Abstract
CD4+ interleukin-17 (IL-17)+ T cells (Th17 cells) have been implicated in allograft rejection of solid organs and several autoimmune diseases. However, the functional role of Th17 cells in the development of acute graft-versus-host disease (GVHD) has not been well-characterized. We detected significant numbers of alloreactive CD4+ donor T cells expressing IL-17, IL-17F, or IL-22 in the lymphoid organs of recipients of an allogeneic bone marrow transplant. We found no differences in GVHD mortality or graft-versus-tumor (GVT) activity between wild type (WT) and IL-17−/− T-cell recipients. However, upon transfer of murine IL-17−/− CD4+ T cells in an allogeneic BMT model, GVHD development was significantly delayed behind recipients of WT CD4+ T cells, yet overall GVHD mortality was unaffected. Moreover, recipients of IL-17−/− CD4+ T cells had significantly fewer Th1 cells during the early stages of GVHD. Furthermore, we observed a decrease in the number of IFN-γ–secreting macrophages and granulocytes and decreased production of proinflammatory cytokines (interferon [IFN]-γ, IL-4, and IL-6) in recipients of IL-17−/− CD4+ T cells. We conclude that IL-17 is dispensable for GVHD and GVT activity by whole T cells, but contributes to the early development of CD4-mediated GVHD by promoting production of proinflammatory cytokines.
Introduction
Thus far, 3 subsets of proinflammatory helper T cells have been described: Th1, Th2, and Th17 cells.1 Naive T cells exposed to interleukin-121 (IL-12) differentiate into Th1 cells, resulting in expression of Tbet and STAT4, and secretion of interferon (IFN)–γ.2 Th1 cells are essential for the clearance of intracellular bacteria and negatively regulate the development of Th2 and Th17 cells.2 IL-4 activates a Th2 program, characterized by expression of STAT6 and GATA3 and secretion of IL-4, IL-5, and IL-13, which is important for humoral immunity.2 Finally, naive T cells exposed to TGF-β, IL-6, and IL-21 differentiate into Th17 cells by activating RORγt, RORα, STAT3, STAT4, and IRF4 transcription factors.3-6 Th17 cells produce high levels of IL-17A (IL-17), IL-17F, IL-21, and IL-22, and have been shown to contribute to mucosal immunity.7-9 Specifically, IL-17 is important in the control or clearance of various pathogens, including Klebsiella pneumoniae,10 Citrobacter rodentium,3 Borrelia burgdorferi,11 and Candida albicans.12 In addition, Th17 cells have been implicated in allograft rejection of solid organs13-15 and several autoimmune diseases.1,16-19 IL-17, otherwise known as IL-17A, is the best-characterized member of the IL-17 family (IL-17A through F). IL-17 is a disulfide-linked homodimeric glycoprotein consisting of 155 amino acids with a molecular weight of 35 kDa.20 IL-17F shares the greatest homology with IL-17 (55%). Th17 cells produce IL-17 and IL-17F while innate immune cells produce other IL-17 family members. IL-17 and IL-17F can exist as homodimers or heterodimers.21 IL-17 homodimers are efficient at inducing chemokine production by epithelial cells. The IL-17 receptor (IL-17RA) is a type 1 transmembrane protein, and its mRNA is expressed in the lungs, kidneys, liver, and spleen as well as in isolated fibroblasts, epithelial cells, mesothelial cells, and various myeloid cells in rats and mice.20 Th17 cells have been identified as the primary source of IL-22, an IL-10 family member.8,22 IL-22 can stimulate proliferation, abnormal differentiation, and migration of various epithelial cells.23 Its up-regulation has been associated with several autoimmune diseases, including psoriasis, Crohn's disease, and ulcerative colitis, as well as murine models of inflammatory bowel disease.24-27
Allogeneic bone marrow transplantation (BMT) is a potentially curative therapy for various hematopoietic malignancies and immunologic diseases.28,29 GVHD is a major complication of allogeneic BMT and causes significant morbidity and mortality. GVHD is the result of alloreactive donor T cells recognizing alloantigens on normal host tissues and mounting an attack against the host.28,30 During GVHD, donor T cells specifically target the intestines, skin, and liver,28 however, alloreactive T cells also contribute to graft-versus-tumor (GVT) activity, which may prevent tumor relapse.28
During the early stages after BMT, alloreactive CD4+ T cells secrete proinflammatory cytokines that play an important role in the pathophysiology of GVHD by causing activation of immune cells and by direct tissue damage.28,31,32 Both Th1 and Th2 cells are generated in response to alloantigens33,34 and contribute to the development of GVHD.33 Recipients of STAT4−/− (Th1-deficient) splenocytes have decreased GVHD mortality and lack signs of colitis and weight loss, but have severe skin GVHD and profound scaling while WT controls and STAT6−/− (Th2-deficient) recipients do not.35 Recipients of STAT6−/− cells develop less GVHD than WT and STAT4−/− recipients, and have decreased liver and skin damage but increased symptoms of colitis.35 These data suggest that Th2 cells contribute to hepatic and skin GVHD while Th1 cells contribute to or mediate intestinal GVHD. However, the functional role of Th17 cells in the development of acute GVHD has not been well characterized.
In this study, we examine whether Th17 cells are generated during GVHD and which Th17 cytokines are produced after BMT. In addition, we used IL-17–deficient mice to assess whether IL-17 is required for the development of GVHD and GVT activity by alloreactive donor T cells.
Methods
Cell lines, antibodies, and flow cytometry
Fluorchrome-labeled antibodies were obtained from Pharmingen (San Diego, CA), R&D Systems (Minneapolis, MN) or Biolegend (San Diego, CA). Flow cytometry was performed as previously described21 using an LSR II cytometer (Becton Dickinson, San Jose, CA) and FlowJo software (TreeStar, Ashland, OR) for data analysis.
GVHD/GVT experiments
Female C57BL/6 (B6) (H-2b), BALB/c (H-2d), and C3FeB6F1 (H-2b/k) were obtained from The Jackson Laboratory (Bar Harbor, ME). IL-17−/− mice were a kind gift from Yoichiro Iwadura (University of Tokyo, Japan). The BMT procedure was performed as previously described.36 Recipients were monitored for survival, weight loss and clinical GVHD as previously described.36 During GVT experiments, mice were administered A20 lymphoma cells at the time of transplantation. BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. BMT recipients were killed for blinded histopathologic and flow cytometric analyses.
Intracellular staining
Lymphoid organs from GVHD mice were removed and processed into a single cell suspension. Absolute numbers for each organ were determined using a hemacytometer. For cytokine staining, cells were stimulated in vitro with 50 ng/mL PMA (Phorbol Myristate Acetate; Sigma-Aldrich, St Louis, MO), 750-ng/mL Ionomycin (Sigma-Aldrich) and 5 μL Golgi Plug (BD Biosciences) in a 96-well plate, and incubated at 37°C for 4 to 5 hours before staining. Surface staining was performed for 15 to 20 minutes in FcR block with the corresponding cocktail of antibodies. Cells were washed and resuspended in fixation/permeabilization solution (BD Cytofix/Cytoperm kit; BD Pharmingen) and intracellular staining was performed per the manufacturer's protocol. Gates were determined by isotype staining performed on the same organ and time point.
Cytometric bead array and ELISA
Lymphoid organs from GVHD mice were removed, processed into a single cell suspension, and stimulated in vitro with 50 ng/mL PMA (Sigma-Aldrich) and 750 ng/mL Ionomycin (Sigma-Aldrich) in a 96-well plate and incubated at 37°C for 4 hours. Supernatant was removed and a BD cytometric bead array (CBA) or an ELISA (R&D Systems) was performed according to the manufacturer's protocol.
Histopathologic analysis of GVHD target organs
Mice were killed after BMT, and small bowel, large bowel, liver, and skin were removed and formalin-preserved, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Scoring was done as previously described.36
Statistics
All values shown in graphs represent the mean of each group plus or minus SEM. Survival data were analyzed with the Mantel-Cox log-rank test. For all other analyses, nonparametric unpaired Mann-Whitney U test was used. Samples that generated values of more than 2 standard deviations from the mean were removed.
Results
Th17 cells are found in lymphoid organs during GVHD
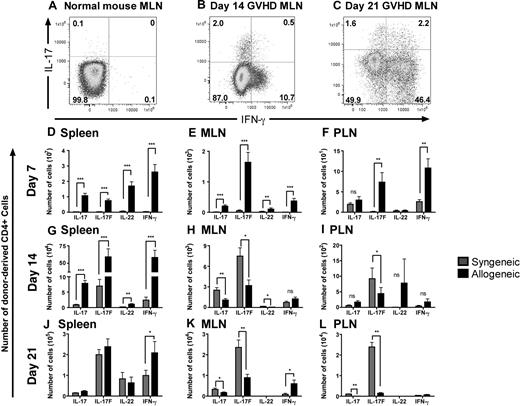
To assess whether Th17 cells are generated during GVHD, we used a major histocompatibility complex (MHC) class I/II–disparate allogeneic BMT model (B6→BALB/c) and transplanted lethally irradiated (850 cGy, split dose) BALB/c mice with 5 × 106 B6 T cell–depleted bone marrow (TCD-BM) and 0.5 × 106 CD4+ B6 T cells. The spleen, mesenteric lymph nodes (MLN), and peripheral lymph nodes (PLN) were harvested on days 7, 14, and 21 after BMT and donor-derived CD4+ T cells were analyzed for cytokine expression. We observed both an IL-17 single-positive and an IL-17+IFN-γ+ double positive population in all 3 lymphoid organs of allogeneic BM transplant recipients; however, these populations were not seen in untreated B6 mice (Figure 1A-C) or in transplanted mice without GVHD (data not shown). We confirmed this observation with nylon wool-passed (NWP) B6 donor T cells in the B6→BALB/c model and in a second allogeneic BMT model (B6→C3FeB6F1, data not shown). These data suggest that donor-derived Th17 cells, as well as CD4+IL-17+IFN-γ+ cells are generated during GVHD. These results are in agreement with a previous study that showed the presence of Th17 and CD4+IL-17+IFN-γ+ cells in a model of chronic GVHD.37
Th17 cells are found in lymphoid organs after BMT. Lethally irradiated (850 cGy) BALB/c mice were reconstituted with 5 × 106 WT TCD-BM and 0.5 × 106 CD4+ T cells. One representative staining for intracellular IFN-γ and IL-17 on CD4+ T cells from (A) untreated B6 mice (n = 5) and donor-derived CD4+ T cells from the MLN from mice with GVHD (B) on day 14 (n = 8) and (C) day 21 after BMT (n = 9). The absolute number of donor-derived CD4+ T cells expressing IL-17, IL-17F, or IL-22 in the (D-F) spleen, (G-I) MLN, and (J-L) PLN on days 7 (syngeneic n = 10; allogeneic n = 16), 14 (syngeneic n = 10; allogeneic n = 14), and 21 (syngeneic n = 5; allogeneic n = 14) after BMT. The mean and SD of each group is shown. Data represent 2 combined experiments. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
Th17 cells are found in lymphoid organs after BMT. Lethally irradiated (850 cGy) BALB/c mice were reconstituted with 5 × 106 WT TCD-BM and 0.5 × 106 CD4+ T cells. One representative staining for intracellular IFN-γ and IL-17 on CD4+ T cells from (A) untreated B6 mice (n = 5) and donor-derived CD4+ T cells from the MLN from mice with GVHD (B) on day 14 (n = 8) and (C) day 21 after BMT (n = 9). The absolute number of donor-derived CD4+ T cells expressing IL-17, IL-17F, or IL-22 in the (D-F) spleen, (G-I) MLN, and (J-L) PLN on days 7 (syngeneic n = 10; allogeneic n = 16), 14 (syngeneic n = 10; allogeneic n = 14), and 21 (syngeneic n = 5; allogeneic n = 14) after BMT. The mean and SD of each group is shown. Data represent 2 combined experiments. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
Donor CD4+ T cells produce IL-17, IL-17F, and IL-22 after syngeneic or allogeneic BMT
To further characterize the cytokine profile of alloreactive CD4+ T cells, we quantified the number of donor-derived CD4+ T cells expressing IL-17, IL-17F, or IL-22 in both allogeneic (B6→BALB/c) and syngeneic (Ly5.1→B6) BMT recipients on days 7, 14, and 21 after BMT. We found donor-derived CD4+ T cells expressing these cytokines in the spleen, MLN, and PLN in both syngeneic and allogeneic BMT recipients (Figure 1D-L). On day 7 after BMT, we found the numbers of donor-derived CD4+ T cells secreting Th17 cytokines or IFN-γ in the spleen and MLN in recipients of an allogeneic BMT to be significantly greater than in recipients of a syngeneic BMT. In addition, we found increased IL-17F+ or IFN-γ+ CD4+ T cells in the PLN of allogeneic recipients on day 7. Interestingly, on day 14, splenic donor-derived CD4+ T cells from allogeneic recipients displayed a similar phenotype as seen on day 7 (Figure 1G); however, the cytokine profile of donor-derived CD4+ T cells in the lymph nodes changed on day 14. We found greater numbers of CD4+ T cells expressing all 3 Th17 cytokines in the MLN and IL-17F+ cells in the PLN from syngeneic recipients than in allogeneic recipients (Figure 1H,I). This phenotype persisted on day 21 in the MLN and PLN, demonstrating an increase in IL-17+ and IL-17F+ donor-derived T cells in syngeneic recipients over allogeneic recipients (Figure 1K,L). Furthermore, on day 21 after BMT, the number of donor-derived CD4+ T cells producing Th17 cytokines in the spleen was similar between allogeneic and syngeneic BMT recipients, while the number of IFN-γ–expressing cells remained high in the allogeneic group (Figure 1J). These data suggest that donor-derived CD4+ T cells produce IL-17, IL-17F, or IL-22 after both syngeneic and allogeneic BMT, but production of these IL-17 family cytokines is significantly increased during the early stage of GVHD.
Th17 cells play a role in the early stages of CD4-mediated GVHD
To assess the functional role of CD4+ IL-17+ alloreactive T cells during GVHD, we used 2 MHC class I/II disparate allogeneic BMT models: B6→BALB/c and B6→C3FeB6F1. We observed no difference in survival between recipients of whole T cells isolated from IL-17−/− or WT mice in either model tested (Figure 2A,C). To evaluate the role of IL-17 in CD4-mediated GVHD, we transferred either IL-17−/− CD4+ T cells or WT CD4+ T cells along with WT B6 TCD-BM and monitored animals for survival. We found that recipients of IL-17−/− CD4+ T cells had a significant (P = .04) delay in the development of GVHD compared with recipients of WT CD4+ T cells (Figure 2B). By day 20 after allogeneic BMT, 56% of mice transplanted with WT CD4+ T cells were dead, while only 17% of mice that received IL-17−/− CD4+ T cells were dead (Figure 2B). Although recipients of IL-17−/− CD4+ T cells developed GVHD at a slower rate than WT recipients, these mice eventually succumbed to GVHD, and there was no difference in overall survival by day 90 after BMT (Figure 2B).
Th17 cells play a role in the early stage of CD4-mediated GVHD. (A) Lethally irradiated (850 cGy) BALB/c mice were reconstituted with 5 × 106 WT B6 TCD-BM alone, or with TCD-BM and 106 nylon wool–passed (NWP) WT B6 T cells or IL-17−/− T cells. Data represent 3 combined experiments: mice either received TCD-BM only (n = 15; dotted line, black square), IL-17−/− T cells + TCD-BM (n = 30; gray line, gray triangles) or WT T cells + TCD-BM (n = 30; solid line, black squares). (B) Lethally irradiated BALB/c mice received 5 × 106 WT B6 TCD-BM and 0.5 × 106 CD4+ WT B6 T cells or IL-17−/− T cells. Data represent 4 combined experiments: mice received either TCD-BM only (n = 30) (dotted line, black square), IL-17−/− CD4+ T cells + TCD-BM (n = 35) or WT B6 CD4+ T cells + TCD-BM (n = 45), P < .04. (C) Lethally irradiated (1300 cGy) C3FeB6F1 mice were reconstituted with 5 × 106 WT B6 TCD-BM and 2 × 106 NWP T cells WT B6 T cells or IL-17−/− T cells. Data represent 2 combined experiments: mice received TCD-BM only (n = 10; dotted line, black square), IL-17−/− T cells + TCD-BM (n = 20; gray line, gray triangles) or WT T cells + TCD-BM (n = 20; solid line, black squares). (D) Lethally irradiated BALB/c mice were reconstituted with 5 × 106 WT B6 TCD-BM (n = 20; dotted line, black square), or 5 × 106 WT B6 TCD-BM + 0.5 × 106 A20 cells (n = 20; gray line, gray circles), and 0.5 × 106 NWP WT B6 T cells + 0.5 × 106 A20 cells (n = 40; solid line, black squares) or IL-17−/− T cells + 0.5 × 106 A20 cells (n = 40; gray line, gray triangles). Data represent 4 combined experiments. Survival curves are shown.
Th17 cells play a role in the early stage of CD4-mediated GVHD. (A) Lethally irradiated (850 cGy) BALB/c mice were reconstituted with 5 × 106 WT B6 TCD-BM alone, or with TCD-BM and 106 nylon wool–passed (NWP) WT B6 T cells or IL-17−/− T cells. Data represent 3 combined experiments: mice either received TCD-BM only (n = 15; dotted line, black square), IL-17−/− T cells + TCD-BM (n = 30; gray line, gray triangles) or WT T cells + TCD-BM (n = 30; solid line, black squares). (B) Lethally irradiated BALB/c mice received 5 × 106 WT B6 TCD-BM and 0.5 × 106 CD4+ WT B6 T cells or IL-17−/− T cells. Data represent 4 combined experiments: mice received either TCD-BM only (n = 30) (dotted line, black square), IL-17−/− CD4+ T cells + TCD-BM (n = 35) or WT B6 CD4+ T cells + TCD-BM (n = 45), P < .04. (C) Lethally irradiated (1300 cGy) C3FeB6F1 mice were reconstituted with 5 × 106 WT B6 TCD-BM and 2 × 106 NWP T cells WT B6 T cells or IL-17−/− T cells. Data represent 2 combined experiments: mice received TCD-BM only (n = 10; dotted line, black square), IL-17−/− T cells + TCD-BM (n = 20; gray line, gray triangles) or WT T cells + TCD-BM (n = 20; solid line, black squares). (D) Lethally irradiated BALB/c mice were reconstituted with 5 × 106 WT B6 TCD-BM (n = 20; dotted line, black square), or 5 × 106 WT B6 TCD-BM + 0.5 × 106 A20 cells (n = 20; gray line, gray circles), and 0.5 × 106 NWP WT B6 T cells + 0.5 × 106 A20 cells (n = 40; solid line, black squares) or IL-17−/− T cells + 0.5 × 106 A20 cells (n = 40; gray line, gray triangles). Data represent 4 combined experiments. Survival curves are shown.
We performed histopathologic analyses of GVHD target organs, including the liver, skin, small-, and large-bowel. We found no significant differences between recipients of IL-17−/− and WT CD4+ T cells on days 14 and 20. However, in recipients of IL-17−/− CD4+ T cells, there was a trend toward less ulceration in the small bowel on day 21 and in the large bowel on days 14 and 21 (data not shown). These data suggest that IL-17 contributes to the early development of CD4-mediated GVHD, but IL-17+ CD4+ T cells do not significantly contribute to GVHD target organ damage.
To assess whether IL-17 production by donor T cells contributes to the GVT response, we transplanted lethally irradiated BALB/c mice with 5 × 106 TCD-BM, 0.5 × 106 WT T cells or IL-17−/− T cells, and 106 A20 lymphoma cells (Figure 2D). We observed no differences in the survival between IL-17−/− T cell recipients and WT T cell recipients, suggesting that recipients of IL-17−/− T cells mediate GVT activity. We conclude that IL-17 is dispensable for GVHD and GVT activity after transfer of whole donor T cells, but plays a role in early development of GVHD in recipients of selected CD4+ T cells.
Recipients of IL-17−/− CD4+ T cells have a decrease in splenic Th1 cells and produce decreased levels of proinflammatory cytokines
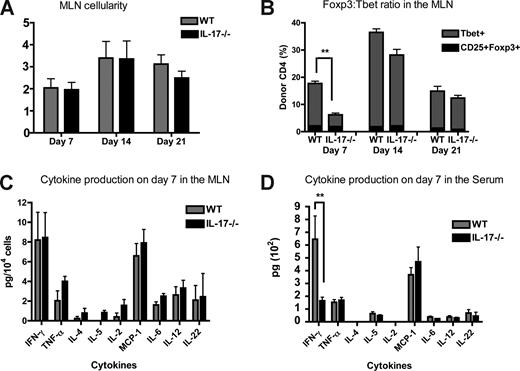
To determine how IL-17 modulates the immune response during the early stages of CD4-mediated GVHD development, we analyzed lymphoid organs from BMT recipients (B6→BALB/c with B6 TCD-BM and 0.5 × 106 CD4 + WT or IL-17−/− T cells) on days 7, 14, and 21 after BMT. By day 7 after allogeneic BMT, more than 95% of the spleens are of donor origin (data not shown). We found no differences in splenic cellularity between recipients of WT versus IL-17−/− CD4+ T cells (Figure 3A). However, we observed a significant decrease in the percentage of donor-derived CD4+ Tbet+ cell in recipients of IL-17−/− CD4+ T cells on day 14 after BMT (Figure 3B). Although Th17 cells can express Tbet,18 the difference in Tbet + CD4 + cells in recipients of IL-17−/− versus WT CD4+ T cells is greater than could be explained by a lack of Th17 cells alone. Importantly, we observed no difference in the percentage of T regulatory cells (Tregs; CD4+CD25+Foxp3+) in recipients of IL-17−/− CD4+ T cells compared with WT CD4+ T cells in the spleen (Figure 3B). Interestingly, the decrease in frequency of Th1 cells in the spleen on day 14 results in a 1:1 ratio of Treg:Th1 cells (Figure 3B). This change in the CD4+ T cell ratio may facilitate Treg-mediated control of Th1 cells in the early stage of GVHD.
Recipients of IL-17−/− CD4+ T cells have a decrease in splenic Th1 cells and produce decreased levels of proinflammatory cytokines. Lethally irradiated BALB/c mice were reconstituted with 5 × 106 WT TCD-BM and 0.5 × 106 WT or IL-17−/− CD4+ T cells. Spleens from recipient mice were harvested on days 7, 14, and 21 after BMT. (A) The absolute number of splenoctyes in recipients of CD4+ WT (gray) versus CD4+IL-17−/− (black). The mean of each group is shown; data represent 3 combined experiments on days 7 (n = 48) and 14 (n = 28) and one experiment on day 21 (n = 9). (B) The percentage of donor-derived CD4+CD25+Foxp3+ cells (black) and donor-derived CD4+Tbet+ cells (gray) in the spleen was determined using flow cytometry. The mean of each group is shown; data represent one of 3 experiments (days 7 and 14), day 7 (n = 6), day 14 (n = 9), or day 21 (n = 9; 1 experiment on day 21). (C) The number of IFN-γ+ cells on day 7 was determined by intracellular cytokine staining in recipients of CD4+ WT (gray) and CD4+ IL-17−/− (black) T cells. Two combined experiments (n = 12). (D) Splenocytes from recipients of WT B6 CD4+ T cells (gray) and IL-17−/− CD4+ T cells (black) on day 7 after BMT were stimulated in vitro for 4 hours, the supernatant was collected, and a CBA or an ELISA was performed to determine cytokine levels. Graphs represent the amount of cytokine secreted per 104 cells stimulated. The mean of each group is shown (n = 8); data represent one experiment of 2. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
Recipients of IL-17−/− CD4+ T cells have a decrease in splenic Th1 cells and produce decreased levels of proinflammatory cytokines. Lethally irradiated BALB/c mice were reconstituted with 5 × 106 WT TCD-BM and 0.5 × 106 WT or IL-17−/− CD4+ T cells. Spleens from recipient mice were harvested on days 7, 14, and 21 after BMT. (A) The absolute number of splenoctyes in recipients of CD4+ WT (gray) versus CD4+IL-17−/− (black). The mean of each group is shown; data represent 3 combined experiments on days 7 (n = 48) and 14 (n = 28) and one experiment on day 21 (n = 9). (B) The percentage of donor-derived CD4+CD25+Foxp3+ cells (black) and donor-derived CD4+Tbet+ cells (gray) in the spleen was determined using flow cytometry. The mean of each group is shown; data represent one of 3 experiments (days 7 and 14), day 7 (n = 6), day 14 (n = 9), or day 21 (n = 9; 1 experiment on day 21). (C) The number of IFN-γ+ cells on day 7 was determined by intracellular cytokine staining in recipients of CD4+ WT (gray) and CD4+ IL-17−/− (black) T cells. Two combined experiments (n = 12). (D) Splenocytes from recipients of WT B6 CD4+ T cells (gray) and IL-17−/− CD4+ T cells (black) on day 7 after BMT were stimulated in vitro for 4 hours, the supernatant was collected, and a CBA or an ELISA was performed to determine cytokine levels. Graphs represent the amount of cytokine secreted per 104 cells stimulated. The mean of each group is shown (n = 8); data represent one experiment of 2. *P ≤ .05, **P ≤ .01, ***P ≤ .001.
We also analyzed IFN-γ production by leukocytes. Macrophages, granulocytes, dendritic cells, natural killer (NK) cells, CD8+, and CD4+ cells in the spleen were analyzed for IFN-γ expression on days 7 and 14 after BMT. Recipients of IL-17−/− CD4+ T cells had a significant decrease in the number of IFN-γ+ macrophages, granuloyctes and CD4+ T cells on day 7 after BMT (Figure 3C). However, by day 14, there were no differences in the number of IFN-γ + cells in the spleen from recipients of WT versus IL-17−/− CD4+ T cells (data not shown). To assess whether IL-17 production by donor T cells affects IFN-γ production, we stimulated whole splenocytes harvested from WT and IL-17−/− recipients on days 7 and 14 after BMT and measured the cytokine levels in the supernatant. Recipients of IL-17−/− CD4+ T cells secreted lower levels of IFN-γ, IL-4, and IL-6 in the spleen on day 7 but not on day 14 (Figure 3D and data not shown). These data suggest that the decreased in IFN-γ+ CD4+ T cells, macrophages and granulocytes lead to a dramatic decrease in the amount of IFN-γ produced by splenocytes.
In addition, we found no differences in the expression of costimulatory molecules (CD28, GITR, ICOS), gut homing markers (CCR6, CCR9, LPAM) or naive/memory T-cell markers (CD44, CD62L) on donor T cells between recipients of IL-17−/− CD4+ T cells and WT CD4+ T cells (data not shown). Furthermore, we found no differences in the expression of costimulatory molecules (CD80, CD86) on myeloid cells (data not shown). We conclude that IL-17 may contribute to the recruitment or priming of Th1 cells and may be important for the proinflammatory cytokine milieu in the spleen during the early stages of GVHD.
Recipients of IL-17−/− CD4+ T cells have decreased percentages of CD4+ T cells in the MLN and decreased IFN-γ levels in the serum on day 7 after BMT
We harvested MLN from recipients of WT and IL-17−/− CD4+ T cells on days 7, 14 and 21 after BMT and found no differences in cellularity between recipients of WT and IL-17−/− CD4 T cells. However, we observed a decrease in the percentage of donor-derived Th1 cells on day 7 in recipients of IL-17−/− CD4+ T cells (Figure 4A,B). Interestingly, within the MLN, the Treg compartment was unaffected by transfer of IL-17−/− CD4+ T cells (Figure 4B). Recipients of IL-17−/− CD4+ T cells displayed a similar phenotype in the MLN on day 7 as seen in the spleen on day 14, with a decrease in the Th1 population, resulting in a 1:1 ratio of Treg:Th1 cells on day 7 (Figure 4B). We found no differences in IFN-γ expression by macrophages, granulocytes, dendritic cells, NK cells, CD8+, and CD4+ T cells (data not shown), or in the levels of IFN-γ produced upon stimulation of whole MLN (Figure 4C; data not shown). Interestingly, we do not see a correlation between the decrease in the number of CD4+ Tbet+ cells and IFN-γ+ CD4+ T cells in the MLN. However, we observed decreased levels of IFN-γ in the serum from recipients of IL-17−/− CD4+ T cells on day 7 after BMT (Figure 4D). Finally, there were no differences in the frequency of Th1 cells, Tregs, or IFN-γ levels in the PLN from recipients of WT and IL-17−/− CD4+ T cells (data not shown). We conclude that recipients of IL-17−/− CD4+ T cells have significantly fewer Th1 cells and lower IFN-γ serum levels on day 7 after BMT compared with recipients of WT CD4+ T cells.
Recipients of IL-17−/− CD4+ T cells have decreased frequency of CD4+ Tbet+ cells in the MLN and decreased IFN-γ levels in the serum on day 7 after BMT. Lethally irradiated BALB/c mice were reconstituted with 5 × 106 WT TCD-BM and 0.5 × 106 WT or IL-17−/− CD4+ T cells. MLN from recipient mice were harvested on days 7, 14, and 21 after BMT. (A) The absolute number of MLN from recipients of CD4+ WT (gray) and CD4+IL-17−/− (black) T cells. The mean of each group is shown; data represent 3 combined experiments on days 7 (n = 48) and 14 (n = 28), and 1 experiment on day 21 (n = 9). (B) The percentage of donor-derived CD4 + CD25 + Foxp3 + cells (black) and donor-derived CD4+Tbet+ cells (gray) in the MLN was determined using flow cytometry. The mean of each group is shown, and data represent one of 3 experiments (days 7 and 14), day 7 (n = 6), day 14 (n = 9) or a single experiment (day 21, n = 9). (C) MLN from recipients of WT B6 CD4+ T cells (gray) and IL-17−/− CD4+ T cells (black) on day 7 after BMT were stimulated in vitro for 4 hours, the supernatant was collected, and a CBA or an ELISA was performed to determine cytokine levels. Graphs represent the amount of cytokine secreted per 104 cells stimulated. The mean of each group is shown (n = 8), and data represent one experiment of 2. (D) Cytokine levels in the serum on day 7 after BMT were measured by CBA and ELISA. Shown is the mean for each group (n = 7), one representative experiment of 3. **P ≤ .01.
Recipients of IL-17−/− CD4+ T cells have decreased frequency of CD4+ Tbet+ cells in the MLN and decreased IFN-γ levels in the serum on day 7 after BMT. Lethally irradiated BALB/c mice were reconstituted with 5 × 106 WT TCD-BM and 0.5 × 106 WT or IL-17−/− CD4+ T cells. MLN from recipient mice were harvested on days 7, 14, and 21 after BMT. (A) The absolute number of MLN from recipients of CD4+ WT (gray) and CD4+IL-17−/− (black) T cells. The mean of each group is shown; data represent 3 combined experiments on days 7 (n = 48) and 14 (n = 28), and 1 experiment on day 21 (n = 9). (B) The percentage of donor-derived CD4 + CD25 + Foxp3 + cells (black) and donor-derived CD4+Tbet+ cells (gray) in the MLN was determined using flow cytometry. The mean of each group is shown, and data represent one of 3 experiments (days 7 and 14), day 7 (n = 6), day 14 (n = 9) or a single experiment (day 21, n = 9). (C) MLN from recipients of WT B6 CD4+ T cells (gray) and IL-17−/− CD4+ T cells (black) on day 7 after BMT were stimulated in vitro for 4 hours, the supernatant was collected, and a CBA or an ELISA was performed to determine cytokine levels. Graphs represent the amount of cytokine secreted per 104 cells stimulated. The mean of each group is shown (n = 8), and data represent one experiment of 2. (D) Cytokine levels in the serum on day 7 after BMT were measured by CBA and ELISA. Shown is the mean for each group (n = 7), one representative experiment of 3. **P ≤ .01.
Discussion
In this study we describe the expression of IL-17, IL-17F, and IL-22 by CD4+ donor-derived T cells; the functional role of T cell–derived IL-17 in GVHD and GVT; and the mechanism by which IL-17 contributes to the early stages of CD4-mediated GVHD through the regulation of Th1 cells and production of proinflammatory cytokines. Although a role for Th17 cells has been implicated in a variety of T-cell–mediated diseases, the functional role of Th17 cells in GVHD has not been well characterized.
We observed a population of donor-derived CD4+ T cells that produced both IL-17 and IFN-γ in lymphoid organs at days 14 and 21 after allogeneic BMT in 2 different models (Figure 1B,C and data not shown). This is in agreement with other studies that have demonstrated that in vivo IL-17–producing cells can also produce IFN-γ,38 suggesting that IFN-γ and IL-17 production by activated CD4+ T cells is not mutually exclusive. As GVHD progresses in allogeneic BMT recipients, donor-derived CD4+ T cells expressing IL-17, IL-17F, or IL-22 increase in the spleen, MLN, and PLN (Figure 1D-L), suggesting that Th17 cells are generated in response to alloantigens during GVHD.
Interestingly, we also noted significant numbers of donor-derived nonalloreactive CD4+ T cells expressing IL-17, IL-17F, or IL-22 after syngeneic BMT (Figure 1D-L). This may be a result of increased immune reconstitution of syngeneic hosts compared with allogeneic hosts with GVHD. Moreover, Yang et al39 observed that IL-17 and IL-17F are expressed at different ratios in different T-cell populations in the spleen during normal homeostasis and in the central nervous system (CNS) and intraepithelial lymphocytes (IEL) from mice with experimental autoimmune encephalomyelitis (EAE; mouse model of MS). IL-17 and IL-17F have been shown to have different functions in vivo. IL-17−/− mice have a significant delay in EAE onset after disease induction, whereas IL-17F−/− mice demonstrate only a moderate improvement in recovery over IL-17−/− mice,39 suggesting that IL-17F has a minor role in neuronal inflammation. In contrast, IL-17 was shown to positively regulate asthmatic allergic responses by enhancing Th2 cytokine production, whereas IL-17F was shown to negatively regulate allergic asthma by suppressing Th2 cytokine expression and eosinophil function.6,39 Finally, in a murine colitis model, IL-17 has been shown to play a protective role, while IL-17F exacerbates intestinal inflammation.39 These studies suggest that IL-17 and IL-17F expression are induced in response to different stimuli and mediate different functions, therefore, supporting our data that IL-17, IL-17F, and IL-22 are differentially regulated in various lymphoid tissues in BMT recipients with or without GVHD. The factors mediating the differential regulation of Th17-related cytokines and their biologic significance remain to be determined.
We found no difference in the survival of recipients of nonselected IL-17−/− or WT T cells, however, transfer of CD4+IL-17−/− T cells resulted in a delay in the onset of GVHD (Figure 2A-C). This suggests that alloreactive CD8+ T cells are not affected directly or indirectly by IL-17 deficiency and can compensate for the initial decrease in alloreactivity by IL-17−/− CD4+ T cells in our GVHD models. Moreover, the GVT response in our model is mostly CD8-mediated and the intact GVT achieved by IL-17−/− T cells supports our notion that CD8 alloreactive T cells function independently of IL-17. We conclude that IL-17 plays a role in the early onset of GVHD in recipients of selected CD4+ T cells, but is dispensable for GVHD development upon transfer of whole T cells.
We also observed that the number of splenic IFN-γ+ macrophages, granulocytes and CD4+ T cells in recipients of IL-17−/− CD4+ T cells was less than that observed in WT recipients (Figure 3C). These data correlated with a significant decrease in the amount of IFN-γ released in the supernatant upon stimulation of spleen cells (P < .001) from recipients of IL-17−/− T cells compared with WT recipients (Figure 3D). These data suggest that the decrease in the number of IFN-γ+ myeloid cells and Th1 cells may lead to a substantial decrease in the amount of IFN-γ produced. Collectively, these data imply that IL-17 regulates IFN-γ secretion of myeloid cells, which is in agreement with a study in a murine cardiac allograft model, which demonstrated that IL-17 modulates the maturation and activation state of myeloid cells, including dendritic cells.13 Studies using tumor-specific T cells demonstrated that Th17 polarized cells mediated enhanced tumor rejection compared with Th1 and Th0 polarized cells, which is dependent on IFN-γ production.40 These findings suggest that IL-17 is a potent inducer of IFN-γ and may require IFN-γ to mediate inflammatory effects.
Recipients of IL-17−/− CD4+ T cells have a decrease in the frequency of donor-derived Th1 cells in both the MLN and spleen on days 7 and 14, respectively (Figures 3B,4B). These data correlate with other studies regarding IL-17 and recruitment of Th1 cells. Khader et al41 demonstrated that IL-17 signaling is not required for primary control of Mycobacterium tuberculosis; however, IL-17 enhances recruitment of Th1 cells by inducing chemokine expression. Moreover, IL-17−/− mice immunized with MOG and CFA (complete Freund's adjuvent containing 0.5 mg/mL Mycobacterium tuberculosis) in EAE studies have decreased frequencies of CD4+ IFN-γ+ cells in the spleen on day 7 after immunization than do WT controls.39 Collectively, these findings suggest that IL-17 may directly or indirectly modulate Th1 cells.
In addition to changes in IFN-γ production, we also observed a decrease in IL-4 and IL-6 levels in the spleen on day 7 in recipients of IL-17−/− CD4+ T cells (Figure 3D). Others have found that IL-17 treatment of keratinocytes induces IL-6 production42 ; which could explain why in the absence of T cell–derived IL-17, the levels of IL-6 are decreased during GVHD. These data suggest that IL-17 production by donor-derived T cells contributes to the proinflammatory cytokine milieu during the early stage GVHD.
Our data differ from those by Yi et al,43 who found that transplantation of IL-17−/− spleen cells in the B6 into BALB/c model results in accelerated GVHD. Several important differences between the experimental designs might have contributed to the contrasting results. Although both studies use the B6 into BALB/c model, Yi et al find that sublethal irradation along with IL-17−/− spleens cells exacerbate GVHD, whereas we demonstrate that lethal irradiation and IL-17−/− CD4+ T cells delay GVHD. One important difference in these studies is the conditioning regimen. It is unclear whether Yi et al use a sublethal dose or a single dose of 800 cGy. However, they state in the text that they administer a “sublethal radiation dose, 2.5 × 106 TCD-BM cells and spleen cells” to BMT recipients, whereas we administered a split dose of lethal radiation, 5 × 106 TCD-BM and CD4-selected T cells. Differences in conditioning regimen will significantly impact radiation-induced gut damage and the “cytokine storm.”28,29,44 The conditioning regimen induces inflammatory cytokines, resulting in increased expression of adhesion molecules, costimulatory molecules, and MHC antigens.44 Danger signals expressed by injured host tissue signal for activation of host antigen-presenting cells (APCs), amplifying antigen presentation to allogeneic donor T cells.44 In the study by Yi et al, they suggest that IL-17 regulates Th1 differentiation by influencing host DCs early after BMT, and this effect is dependent on IL-12. Many studies have shown that Th1 differentiation is mediated by IL-12, which is primarily secreted by APCs. The different conditioning regimens may result in differences in the activation state and cytokine production of host APCs.
A further discrepancy is the survival kinetics of the individual GVHD models. Yi et al show that 100% of mice transplanted with 0.5 × 106 purified allogeneic WT B6 T cells (both CD4 and CD8 T cells) are still alive on day 50 after BMT, whereas we routinely observe that greater than 50% of recipient animals are dead by day 50.36,45 In addition, when Yi et al transfer whole spleen cells from WT B6 mice at several different doses (1.25 × 106 and 2.5 × 106), more than 70% of recipient mice survive longer than 50 days. Collectively, these data suggest that the discrepancies in the BMT procedure, such as radiation dose, result in different kinetics of GVHD development.
Another important difference between these 2 studies is the preparation of donor T cells. We used purified T cells, whereas Yi et al used whole spleen cells. A variety of T-cell subsets secrete IL-17 in the spleen. Stark et al46 determined that 60% of IL-17–producing cells in a B6 spleen are γδ T cells, 25% are NKT-like cells and only 15% are CD4+ T cells. In addition CD8 + T cells, astrocytes, and oligodentrocytes have been reported to express IL-17 in multiple sclerosis patients.47 Moreover, Uhlig and colleagues used a T-cell–independent colitis model and demonstrated that IL-23 regulates intestinal inflammation, which was associated with down-regulation of IL-17 in anti–IL-23-treated animals, suggesting that the source of IL-17 is a non–T-cell source.48 Furthermore, Alber and colleagues observed CD4−γδ TCR+ and CD4−γδ TCR− cells produce IL-17 during Salmonella infection.49 These data show that many cell types produce IL-17 in addition to T cells, suggesting that the data generated by Yi et al might be dependent on IL-17 deficiency in T cells and non-T cells, whereas our study investigates the specific role of CD4+ IL-17–producing cells in the development of GVHD.
In conclusion, Th17-related cytokines are produced by donor-derived CD4+ T cells during the development of GVHD. IL-17 production by donor T cells is not required for GVHD in recipients of nonselected donor T cells; however, it contributes to the development of GVHD in recipients of CD4+ T cells by increasing the production of proinflammatory cytokines and recruiting or priming Th1 cells in lymphoid organs during the early phase after BMT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by National Institutes of Health grants RO1-HL069929 (MvdB), RO1-CA107096 (MvdB), RO1-AI080455 (M.R.M.v.d.B.), PO1-CA33049 (M.R.M.v.d.B.), and T32-AI0762 (L.W.K.). Support was also received from the Ryan Gibson Foundation, the Elsa U. Pardee Foundation, the Byrne Fund, the Emerald Foundation, and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research (M.R.M.v.d.B.).
National Institutes of Health
Authorship
Contribution: L.W.K. designed and conducted experiments, analyzed data, and wrote the paper. G.L.G. helped design experiments and edited the paper. C.G.K., D.Y.S., O.M.S., C.L., A.M.H., J.G., and N.M.M. performed research. C.L. did histopathologic analyses of organs. Y.I. provided vital new reagents. G.H. analyzed the data. M.R.M.v.d.B. designed experiments and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel R. M. van den Brink, Memorial Sloan-Kettering Cancer Center, 418 East 69th Street, Zuckerman Research Center 1419, New York, NY 10021; e-mail: vandenbm@mskcc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal