriction and may represent a monotypic cell population, found in small clusters surrounding or replacing follicles, in MCD. Therefore, important features to subclassify these neoplasms include the stage of differentiation of the putative cell of origin and association with viruses. Lymphomas with plasmablastic differentiation are a heterogeneous group of neoplasms with different clinicopathological characteristics and different associations with specific viruses. DLBCL-IB indicates diffuse large B-cell lymphoma-immunoblastic; EBV, Epstein-Barr virus; KSHV, Kaposi sarcomaherpesvirus; MCD, multicentric Castleman disease; PBL, plasmablastic lymphoma; PC, plasma cell; and PEL, primary effusion lymphoma.
Virology aspects: mechanisms that lead to lymphomagenesis
EBV and lymphoma in AIDS patients
Diffuse large cell B-cell lymphoma.
The transforming EBV protein LMP-1 is frequently expressed in DLBCL.59,60 LMP-1 plays a crucial role in the transformation of B-lymphocytes by EBV (reviewed in Young and Rickinson61 ). Thus, LMP-1 transforms rodent fibroblasts,62 transgenic mice that express LMP-1 in B cells show increased development of B-cell lymphomas,63 and LMP-1 deletion mutants of EBV are compromised in their ability to immortalize human primary B cells.64 LMP-1 activates the NFkB as well as the JNK and p38 pathways,65-67 by recruiting cellular TRAF 1–3 and TRADD molecules to 2 short sequence motifs, CTAR-1 and CTAR-2, respectively, in the cytoplasmic domain of the LMP-1 molecule.68-70 In B cells, LMP-1 increases the expression of the antiapoptotic proteins A20 and bcl-2, the adherence molecule CD54/ICAM-1, the cell-cycle regulator p27Kip,71 and many others (reviewed in Brinkmann and Schulz72 ). In DLBCL, expression of LMP-1 correlates inversely with the expression of BCL6, a marker for germinal center B cells, suggesting that, among DLBCLs, the impact of EBV LMP-1 is likely to be strongest in tumors representing a post–germinal center plasmacytic differentiation profile.73 In addition, knockdown of LMP-1 in cell lines derived from AIDS-DLBCL results in apoptosis, indicating that this viral oncoprotein plays a role in lymphoma pathogenesis.74
EBV-associated DLBCLs have therefore been considered as EBV-driven lymphoproliferations occurring in the context of a defective T-cell immunity against EBV.75 However, unlike EBV-driven lymphoproliferative disease in transplant recipients, which includes monoclonal, oligoclonal, as well as polyclonal B-cell proliferations, DLBCL is always monoclonal. This suggests that, in addition to the effects contributed by EBV LMP-1, additional factors such as genetic damage are likely to contribute to the pathogenesis of AIDS-DLBCL.
Burkitt lymphoma.
About 30% to 60% of AIDS-BLs are EBV positive and the transforming EBV protein LMP-1 is not expressed in BL.61 Although not essential in the pathogenesis of BL, EBV supports tumor development. EBNA-1, a viral protein required for the replication and maintenance of the latent viral episomal DNA, is found consistently in BL cells.61 EBNA-1 transgenic mice develop B-cell lymphoma with a very long latency,76 and EBNA-1 and c-myc may cooperate.77 The presence of latent EBV in BL cells has been shown to promote genetic instability,78 suggesting a mechanism by which latent EBV could contribute to genetic alterations required for the development of BL. In addition, some latent EBV transcription patterns found in BL produce viral proteins that are likely to protect BL cells from apoptosis induced by deregulated c-myc expression.79 The importance of apoptosis protection during B-cell immortalization has recently been highlighted by the failure of EBV deletion mutants lacking both viral bcl-2 homologues (BALF1, BHRF1) to efficiently immortalize human B cells.80 Given the strong apoptotic effects of overexpressed c-myc, the role of EBV in some cases of BL could therefore consist of protecting BL cells against this side effect of c-MYC translocation.
Hodgkin lymphoma.
The latent EBV proteins EBNA-1, LMP-1, and LMP2A are expressed in the RS cells, the malignant cell population of this tumor.81 RS cells are derived from B cells that have passed through the germinal center, as shown by the presence of somatic mutations in the rearranged Ig variable region of their immunoglobulin genes.82 Notably, many of these hypermutations are incompatible with the expression of a functional B-cell receptor (BCR), suggesting that RS cells may have developed from germinal center B cells that should have been eliminated by apoptosis, but managed to survive.83,84
LMP2A interferes with normal B-cell development, allows BCR-negative B cells to leave the bone marrow/colonize peripheral lymphoid organs,85 and induces a transcriptome pattern in B cells, which resembles that of HL RS cells.86 Following EBV infection, LMP2A is essential for the survival and continued proliferation of germinal center B cells lacking a functional B-cell receptor.87,88 LMP2A may therefore promote the survival of “crippled” germinal center B cells and could thus aid their development into RS cells.
LMP-1 may also induce an “HL-like” transcriptional program in germinal center B cells.89 Among the cellular genes up-regulated by LMP-1 in HL cells is bmi-1, a polycomb family member known to cause lymphoma in transgenic mice and to down-regulate the ATM tumor suppressor.90 EBNA-1 was shown to induce CCL-20 secretion in RS cell lines and to thereby promote the migration of regulatory T cells, which could be envisaged to downmodulate EBV-specific T-cell responses.91 Protein tyrosine phosphatase receptor kappa (PTPRK) suppresses the growth of HL cell lines and is downmodulated by EBV.92 These results provide suggestions of how EBV LMP-1, LMP2A, and EBNA-1 may contribute to the development of RS cells.
Kaposi sarcoma herpesvirus and AIDS lymphoma
PEL.
PEL cells contain multiple copies (in the order of 50–150 copies/cell) of episomal KSHV genomes.93 In most cells, a latent viral gene expression pattern involves the expression of the LANA, a viral D-type cyclin homologue (vcyc), a viral homologue of FLICE inhibitory protein (vFLIP), a pre-miRNA transcript encoding 11 viral miRNAs, as well as vIRF3/K10.5/LANA-2.94-101 In addition, a homologue of IL-6 (vIL-6) is also expressed in some PEL cells.54,102,103
A detailed description of the functional properties of individual KSHV proteins can be found in recent review articles.101,104,105 The evidence for an involvement of the above-cited 5 to 6 KSHV genes in the pathogenesis of PEL is as follows: gene silencing experiments using shRNA or siRNA106,107 indicate that silencing of vFLIP and/or vcyc leads to increased apoptosis of PEL cell lines. Since vcyc and vFLIP are translated from the same bicistronic latent transcript, silencing of vcyc also lead to the silencing of vFLIP, and vice versa.106,107 However, apoptosis was found to be due to vFLIP suppression as reconstitution with transfected vcyc does not rescue the cells from apoptosis.106 vFLIP is a potent activator of the NFkB pathway,108-111 and NFkB inhibition also induces apoptosis in PEL cells,112 providing additional evidence that vFLIP is essential for the survival of PEL cells. Its downstream effects include the induction of IL-6, an important growth factor for B cells, as well as the cellular antiapoptotic factors cFLIPL, cIAP-1, and cIAP-2.106,111
The viral D-type cyclin homologue vcyc associates with cdk2, cdk4, and cdk6 but appears to promote phosphorylation of its targets mainly in concert with cdk6.94-101,103-113 Its targets include not only RB, but also other cellular targets including histone H1, Id2, CDC6, cdc25A, Orc-1, the antiapoptotic protein bcl-2, and the cdk inhibitors p27Kip and p21CIP.113,114 Phosphorylation of p27Kip by the vcyc/cdk6 complex on Ser10 during latency leads to sequestration of p27Kip in the cytoplasm, thereby allowing PEL cells to proliferate in the presence of high p27Kip levels.113 Likewise, phosphorylation of p21CIP1 on serine 130 by vcyc allows vcyc to bypass the p21CIP1-mediated G1 arrest.114 Vcyc has been shown to promote S phase entry and also to induce apoptosis in cells with high cdk6 expression, which can be counteracted by the action of the viral bcl-2 homologue, vbcl-2115 ; it can induce a DNA damage response in endothelial cells.116 It is likely that some of these biochemical features of vcyc will play a role in PEL pathogenesis.
KSHV LANA also interacts with several cellular components that have been linked to cancer development. It binds to and antagonizes p53 and Rb, sequestrates GSK-3β and thereby stabilizes β-catenin as well as c-myc, and additionally activates c-myc–mediated transcription by promoting its phosphorylation.117-120 A DNA damage response pathway appears to be active in PEL cells, which is normally balanced by the ability of LANA to interact with p53 and its E3 ligase, mdm2; disruption of the LANA/p53/mdm2 complex by nutlin 3a, an inhibitor of the p53-mdm2 interaction, induces apoptosis in KSHV-infected PEL cell lines, but not in EBV-transformed LCLs.121 This observation underlines the importance of the LANA/p53/mdm2 complex in PEL cells for their survival. However, in contrast to vFLIP, silencing of LANA only reduced the KSHV genome copy number without affecting cell survival.
KSHV vIRF3 interferes with p53-induced transcription as well as p53- and protein kinase R (PKR)–mediated apo-ptosis97-101,103-122 ; its silencing by siRNA/shRNA in PEL cell lines results in an increased apoptosis and caspase 3/7 activity, suggesting that this protein also contributes to the survival of PEL cells.123
A fourth latent transcript in PEL cells serves as a precursor RNA for 12 microRNAs (miRNAs), small 19- to 23-nt RNAs that regulate cellular mRNA turnover or stability.98-100 One of these, miR-K12-11, has been found to target the same cellular mRNAs as miR-155/BIC, a cellular miRNA regulating the germinal center reaction during B-cell maturation.124-126 Both miR-K12-11 and miR-155 down-regulate several proapoptotic cellular genes, such as LDOC1, Bim, BCLAF1 (Bcl2-associated transcription factor 1), and the NFkB regulator BAZF.125,126 MiR-K12-11 may therefore be involved in the late stages of B-cell differentiation, could contribute to the plasmablastic phenotype of PEL cells, or could play a role in the protection of PEL cells against apoptosis.
The viral IL-6 homologue, vIL-6, is expressed in a subpopulation of PEL cells in vivo and in many KSHV-infected B cells in MCD lymphoid follicles.54,103,127 It induces proliferation, angiogenesis, and hematopoiesis in IL-6–dependent cell lineages127-129 and serves as an autocrine factor in PEL cell lines130 ; it also induces vascular endothelial growth factor (VEGF), which has been implicated in the pathogenesis of PEL and KS.131 A single chain antibody to vIL-6 blocking its interaction with the IL-6 receptor complex was found to inhibit the proliferation of a PEL cell line and to inhibit vIL-6–induced STAT 3 phosphorylation in vIL-6–transfected cells.132 Therefore vIL-6 may contribute to PEL cell proliferation and to the angiogenesis noted in patients with this lymphoma.
MCD.
Among KSHV-associated pathologies, MCD appears to be the one with the highest number of productively infected cells.53,103 A sizeable fraction of MCD B cells expresses vIL-6 and it is thought that its downstream effects on B-cell proliferation and VEGF secretion play a role in their proliferation and in the strong angiogenic component characteristic for MCD lesions. In patients with MCD, exacerbations of the disease were reported to correlate with increased viral load and increased IL-6 and IL-10 levels, underlining the importance of productive viral replication and cellular cytokines in the pathogenesis of this disorder.133
Conclusion
From the direct transforming role of EBV in many transplant-associated and some AIDS lymphomas, via the impact of EBV and KSHV on the DNA damage response in infected B cells, the contribution of individual EBV and KSHV proteins to protection against apoptosis and promotion of cell survival to possible direct effects on B-cell differentiation and maturation, these viruses have developed multiple strategies that allow them to act as cofactors in lymphoma development acting in concert with immune suppression and presumably other oncogenic factors (Figure 7).
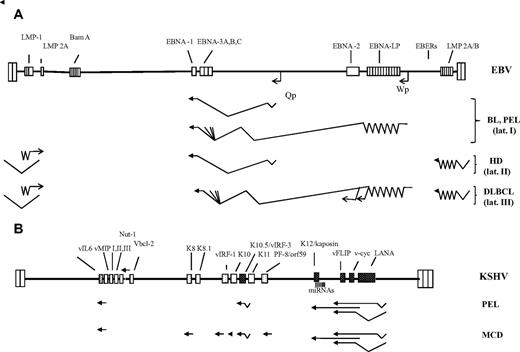
A diagram of the genomes of EBV and KSHV/HHV8 and their expression pattern in EBV- and KSHV-associated AIDS lymphoma. (A) The EBV genome is shown in the same orientation as the KSHV genome. The terminal repeats of the 2 viral genomes are indicated by a pair of taller boxes at the end of the long unique coding region. EBV adopts its most restricted pattern of gene expression (latency pattern I) in BL and PEL cells; this involves expression of EBNA-1 from the Qp promoter, of the untranslated EBERs, and probably of a group of transcripts from the Bam A region. In some BL tumors, the use of an alternative latent EBV promoter, Wp, leads to the expression of EBNA-3A,B,C in the absence of EBNA-2 and LMP-1, resulting a significant protection against apoptosis of c-myc–expressing cells.79 In HL, EBV adopts latency pattern II, which involves expression of EBNA-1, the EBERs, and the 2 latent membrane proteins, LMP-1 and LMP-2A. LMP-2A is translated from a transcript that spans the terminal repeats in the circular viral episome found during latency. In DLBCL, EBV latency pattern III includes the expression of EBNA-2, EBNA-LP, EBNA-3A,B,C from the latent Wp promoter, as shown. Details on the function of EBNA-2, EBNA-LP, and EBNA-3A,B,C can be found in a recent review.61 (B) KSHV/HHV8 genome and viral genes expressed in PEL or MCD. The latent KSHV genes LANA, v-cyc, vFLIP, K12/kaposin, and vIRF-3/K10.5/LANA-2 are shown as black stippled boxes; the position of the KSHV miRNAs is indicated by vertical lines. These genes are expressed in the majority of tumor cells in vivo and in PEL cell lines. Viral genes expressed in only a subpopulation of PEL or MCD cells (eg, vIL6) are cross-hatched, and those expressed only during the later stages of the productive (lytic) viral replication cycle are stippled. The viral gene expression pattern in PEL is more restricted than in MCD. Whereas LANA, vcyc/vFLIP, the miRNAs, and vIRF-3/K10.5/LANA-2 are expressed in most, vIL6 is expressed only in a small proportion of lymphoma cells. Other lytic genes are only rarely expressed and are therefore not shown in this diagram for PEL. In MCD, several lytic KSHV genes are expressed in a few cells, suggesting noticeable productive viral replication in this condition.
A diagram of the genomes of EBV and KSHV/HHV8 and their expression pattern in EBV- and KSHV-associated AIDS lymphoma. (A) The EBV genome is shown in the same orientation as the KSHV genome. The terminal repeats of the 2 viral genomes are indicated by a pair of taller boxes at the end of the long unique coding region. EBV adopts its most restricted pattern of gene expression (latency pattern I) in BL and PEL cells; this involves expression of EBNA-1 from the Qp promoter, of the untranslated EBERs, and probably of a group of transcripts from the Bam A region. In some BL tumors, the use of an alternative latent EBV promoter, Wp, leads to the expression of EBNA-3A,B,C in the absence of EBNA-2 and LMP-1, resulting a significant protection against apoptosis of c-myc–expressing cells.79 In HL, EBV adopts latency pattern II, which involves expression of EBNA-1, the EBERs, and the 2 latent membrane proteins, LMP-1 and LMP-2A. LMP-2A is translated from a transcript that spans the terminal repeats in the circular viral episome found during latency. In DLBCL, EBV latency pattern III includes the expression of EBNA-2, EBNA-LP, EBNA-3A,B,C from the latent Wp promoter, as shown. Details on the function of EBNA-2, EBNA-LP, and EBNA-3A,B,C can be found in a recent review.61 (B) KSHV/HHV8 genome and viral genes expressed in PEL or MCD. The latent KSHV genes LANA, v-cyc, vFLIP, K12/kaposin, and vIRF-3/K10.5/LANA-2 are shown as black stippled boxes; the position of the KSHV miRNAs is indicated by vertical lines. These genes are expressed in the majority of tumor cells in vivo and in PEL cell lines. Viral genes expressed in only a subpopulation of PEL or MCD cells (eg, vIL6) are cross-hatched, and those expressed only during the later stages of the productive (lytic) viral replication cycle are stippled. The viral gene expression pattern in PEL is more restricted than in MCD. Whereas LANA, vcyc/vFLIP, the miRNAs, and vIRF-3/K10.5/LANA-2 are expressed in most, vIL6 is expressed only in a small proportion of lymphoma cells. Other lytic genes are only rarely expressed and are therefore not shown in this diagram for PEL. In MCD, several lytic KSHV genes are expressed in a few cells, suggesting noticeable productive viral replication in this condition.
Treatment strategies
Combined chemotherapy and rituximab
Before the introduction of highly active antiretroviral therapy (HAART), several studies demonstrated that the use of aggressive chemotherapy regimens leads to high mortality rate because of the incidence of opportunistic infections (OIs). Therefore low-dose M-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone)134 or CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone)135 were considered the gold standard in the treatment of these patients even if other reports suggested a superiority of continuous infusional chemotherapy regimens (CDE [cyclophosphamide, doxorubicin, etoposide] or EPOCH [cyclophosphamide, doxorubicin, etoposide, vincristine, prednisone]).136,137
The introduction of rituximab has significantly improved survival from NHL in the general population,138-141 and based on these data, several authors have explored the feasibility and effectiveness of rituximab plus chemotherapy in patients with HIV-NHL. All published data suggest the high activity of rituximab plus chemotherapy in comparison with historical control with chemotherapy alone (see Table 1 for the comparison of R-CDE vs CDE) even if a slight increase in the rate of OIs has been reported.141-146 Table 2 summarizes the results of these studies.
Comparison between CDE and R-CDE
| . | CDE (95% CI) . | R-CDE (95% CI) . |
|---|---|---|
| No. of patients | 55 | 74 |
| Median age, y | 40 | 38 |
| Median CD4 count/μL | 227 | 161 |
| Histology, % | ||
| Burkitt or Burkitt-like | 22 | 28 |
| Diffuse large cell or variants | 78 | 72 |
| Age-adjusted IPI, % | ||
| Low or low intermediate | 42 | 43 |
| High or high intermediate | 58 | 57 |
| Complete remission rate, % | 45 (30–58) | 70 (59–81) |
| Disease-free survival at 2 y, % | 38 (25–51) | 59 (47–71) |
| Overall survival at 2 y, % | 45 (20–58) | 64 (52–76) |
| . | CDE (95% CI) . | R-CDE (95% CI) . |
|---|---|---|
| No. of patients | 55 | 74 |
| Median age, y | 40 | 38 |
| Median CD4 count/μL | 227 | 161 |
| Histology, % | ||
| Burkitt or Burkitt-like | 22 | 28 |
| Diffuse large cell or variants | 78 | 72 |
| Age-adjusted IPI, % | ||
| Low or low intermediate | 42 | 43 |
| High or high intermediate | 58 | 57 |
| Complete remission rate, % | 45 (30–58) | 70 (59–81) |
| Disease-free survival at 2 y, % | 38 (25–51) | 59 (47–71) |
| Overall survival at 2 y, % | 45 (20–58) | 64 (52–76) |
Data are from Spina et al.141
CDE indicates cyclophosphamide, doxorubicin, etoposide; and R, rituximab.
Rituximab and chemotherapy in HIV-related non-Hodgkin lymphomas: review of the literature
| . | R-CDE141 . | R-CHOP143 . | R-CHOP144 . | R-CHOP145 . | R-EPOCH146 . |
|---|---|---|---|---|---|
| No. of patients | 74 | 61 | 95 | 60 | 51 |
| Stage III-IV (%) | 70 | 69 | 80 | 63 | 70 |
| Histology, % | |||||
| Diffuse large cell or variants | 72 | 72 | 81 | 100 | 74 |
| Burkitt or Burkitt-like | 28 | 26 | 8 | 0 | 26 |
| PBL of the oral cavity type | 0 | 2 | 1 | 0 | 0 |
| IPI at least 2, % | 57 | 48 | 58 | 64 | 69 |
| Median CD4/dL | 161 | 172 | 128 | 152 | 181 |
| Complete remission rate, % | 70 | 77 | 57 | 66 | 69 |
| Febrile neutropenia, % | 31 | 25 | 32 | NA | 16 |
| Deaths from infections, % | 7 | 2 | 11 | 5 | 10 |
| . | R-CDE141 . | R-CHOP143 . | R-CHOP144 . | R-CHOP145 . | R-EPOCH146 . |
|---|---|---|---|---|---|
| No. of patients | 74 | 61 | 95 | 60 | 51 |
| Stage III-IV (%) | 70 | 69 | 80 | 63 | 70 |
| Histology, % | |||||
| Diffuse large cell or variants | 72 | 72 | 81 | 100 | 74 |
| Burkitt or Burkitt-like | 28 | 26 | 8 | 0 | 26 |
| PBL of the oral cavity type | 0 | 2 | 1 | 0 | 0 |
| IPI at least 2, % | 57 | 48 | 58 | 64 | 69 |
| Median CD4/dL | 161 | 172 | 128 | 152 | 181 |
| Complete remission rate, % | 70 | 77 | 57 | 66 | 69 |
| Febrile neutropenia, % | 31 | 25 | 32 | NA | 16 |
| Deaths from infections, % | 7 | 2 | 11 | 5 | 10 |
PBL indicates plasmablastic lymphoma; R-CDE, rituximab–cyclophosphamide, doxorubicin, etoposide; R-CHOP, rituximab–cyclophosphamide, vincristine, doxorubicin, prednisone; and R-EPOCH, rituximab–cyclophosphamide, doxorubicin, etoposide, vincristine, prednisone.
Current treatment of BL and DLBCL in the HAART era
In the HAART era the treatment of BL remains a big challenge. In fact, HAART has improved the NHL course, with the only exception being BL, which has turned out to be clinically more aggressive than DLBCL. This is related to the strong positive effect HAART has on the outcome of DLBCL, whereas the outcome for BL is unchanged.147,148 Since this lymphoma subtype affects survival, a question has arisen whether it should be treated more aggressively. A retrospective analysis has been conducted on the feasibility of intensive aggressive chemotherapy regimens, which are usually used in the treatment of BL in the general population, and also in HIV patients. American and Spanish investigators report a 63% to 68% CR rate, a 46% to 60% failure-free survival rate at 2 years, and the same toxicity as in the general population, which confirms the feasibility of aggressive regimens also in the HIV setting.149-151
Treatment of unusual entities
Few data have been reported on the treatment of other rare entities, that is, PEL and PBL of the oral cavity type. The classic PEL presents in advanced course of HIV infection with a typical abundant effusion. All published data showed that despite the use of standard chemotherapy regimens (CHOP or CHOP-like) the prognosis is poor in comparison with that of other HIV-NHL subtypes.6,46,152 Considering the aggressiveness of PEL and its pathogenesis, several nonchemotherapy approaches have been tested, including HAART alone or antiviral treatment (ie, cidofovir, AZT, IFN) with promising results.153-155
Perspectives and conclusions
Lymphoma progression is the leading cause of death in 35% to 55% of the patients with HIV-NHL receiving chemotherapy, of whom approximately half need second-line chemotherapy following progression or relapse of the disease. To date, the results achieved by salvage therapies that do not include a standard high-dose chemotherapy regimen with peripheral blood stem cell (PBSC) transplantation have been very frustrating (median survival, 2–4 months).158 With the introduction of HAART into clinical practice, more aggressive treatment protocols can be taken into consideration, whose effectiveness has already been documented in HIV-negative patients. Preliminary studies support the feasibility of high-dose chemotherapy in combination with PBSC transplantation in HIV-NHL, proving that PBSC collections are adequate, anchoring rates are similar to those recorded in HIV-negative patients, and high-dose chemotherapy is well tolerated with no increase in the incidence of OIs.159,160 Within the GICAT, a study has been performed on a group of patients with NHL or refractory or recurred HL: the results support the feasibility of an adequate peripheral blood stem cell collection, with no transplantation-related mortality and a very good outcome—60% of the patients being alive and disease-free.161 The long-term follow-up of patients who underwent high-dose chemotherapy and PBSC support confirms that the outcome of HIV patients with chemosensitive disease is superimposable to that of HIV-negative patients.162
It can be drawn from the above data that prognosis for patients with lymphomas and concomitant HIV infection could be improved using better combined chemotherapy protocols incorporating anticancer treatments and antiretroviral drugs. We envision that in the future, therapies will be developed that target specific viral oncogenes to which the lymphoma cells are addicted (ie, EBV LMP1, KSHV vFLIP), and that these will provide therapeutic benefit. For the time being, the administration of HAART during chemotherapy can improve control of the underlying HIV infection. The inclusion of hematopoietic growth factors in the treatment of this patient group makes it possible to increase chemotherapy doses and prolong the administration of antiretroviral drugs with the intent to improve survival. At the present time, we strongly recommend that patients with lymphoma and HIV infection should be treated as patients with lymphoma of the general population.
Acknowledgments
This work was supported in part by a grant from the Ministero della Salute (Rome, Italy) within the framework of the Progetto Integrato Oncologia-Advanced Molecular Diagnostics project (RFPS-2006-2-342010.7; A.C.), and by the European Union Integrated project INCA (LSHC-CT-2005-18704; T.F.S.).
Authorship
Contribution: A.C. designed the review; and all authors contributed to writing and proofreading of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonino Carbone, Chairman of the Department of Pathology, National Cancer Institute, via Venezian, 1, 20133 Milan, Italy; e-mail: antonino.carbone@istitutotumori.mi.it.