In this issue of Blood, Chun and colleagues define snrk-1 as a novel participant in angioblast migration and arteriovenous speciation.
Sucrose nonfermenting-related kinase-1 (snrk-1) has been found to be essential for signaling through notch activation, and the kinase function of snrk-1 has been found essential for snrk-1 activity. Chun et al initially isolated snrk-1 as a candidate through examination of expressed sequence tags that were highly expressed in zebrafish vasculature. Snrk-1 was found to be expressed in axial vessels and neural structures, tissues with cells known for their ability to migrate. High-level expression of snrk-1 was found in human vascular tissues, including placenta and hemangioma of infancy.
Morpholino-mediated knockdown of snrk-1 resulted in decreased numbers and mislocalization of zebrafish angioblasts. Mutation of the kinase domain of snrk-1 decreased angioblast number and migration, indicating that the kinase function was essential for these functions. Snrk-1 mRNA depletion in human endothelial cells led to decreased migration, suggesting that these effects were not zebrafish-specific. Finally, snrk-1 morpholinos blocked the activity of notch 2 on arteriovenous speciation. These findings have implications not only on developmental angiogenesis, but on pathologic angiogenesis as well.
Snrk-1 was found to be high in infantile hemangiomas. The signaling properties of hemangiomas have been found to be dependent on an Akt/reactive oxygen signaling pathway, in that reactive oxygen/rac/NADPH oxidase signaling drives NFkB and inhibition of these pathways results in the blockade of experimental hemangiomas in murine models.1 This phenotype, which is not limited to hemangiomas, is called the reactive oxygen driven tumor.2 Snrk-1 is likely required for the maintenance of hemangiomas.
What is known about the function of snrk-1? Snrk-1 falls under the class of AMP-activated protein kinases (AMPKs) that have been extensively studied in plants. Snrk-1 and AMPK family members play a critical role in carbon metabolism and determine levels of sugar storage in response to plant stress. In the humble potato, a potential substrate of snrk-1 is NADPH oxidase (RBOH; respiratory burst oxidase homolog).3 It is likely that snrk-1 plays an evolutionary-conserved role in the animal kingdom as well, adapting to low glucose levels/high reactive oxygen that may occur during physiologic and pathologic angiogenesis.4 Renal cell carcinoma cells have been found to express high levels of reactive oxygen, hypoxia inducible factor 2, and notch signaling, thus classifying many renal cell carcinomas as reactive oxygen driven tumors. Snrk-1 may be an essential part of this phenotype.5
In a companion paper, Pramanik and colleagues demonstrate an extremely high prevalence of the dusp5 S147P mutation, which destabilizes dusp5 and the snrk-1 R248Q mutation, which impairs the kinase function of snrk-1. The confluence of both mutations is extremely high in venous malformations. The impaired snrk-1 activity might result in increased cells with venous speciation, while the loss of dusp5 might result in increased Akt/MAPK activation, preventing apoptosis and allowing abnormal persistence of venous endothelium. Further work remains to determine whether loss of dusp5 results in activation of S6 kinase/mTOR activation, as does the loss of another phosphatase, PTEN.
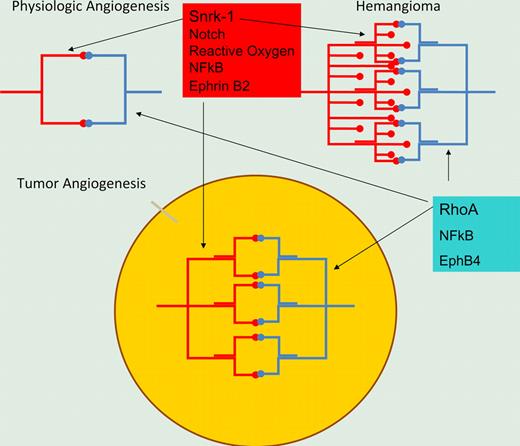
Illustration of 2 signaling pathways in physiologic and pathologic angiogenesis. Snrk-1 marks an arterially differentiated vasculature involving notch signaling, while alternative rho-mediated signaling may be present in venous differentiation of microvasculature. Inhibition of both pathways may be required for effective angiogenesis inhibition.
Illustration of 2 signaling pathways in physiologic and pathologic angiogenesis. Snrk-1 marks an arterially differentiated vasculature involving notch signaling, while alternative rho-mediated signaling may be present in venous differentiation of microvasculature. Inhibition of both pathways may be required for effective angiogenesis inhibition.
While the endothelium involved in pathologic angiogenesis is complex, at least 2 signaling pathways can be dissected out. One is the reactive oxygen-HIF2a-notch-snrk-1 pathway, which is elegantly demonstrated by Chun et al. The second involves aberrant regulation of rho and has been demonstrated by Pramanik et al to be physiologically required in physiologic angiogenesis and by Ghosh et al to be present in tumor-derived endothelial cells.6,7 It is likely that these pathways contribute to tumor angiogenesis and provide a potential explanation of why anti-angiogenic agents as monotherapy have not been entirely successful in the clinic. Agents may target one signaling pathway but leave tumor-derived endothelial cells intact that use a second pathway. This study provides a rationale for several further studies. First, what are the substrates of snrk-1 that provide resistance to apoptosis as well as endothelial migration? Second, what are the upstream events that activate snrk-1? Finally, can small molecules be developed that inhibit snrk-1 kinase activity, and would such compounds have therapeutic benefit in vivo?
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal