Abstract
Reactive species derived from cell oxygenation processes play an important role in vascular homeostasis and the pathogenesis of many diseases including retinopathy of prematurity. We show that CYP1B1-deficient (CYP1B1−/−) mice fail to elicit a neovascular response during oxygen-induced ischemic retinopathy. In addition, the retinal endothelial cells (ECs) prepared from CYP1B1−/− mice are less adherent, less migratory, and fail to undergo capillary morphogenesis. These aberrant cellular responses were completely reversed when oxygen levels were lowered or an antioxidant added. CYP1B1−/− ECs exhibited increased oxidative stress and expressed increased amounts of the antiangiogenic factor thrombospondin-2 (TSP2). Increased lipid peroxidation and TSP2 were both observed in retinas from CYP1B1−/− mice and were reversed by administration of an antioxidant. Reexpression of CYP1B1 in CYP1B1−/− ECs resulted in down-regulation of TSP2 expression and restoration of capillary morphogenesis. A TSP2 knockdown in CYP1B1−/− ECs also restored capillary morphogenesis. Thus, CYP1B1 metabolizes cell products that modulate intracellular oxidative stress, which enhances production of TSP2, an inhibitor of EC migration and capillary morphogenesis. Evidence is presented that similar changes occur in retinal endothelium in vivo to limit neovascularization.
Introduction
Pathologic angiogenesis is associated with major blinding diseases including retinopathy of prematurity, diabetic retinopathy, and age-related macular degeneration (AMD).1 This neovascularization is driven by the hypoxic stimulus due to loss of existing vessels. The oxygen-induced ischemic retinopathy (OIR) model in mouse recapitulates this condition, whereby exposure to hyperoxia results in loss of existing retinal vessels promoting ischemia-mediated retinal neovascularization.2 Reactive oxygen species (ROS) play an important role during angiogenesis, and their aberrant production is linked to retinopathy of prematurity and diabetic retinopathy.3,4 In fact, antioxidants inhibit microvascular degeneration in models of diabetes and OIR.4-6 The cellular mechanisms, which modulate intracellular oxidative stress, however, are not fully characterized.
CYP1B1 is a member of the cytochrome P450 family of proteins. It is expressed in extrahepatic epithelial and particularly mesenchymal cells and exhibits a developmentally regulated expression pattern. This family of enzymes catalyzes a wide array of mono-oxygenase reactions targeting both foreign and endogenous lipophilic compounds including fat-soluble vitamins, steroid hormones, and polyunsaturated fatty acid (PUFA) products.7 Many of these enzymes can function with low specificity to initiate inactivation or excretion pathways, but also function with high specificity and activity to synthesize physiologically active chemicals such as steroid hormones.8,9 Recent studies indicate that expression of CYP enzymes in the cardiovascular system and their metabolites from arachidonic acid, play a crucial role in the modulation of vascular tone, blood flow, and angiogenesis.10-12
CYP1B1 expression is conserved in the early embryo across several species during the development of the neural crest, hind brain, and eyes.13 A role in the local synthesis of retinoic acid was recently demonstrated.14 CYP1B1 also metabolizes estrogen and interacts potently with many plant flavanoids that are present in the human diet.15 A physiologic role for CYP1B1 in eye development was demonstrated when loss of activity mutations were detected in individuals with primary congenital glaucoma.16 This disease results from maldevelopment of the anterior chamber angle of the eye. CYP1B1−/− mice, created by targeted gene disruption in embryonic stem cells, exhibited similar abnormalities.17-19 However, the molecular and cellular mechanisms that contribute to this localized and specific developmental defect are not known. These mice exhibit abnormalities in the organization of trabecular meshwork and Schlemm canal.19 The cellular components of these structures share many characteristics of vascular cells.20 Aberrant expression of CYP1B1 in these cells impacts their normal development and function. In addition, the expression of CYP1B1 in vascular cells, and modulation of CYP1B1 by shear stress, suggest an important role for the expression of CYP1B1 in vascular development and homeostasis.21-24 However, the expression and function of CYP1B1 in vascular development, angiogenesis, and, most importantly, endothelial cell (EC) function, require further investigation.
Angiogenesis is a complex and multistep process involving EC migration, proliferation, and ultimately differentiation and formation of patent vessels. The ability to culture ECs has been instrumental in dissecting the molecular mechanisms involved and identification of many of the components of these steps. Using transgenic mice that carry an interferon-γ–inducible temperature-sensitive large T antigen, we isolated retinal ECs from CYP1B1+/+ and CYP1B1−/− mice. Here, we tested the hypothesis that CYP1B1 removes cellular oxygenation products that induce oxidative stress and, thereby, promote release of antiangiogenic factors. We show that the matricellular protein TSP2 is a key antiangiogenic mediator of these changes and that CYP1B1 is an important determinant of EC oxygen sensitivity.
Methods
Animals
All experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care Committee of the University of Wisconsin School of Medicine and Public Health. Oxygen-induced ischemic retinopathy in mice was carried out as previously described.25 Retinal vasculature was evaluated by staining of retinal whole-mount preparations and frozen sections as described below. For EC isolation, immorto mice expressing a temperature-sensitive SV40 large T antigen (Charles River Laboratories, Wilmington, MA), which were backcrossed into C57BL/6 mice in our laboratory, were crossed with CYP1B1−/− mice, generated in a C57BL/6 background as previously described.17 The immorto CYP1B1−/− mice were identified by polymerase chain reaction (PCR) analysis of DNA isolated from tail biopsies. The PCR primer sequences were as follows: immorto forward: 5′-CCT CTG AGC TAT TCC AGA AGT AGT G-3′, immorto reverse: 5′-TTA GAG CTT TAA ATC TCT GTA GGT AG-3′; Neomyacin forward: 5′-TTG GGT GGA GAG GCT ATT CGG CTA TGA-3′, Neomyacin reverse: 5′-GGC GCG AGC CCC TGA TGC TC-3′; CYP1B1 forward: 5′-CTG AGT TGG ACC AGG TTG TGG-3′; CYP1B1 reverse: 5′-CAT GGA TTC TAA ACG ACT AGG-3′.
Visualization of retinal vasculature
Retinal vascular patterns were analyzed using retinal whole mounts or retinal trypsin digests stained with specific antibodies, including anti-collagen IV and CYP1B1 as previously described.25 Retinas were viewed by fluorescence microscopy and images were captured in digital format using a Zeiss microscope (Carl Zeiss, Chester, VA). The retinal trypsin digests were stained with hematoxylin/periodic acid–Schiff (PAS) to visualize retinal vasculature using phase microscopy. Apoptotic cell death was assessed by TdT-dUTP terminal nick-end labeling (TUNEL) staining of retinal trypsin digests and retinal ECs using the Fluorescein In Situ Cell Death Detection Kit as recommended by the supplier (Roche, Indianapolis, IN) and described by us.26 TUNEL-positive cells were counted and calculated as a percentage of total cell number.
Quantification of neovascular proliferative retinopathy
Quantification of vitreous neovascularization on P17 was performed as previously described.25 The neovascular score was defined as the mean number of neovascular nuclei per section found in 8 sections (4 on each side of the optic nerve) per eye.
Cells
Retinal ECs were isolated from immorto CYP1B1+/+ and CYP1B1−/− mice, and maintained at 33°C with 5% CO2 as previously described.27 For details, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Human retinal ECs were a gift of Dr Ram Nagaraj (Case Western Reserve University School of Medicine, Cleveland, OH) and maintained in 1:1 mixture of Dulbecco modified Eagle medium (DMEM; low glucose; Mediatech, Manassas, VA) and Ham F12 nutrient mix (Mediatech) containing 10% fetal bovine serum (FBS), 15 μg/mL EC growth supplement from bovine pituitary (Sigma-Aldrich, St Louis, MO), 1% ITS (insulin/transferrin, selenium; Sigma-Aldrich), 1% antibiotic/antimycotic (Invitrogen, Carlsbad, CA) and 1% GlutaMax (Invitrogen). Cells were maintained at 37°C with 5% CO2 and propagated on 1% gelatin-coated 60-mm dishes. C3H10T1/2 mouse embryonic mesenchymal cells were from ATCC (Manassas, VA) and maintained as recommended by the supplier. For incubation with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; AccuStandard, New Haven, CT), cells were grown in their growth medium containing TCDD at 10−7 M for 24 hours.
Capillary morphogenesis
Capillary morphogenesis in Matrigel (10 mg/mL; BD Biosciences, San Jose, CA) was performed as previously described.27 For inhibitor studies, cells were incubated with a specific inhibitor for 20 minutes before plating on Matrigel. The inhibitors tested were 2,3′,4,5′-tetramethoxystilbene (TMS; 5 μM; Cayman Chemical, Ann Arbor, MI), N-acetylcysteine (NAC; 1 mM; Sigma-Aldrich), L-ascorbic acid (100 μM; Sigma-Aldrich), phenethyl isothiocyanate (1 μM; Sigma-Aldrich), diphenyleneiodonium chloride (1 μM; Sigma-Aldrich), apocynin (100 μM; Sigma-Aldrich), allopurinol (20 μM; Sigma-Aldrich), 17-octadecynoic acid (10 μM; Sigma-Aldrich), diphenyl-1-pyrenlphophine (40 μM; Cayman Chemical), retinol (1 μM; Sigma-Aldrich), all-trans retinoic acid (1 μM; Sigma-Aldrich), and AM580 (1 μM; Sigma-Aldrich). Cells (3 × 105 in 2 mL growth medium) were gently added to the Matrigel-coated plates and transferred to a tissue-culture incubator (20% oxygen). For capillary morphogenesis under hypoxia conditions, Matrigel-coated plates and medium were pre-equilibrated in the hypoxia chamber (2% O2, 5% CO2, and 93% N2) for 24 hours. Cells were prepared in pre-equilibrated hypoxia growth medium and added gently to the pre-equilibrated hypoxia Matrigel-coated plates as described above and transferred to an incubator in a hypoxia chamber (2% oxygen). Cultures were monitored for 6 to 24 hours, and photographed in digital format using a Nikon microscope. For quantitative assessment of the data, the mean numbers of branches, at points of intercepts per 5 representative high-power fields (100×), were determined.
Adenovirus construction and infection of ECs
Replication-deficient adenoviruses expressing mouse full-length CYP1B1 (accession no. NM_009994), under the control of the cytomegalovirus (CMV) promoter, were generated using the pAdTrack-CMV vector and AdEasy System (Agilent/Stratagene, La Jolla, CA) as previously described.28 Retinal ECs were plated in 60-mm dishes and were 80% to 90% confluent by the next day. Cells were incubated with either recombinant adenovirus (5-15 pfU/cell) expressing CYP1B1 sense cDNA or vector control in 1 mL Opti-MEM and 10 μL Lipofectin (Invitrogen) for 5 hours in a tissue-culture incubator. After incubation, cells were fed with EC growth medium and incubated for 2 days. Cell lysates were prepared and evaluated for CYP1B1 expression by Western blotting as described.
Construction and expression of siRNAs
Retroviruses expressing a gene-specific siRNA for mouse CYP1B1 and TSP2 were prepared and tested for knockdown expression as detailed in Document S1. Stable cell populations expressing a specific siRNA were evaluated for their ability to undergo capillary morphogenesis as outlined.
Western blot analysis
For TSP2 protein analysis, cells were plated at 2 × 105 per 60-mm dish and allowed to reach approximately 90% confluence (2 days). Cells were rinsed once with serum-free medium and incubated with serum-free growth medium (complete medium without serum) for 2 days. Conditioned medium was collected and clarified by centrifugation, and protein concentration was determined using the BCA Protein Assay (Pierce, Rockford, IL). Samples (10 μg) were adjusted for protein content and mixed with appropriate volume of 6× sodium dodecyl sulfate (SDS) sample buffer and analyzed by 4% to 20% SDS–polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen). For cell lysates, cells were rinsed with Tris-buffered saline (TBS; 20 mM Tris-HCl, 150 mM NaCl, pH 7.6) once and lysed using 0.1 mL lysis buffer (10 mM Tris-HCl pH 7.6, 1 mM ethylenediaminetetraacetic acid [EDTA], 1% Triton X-100, 1% NP-40, 0.1% SDS, and protease inhibitor cocktail [Roche Applied Science, Indianapolis, IN]). Protein concentration was determined and adjusted as stated above for analysis using 20 μg cell lysate. Proteins were transferred to nitrocellulose membrane and blotted with specific antibodies including anti-TSP2 (1:1000; BD Transduction, San Diego, CA) and anti–prohibitin-1 (1:1000; Thermo Fisher, Waltham, MA) as described previously.27 For reprobing, blots were washed once with TBS with 0.1% Tween-20 (TBST) and stripped by 2 washes (25 mL) with prewarmed stripping solution (62.5 mM Tris-HCl, pH 6.8; 2% SDS; 100 mM β-mercaptoethanol) at 65°C. Blots were then washed twice with TBST (5 minutes each) at room temperature before proceeding with blotting as described above. The antibody to CYP1B1 was developed in rabbits using a bacterially expressed mouse CYP1B1 and shown to react with mouse CYP1B1 as previously described.29 The antibodies to β-catenin or β-actin (Sigma-Aldrich) were used as loading controls.
CYP1B1 activity assay
CYP1B1 activity was determined using the P450 Glo Assay Kit (Promega, Madison, WI) as recommended by the supplier. For details, see Document S1.
Determination of ROS in ECs
The level of cellular ROS was assessed using dihydroethidium (DHE; Invitrogen/Molecular Probes, Eugene, OR). DHE is cell-permeant, weakly blue-fluorescent dihydroethidium. Upon oxidation, red-fluorescent ethidium binds to DNA and accumulates in the nucleus. Cells were plated on gelatin-coated coverslips at 105 cells/mL. After attachment, cells, with or without preincubation with 5 μM TMS for 6 hours, were rinsed with Hank balanced salt solution (HBSS) twice, and incubated with 5 μM DHE for 15 minutes at 37°C in 5% CO2. At the end of the incubation, cells were rinsed with HBSS and examined alive by fluorescence microscopy and images were captured in digital format using a Zeiss microscope (Carl Zeiss). For quantitative assessments, the images were analyzed with Image J software (National Institutes of Health, Bethesda, MD). Values were obtained from mean fluorescent intensity between CYP1B1+/+, CYP1B1+/+ with TMS, and CYP1B1−/− ECs from multiple cells captured within each section. We also used 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) dye (Invitrogen), which is better retained by cells, and oxidation of the probe can be detected by monitoring the increase in fluorescence with a flow cytometer, fluorometer, microplate reader, or fluorescence microscope using excitation source and filters appropriate for fluorescein. DCF is the oxidation product of H2DCFDA and is widely used as a general marker of cellular oxidation by hydroxyl radicals, hydrogen peroxide, and peroxynitrite. CYP1B1+/+ and CYP1B1−/− retinal ECs were plated in a 96-well plate at 5 × 104 cells per well. The next day, cells were rinsed with HBSS, and incubated with H2DCFDA (20 μg/mL) for 30 minutes at 37°C, and fluorescence changes were monitored using a fluorescence plate reader at 10-minute intervals for 1 hour.
Immunohistochemical staining of frozen eye sections
The immunostaining of frozen eye sections was performed as previously described.25 Rabbit anti–mouse TSP2 (a gift of Dr Deane F. Mosher, University of Wisconsin, Madison, WI), rabbit anti-HNE (4-hydroxy-2-nonenal; 1:500 dilution prepared in blocking solution; Alpha Diagnostic International, San Antonio, TX), and rat anti–mouse endoglin (BD Biosciences) were used. Retinal sections were viewed by fluorescence microscopy, and images were captured in digital format using a Zeiss microscope (Carl Zeiss).
Glutathione assay
The retina and retinal EC glutathione levels were determined using the GSH-Glo Glutathione Assay kit from Promega. The luminescence-based assay is based on the conversion of a luciferin derivative into luciferin in the presence of glutathione, catalyzed by glutathione S-transferase (GST). The signal generated in a coupled reaction with firefly luciferase is proportional to the amount of glutathione present in the sample. For retinal ECs, 104 cells were plated per well in 96-well plates. For retinal extract preparation, retinas were dissected out and put in phosphate-buffered saline (PBS) containing 2 mM EDTA (50 μL per retina). After homogenization, extracts were centrifuged, and 50 μL supernatant was loaded per well in 96-well plates to detect glutathione levels. The assay result is normalized using GSH standard solution provided with the kit.
Statistical analysis
Statistical differences between groups were evaluated with the Student unpaired t test (2-tailed). Means plus or minus SDs are shown. P values less than .05 were considered significant.
Results
Attenuation of pathologic angiogenesis in CYP1B1−/− mice
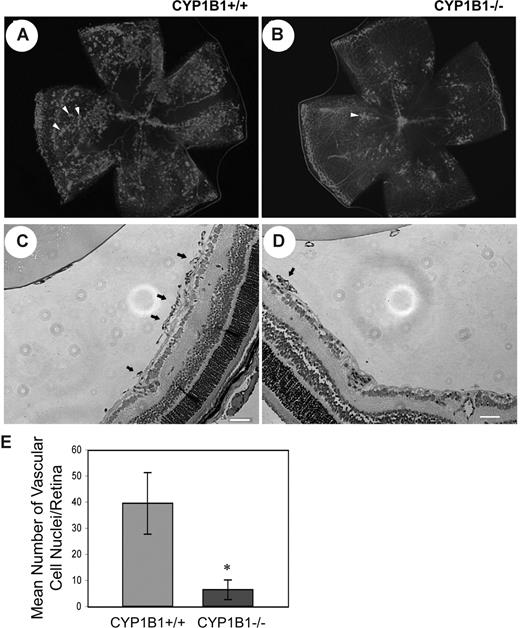
Given the fact that CYP1B1 is expressed in EC and its expression is up-regulated by shear stress,24 we determined the impact lack of CYP1B1 has on pathologic retinal angiogenesis during OIR. The mouse OIR is a highly reproducible model for studying all aspects of angiogenesis. In this model, P7 mice are exposed to 75% oxygen for 5 days, and then returned to room air for 5 days. The exposure of developing retinal vasculature to high oxygen halts growth of additional vessels and promotes loss of existing vessels. Therefore, when animals are returned to room air, the retina becomes ischemic and promotes growth of new blood vessels, which grow into the vitreous and are leaky. Figure 1A and B show collagen IV staining of retinal wholemounts in P17 CYP1B1+/+ and CYP1B1−/− mice subjected to OIR (when maximum preretinal neovascularization occurs), respectively. Figure 1C and D show hematoxylin/PAS- stained eye sections from P17 CYP1B1+/+ and CYP1B1−/− mice subjected to OIR. The quantitative assessment of preretinal neovascularization at P17 is shown in Figure 1E. There were significantly fewer retinal vascular cell nuclei detected on the vitreous side of eyes from P17 CYP1B1−/− mice compared with CYP1B1+/+ mice (n = 20, *P < .05).
Attenuation of pathologic angiogenesis in CYP1B1−/− mice. CYP1B1+/+ and CYP1B1−/− mice were exposed to a cycle of hyperoxia and room air (OIR). The collagen IV–stained of whole mount retinas prepared from P17 CYP1B1+/+ and CYP1B1−/− mice are shown in panels A and B, respectively (×25). The hematoxylin/PAS–stained eye sections prepared from P17 CYP1B1+/+ and CYP1B1−/− mice are shown in panels C and D, respectively (×100). The arrows indicate growth of new vascular tufts, which is significantly diminished in CYP1B1−/− mice. The number of vascular cell nuclei present on the vitreous side of the retina penetrating the inner limiting membrane was determined as described in “Methods” at P17 and presented in panel E. Data in each bar are the mean number of vascular cell nuclei in 5 eyes of 5 mice (error bars indicate SD). Please note that there is a significant decrease in the degree of retinal neovascularization in CYP1B1−/− mice compared with CYP1B1+/+ mice (n = 20, *P < .05).
Attenuation of pathologic angiogenesis in CYP1B1−/− mice. CYP1B1+/+ and CYP1B1−/− mice were exposed to a cycle of hyperoxia and room air (OIR). The collagen IV–stained of whole mount retinas prepared from P17 CYP1B1+/+ and CYP1B1−/− mice are shown in panels A and B, respectively (×25). The hematoxylin/PAS–stained eye sections prepared from P17 CYP1B1+/+ and CYP1B1−/− mice are shown in panels C and D, respectively (×100). The arrows indicate growth of new vascular tufts, which is significantly diminished in CYP1B1−/− mice. The number of vascular cell nuclei present on the vitreous side of the retina penetrating the inner limiting membrane was determined as described in “Methods” at P17 and presented in panel E. Data in each bar are the mean number of vascular cell nuclei in 5 eyes of 5 mice (error bars indicate SD). Please note that there is a significant decrease in the degree of retinal neovascularization in CYP1B1−/− mice compared with CYP1B1+/+ mice (n = 20, *P < .05).
CYP1B1 is constitutively expressed in ECs
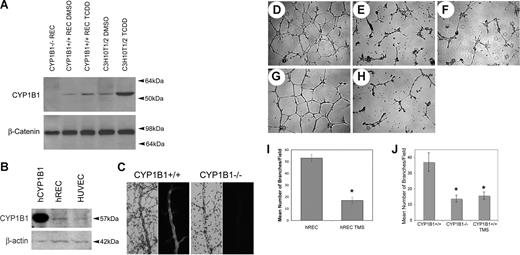
To gain insight into the role CYP1B1 plays in angiogenesis, retinal ECs were isolated from CYP1B1+/+ and CYP1B1−/− immorto mice.27 The CYP1B1+/+ and CYP1B1−/− ECs exhibited a similar morphology when plated on gelatin and expressed significant amounts of platelet/endothelial cell adhesion molecule-1 (PECAM-1) and vascular endothelial (VE)–cadherin on their surface (Figure S1A,B). We next assessed the expression of CYP1B1 in ECs by Western blotting of the lysates prepared from CYP1B1+/+ ECs incubated with DMSO (dimethyl sulfoxide; control) or TCDD (10 nM) for 24 hours. Thus, ECs constitutively expressed CYP1B1, which was further induced in the presence of TCDD, a known inducer of CYP1B1 (Figure 2A). CYP1B1 was absent in the CYP1B1−/− ECs as expected. C3H10T1/2 cells express significant amount of CYP1B1 and were used as positive control.30 CYP1B1 was also expressed in ECs prepared from vascular beds of other mouse tissues including heart, lung, kidney, and aorta (our unpublished data, July 2007). We also observed expression of CYP1B1 in human retinal ECs and human umbilical vein ECs (Figure 2B).
CYP1B1−/− EC fail to undergo capillary morphogenesis in Matrigel. CYP1B1 expression in mouse retinal ECs and C3H10T1/2 cells incubated with and without TCDD (A), and human retinal ECs and umbilical vein ECs (B), were evaluated by Western blot analysis of total cell lysates. The purified human recombinant CYP1B1 protein was used as positive control. β-catenin or β-actin was used as loading control. CYP1B1 or HE/PAS wholemount staining of retinal trypsin digests, prepared from CYP1B1+/+ (right panels) and CYP1B1−/− (left panels) are shown in panel C. Capillary morphogensis of CYP1B1+/+ (D) and CYP1B1−/− (E) retinal ECs were evaluated by plating the cells in Matrigel as described in “Methods.” After 17 hours of incubation, CYP1B1+/+ ECs formed well-organized capillary-like networks, while CYP1B1−/− ECs ability to organize was severely compromised (×40). A similar inhibition can be observed by incubating the CYP1B1+/+ ECs with TMS (F), a specific inhibitor of CYP1B1 activity. Human retinal ECs also form well-organized capillary networks in Matrigel (G), which was attenuated in the presence of TMS (H). The quantitative assessments of the data are shown in panels I and J. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate standard deviation). Please note that the mean number of branch points formed by CYP1B1−/− ECs or CYP1B1+/+ ECs incubated with TMS were significantly lower than those formed by CYP1B1+/+ retinal ECs (n = 3, *P < .05). These experiments were repeated with 3 different preparations of ECs with similar results.
CYP1B1−/− EC fail to undergo capillary morphogenesis in Matrigel. CYP1B1 expression in mouse retinal ECs and C3H10T1/2 cells incubated with and without TCDD (A), and human retinal ECs and umbilical vein ECs (B), were evaluated by Western blot analysis of total cell lysates. The purified human recombinant CYP1B1 protein was used as positive control. β-catenin or β-actin was used as loading control. CYP1B1 or HE/PAS wholemount staining of retinal trypsin digests, prepared from CYP1B1+/+ (right panels) and CYP1B1−/− (left panels) are shown in panel C. Capillary morphogensis of CYP1B1+/+ (D) and CYP1B1−/− (E) retinal ECs were evaluated by plating the cells in Matrigel as described in “Methods.” After 17 hours of incubation, CYP1B1+/+ ECs formed well-organized capillary-like networks, while CYP1B1−/− ECs ability to organize was severely compromised (×40). A similar inhibition can be observed by incubating the CYP1B1+/+ ECs with TMS (F), a specific inhibitor of CYP1B1 activity. Human retinal ECs also form well-organized capillary networks in Matrigel (G), which was attenuated in the presence of TMS (H). The quantitative assessments of the data are shown in panels I and J. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate standard deviation). Please note that the mean number of branch points formed by CYP1B1−/− ECs or CYP1B1+/+ ECs incubated with TMS were significantly lower than those formed by CYP1B1+/+ retinal ECs (n = 3, *P < .05). These experiments were repeated with 3 different preparations of ECs with similar results.
To demonstrate CYP1B1 expression in retinal blood vessels in vivo, we took 2 approaches. In the first approach, we stained retinal blood vessels in trypsin-digested retinal preparations. This leaves the retinal blood vessels intact while digesting all remaining retinal tissue. A strong staining of the retinal blood vessels, especially larger vessels, was observed in CYP1B1+/+ (Figure 2C). No specific staining was observed in the CYP1B1−/− mice, as expected. In the second approach, we stained frozen retinal sections from CYP1B1+/+ and CYP1B1−/− mice. We observed specific staining of retinal blood vessels; again larger blood vessels were more intensely stained, in retinas of CYP1B1+/+ mice. However, some nonspecific staining was also visible. We observed no staining of retinal blood vessels in eyes from CYP1B1−/− mice but similar nonspecific staining was observed (not shown). Thus, CYP1B1 is expressed in retinal vasculature, perhaps in both ECs and supporting perivascular cells, as has been demonstrated here and by others.22-24
CYP1B1−/− ECs failed to undergo capillary morphogenesis
Most ECs rapidly organize and form capillary-like networks when plated on Matrigel. This recapitulates the later stages of angiogenesis, which occur with minimal amounts of cell proliferation. Figure 2D shows that CYP1B1+/+ ECs rapidly organize in Matrigel, forming an extensive capillary-like network. In sharp contrast, the ability of CYP1B1−/− ECs to form such networks was severely compromised (Figure 2E). Similar results to CYP1B1−/− ECs were observed when CYP1B1+/+ cells were incubated with CYP1B1-specific inhibitor TMS (5 μM; Figure 2F).31 Figure 2J shows the quantitative assessment of the data (n = 3, *P < .05). The inability of CYP1B1−/− ECs to undergo capillary morphogenesis was consistent with their reduced adhesive and migratory properties compared with CYP1B1+/+ ECs (Figures S2 and S3) and our in vivo data (Figure 1). Similar results were observed with human retinal ECs. Wild-type human retinal ECs rapidly organized and formed a capillary-like network, very similar to mouse retinal ECs, which was attenuated in the presence of TMS (Figure 2G,H). The quantitative assessment of the data are shown in Figure 2I (n = 3, *P < .05). We next examined the rate of apoptosis in retinal vasculature of CYP1B1+/+ and CYP1B1−/− at different postnatal days and in retinal ECs by TUNEL staining. We observed no significant differences in the rates of apoptosis in retinal vasculature in vivo or retinal ECs in culture (not shown).
Reexpression of CYP1B1 restored capillary morphogenesis in CYP1B1−/− ECs
To demonstrate that changes in cellular functions described above are due specifically to lack of CYP1B1 expression, we restored expression of CYP1B1 in CYP1B1−/− ECs. Adenoviruses expressing mouse CYP1B1 or empty vector were used to infect CYP1B1−/− ECs. Figure 3A shows that these viruses effectively expressed CYP1B1 in ECs in a virus input-dependent manner. We next determined whether the expressed CYP1B1 is enzymatically active. Using a commercially available kit for CYP1B1 activity, we confirmed that the expressed CYP1B1 protein had significant catalytic activity in CYP1B1−/− ECs compared with vector control cells (Figure 3B; n = 3, **P < .05). This activity was comparable with that seen in CYP1B1+/+ ECs, which is enhanced in the presence of TCDD (n = 3,*P < .05).
Expression of CYP1B1 restores the capillary morphogenesis defect observed in CYP1B1−/− EC. (A) Western blot analysis of whole cell lysates (20 μg) from CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1 at different virus input. β-catenin was used for loading control. (B) CYP1B1 activity assay of CYP1B1+/+ (incubated with or without TCDD) and CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1. Data in each bar are the mean relative luminescence (error bars indicate the standard deviation, n = 3, *P [CYP1B1+/+; control vs TCDD] and **P < .05 [CYP1B1−/−; vector vs CYP1B1]). The CYP1B1−/− ECs infected with adenovirus control (5 pfu/cell) (C), adenovirus expressing CYP1B1 (5 pfu/cell) (D), or CYP1B1+/+ ECs with vector control (5 pfu/cell) (E) were plated on Matrigel as described in “Methods” (×40). The capillary morphogenesis by CYP1B1+/+ cells infected with a retrovirus expressing control siRNA (F) or a mouse specific CYP1B1 siRNA 2015 (G) was similarly determined. The quantitative assessments of the data are shown in panels H and I. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate SD). Note that the ability of CYP1B1−/− ECs to organize in Matrigel was significantly improved with reexpression of CYP1B1, while its siRNA knockdown resulted in attenuation of capillary morphogenesis in CYP1B1+/+ retinal ECs (n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.
Expression of CYP1B1 restores the capillary morphogenesis defect observed in CYP1B1−/− EC. (A) Western blot analysis of whole cell lysates (20 μg) from CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1 at different virus input. β-catenin was used for loading control. (B) CYP1B1 activity assay of CYP1B1+/+ (incubated with or without TCDD) and CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1. Data in each bar are the mean relative luminescence (error bars indicate the standard deviation, n = 3, *P [CYP1B1+/+; control vs TCDD] and **P < .05 [CYP1B1−/−; vector vs CYP1B1]). The CYP1B1−/− ECs infected with adenovirus control (5 pfu/cell) (C), adenovirus expressing CYP1B1 (5 pfu/cell) (D), or CYP1B1+/+ ECs with vector control (5 pfu/cell) (E) were plated on Matrigel as described in “Methods” (×40). The capillary morphogenesis by CYP1B1+/+ cells infected with a retrovirus expressing control siRNA (F) or a mouse specific CYP1B1 siRNA 2015 (G) was similarly determined. The quantitative assessments of the data are shown in panels H and I. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate SD). Note that the ability of CYP1B1−/− ECs to organize in Matrigel was significantly improved with reexpression of CYP1B1, while its siRNA knockdown resulted in attenuation of capillary morphogenesis in CYP1B1+/+ retinal ECs (n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.
We next determined whether expression of CYP1B1 affects the ability of CYP1B1−/− ECs to undergo capillary morphogenesis in Matrigel. Figure 3D shows restoration of CYP1B1 expression significantly improved the ability of CYP1B1−/− ECs to undergo capillary morphogenesis compared with CYP1B1−/− vector control cells (Figure 3C). The expression of control adenovirus in CYP1B1+/+ cells did not affect their ability to undergo capillary morphogenesis (Figure 3E). The quantitative determination of the data are shown in Figure 3H (n = 3, *P < .05). Similar results were observed in CYP1B1+/+ cells infected with retroviruses expressing a mouse-specific CYP1B1 siRNA. Down-regulation of CYP1B1 in wild-type retinal ECs resulted in the attenuation of their ability to undergo capillary morphogenesis in Matrigel (Figure 3F,G). The quantitative presentation of the data are shown in Figure 3I (n = 3; *P < .05).
Increased cellular oxidative stress and TSP2 expression in CYP1B1−/− ECs
An important role for alterations in cellular oxidative state has been demonstrated in many vascular diseases. Given the nature of reactions catalyzed by CYP1B1, we hypothesized that CYP1B1 removes cellular oxygenation products that impact endothelium redox homeostasis. We next determined ROS levels in CYP1B1+/+ and CYP1B1−/− ECs using DHE, which develops fluorescence upon reaction with ROS. Figure 4B shows that CYP1B1−/− ECs have increased fluorescence compared with CYP1B1+/+ cells (Figure 4A), indicating higher levels of ROS in these cells. Furthermore, CYP1B1+/+ cells incubated with TMS (Figure 4C), like CYP1B1−/− retinal ECs, showed increased fluorescence in the nucleus. A significant increase in fluorescent intensity of CYP1B1−/− ECs was observed compared with CYP1B1+/+ cells (Figure 4D; n = 20, *P < .05). Similar results were observed using H2DCFDA (not shown).
CYP1B1−/− ECs show higher oxidative stress with increased TSP2 expression. DHE staining of CYP1B1+/+ (A), CYP1B1−/− (B) ECs, and CYP1B1+/+ ECs incubated with TMS (C) (×400) are shown. The quantitative assessment of the is shown in panel D. The data in each bar are the mean fluorescence intensities determined as described in “Methods,” and error bars indicate standard deviation. A significant increased fluorescent intensity was observed in CYP1B1−/− cells (n = 20, *P < .05). CYP1B1+/+ cells incubated with TMS showed higher fluorescence compared with untreated CYP1B1+/+ cells, similar to CYP1B1−/− cells. The level of TSP2 was analyzed by Western blotting of serum-free conditioned medium prepared from CYP1B1+/+ and CYP1B1−/− ECs as described in “Methods” (E). A blot of cell lysates prepared from these cells was probed with β-catenin to control for loading. Western blot analysis of whole cell lysates of CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1 is shown in F. Blots were probed with antibodies to TSP2, CYP1B1, and β-actin to control for loading. Please note expression of CYP1B1 in CYP1B1−/− ECs is inversely correlated with expression of TSP2. Western blot analysis of whole cell lysates from CYP1B1−/− ECs incubated with or without NAC for 2 days is shown in panel G. Blot was probed with TSP2, and β-actin was used as loading control. Western blot analysis of cell lysates prepared from CYP1B1−/− ECs expressing TSP2-specific siRNAs (2574 [I] or 3611 [J]) or control siRNA, probed with anti-TSP2 or β-actin (loading control) is shown in panel H. The capillary morphogenesis of these cells in Matrigel are shown in panels I-K. The quantitative assessment of the data are shown in panel L. Capillary morphogenesis was significantly restored in CYP1B1−/− cells expressing TSP2 siRNAs compared with control vector (n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.
CYP1B1−/− ECs show higher oxidative stress with increased TSP2 expression. DHE staining of CYP1B1+/+ (A), CYP1B1−/− (B) ECs, and CYP1B1+/+ ECs incubated with TMS (C) (×400) are shown. The quantitative assessment of the is shown in panel D. The data in each bar are the mean fluorescence intensities determined as described in “Methods,” and error bars indicate standard deviation. A significant increased fluorescent intensity was observed in CYP1B1−/− cells (n = 20, *P < .05). CYP1B1+/+ cells incubated with TMS showed higher fluorescence compared with untreated CYP1B1+/+ cells, similar to CYP1B1−/− cells. The level of TSP2 was analyzed by Western blotting of serum-free conditioned medium prepared from CYP1B1+/+ and CYP1B1−/− ECs as described in “Methods” (E). A blot of cell lysates prepared from these cells was probed with β-catenin to control for loading. Western blot analysis of whole cell lysates of CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1 is shown in F. Blots were probed with antibodies to TSP2, CYP1B1, and β-actin to control for loading. Please note expression of CYP1B1 in CYP1B1−/− ECs is inversely correlated with expression of TSP2. Western blot analysis of whole cell lysates from CYP1B1−/− ECs incubated with or without NAC for 2 days is shown in panel G. Blot was probed with TSP2, and β-actin was used as loading control. Western blot analysis of cell lysates prepared from CYP1B1−/− ECs expressing TSP2-specific siRNAs (2574 [I] or 3611 [J]) or control siRNA, probed with anti-TSP2 or β-actin (loading control) is shown in panel H. The capillary morphogenesis of these cells in Matrigel are shown in panels I-K. The quantitative assessment of the data are shown in panel L. Capillary morphogenesis was significantly restored in CYP1B1−/− cells expressing TSP2 siRNAs compared with control vector (n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.
Rac1-induced NADPH-dependent production of ROS mediates increased TSP2 expression in human aortic ECs.32 Thus, TSP2 levels may be sensitive to changes in the intracellular oxidative state. We next examined TSP2 expression levels released into medium prepared from CYP1B1+/+ and CYP1B1−/− ECs. Figure 4E shows that CYP1B1−/− ECs produced TSP2 at much higher levels compared with CYP1B1+/+ ECs. This is consistent with our DNA array analysis, which showed a 2.2-fold increase in TSP2 expression (our unpublished data). The Western blot of cell lysates prepared from these plates was probed with β-catenin to control for loading. Most interestingly, the restoration of CYP1B1 expression in CYP1B1−/− ECs resulted in decreased levels of TSP2 protein (Figure 4F; 2.5- and 3.5-fold at 3 pfu/cell and 5 pfu/cell, respectively; determined by densitometry). In addition, incubation of CYP1B1−/− EC with the antioxidant NAC (N-acetyl cysteine) resulted in decreased TSP2 level (Figure 4G; 3.2-fold, determined by densitometry), thus, indicating that TSP2 expression is affected by the oxidative state of retinal ECs. Lack of CYP1B1 did not significantly affect TSP1 expression in these cells (not shown).
To demonstrate that TSP2 is responsible for inhibition of capillary morphogenesis in CYP1B1−/− ECs, we down-regulated TSP2 expression by mouse-specific TSP2 siRNAs (Figure 4H; > 85%, determined by densitometry). The down-regulation of TSP2 in CYP1B1−/− ECs significantly enhanced their ability to undergo capillary morphogenesis compared with control (Figure 4I-K). Figure 4L shows the quantitative evaluation of the data (n = 3, *P < .05). However, inhibition of capillary morphogenesis in wild-type cells with TMS (CYP1B1 inhibitor; Figure 2F,J) did not result in significant changes in TSP2 expression in these short-term assays (not shown).
Antioxidant (NAC) or low oxygen levels overcome CYP1B1 deficiency in ECs
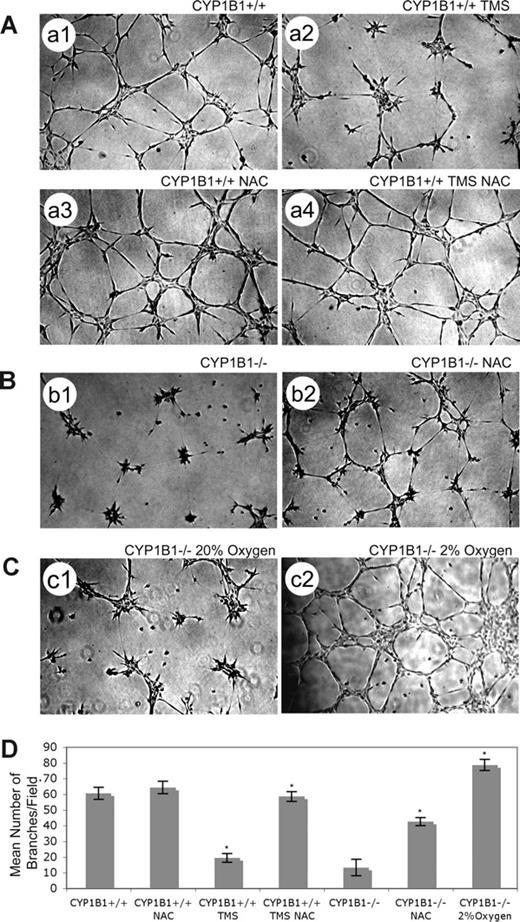
ECs generate ROS products such as isoprostanes in direct proportion to the oxygen concentration. The physiologic range of oxygen in most normal tissues is 2% to 9%.33 Normal cell culture is carried out under physiologic hyperoxia (20% oxygen). To test the hypothesis that lack of CYP1B1 results in accumulation of ROS which then inhibit capillary morphogenesis, we examined the ability of CYP1B1−/− ECs to undergo capillary morphogenesis in the presence of the antioxidant NAC or under hypoxic conditions (2% oxygen) instead of 20% oxygen. The CYP1B1−/− ECs' ability to undergo capillary morphogenesis was significantly improved in the presence of NAC (Figure 5B) and was completely reversed by culturing the cells at 2% oxygen (Figure 5C). For quantitative determination of the data, see Figure 5D (n = 3, *P < .05). The L-ascorbic acid, another antioxidant, was not as effective, while phenethyl isothiocyanate (inhibits oxidative stress through induction of antioxidant enzymes) and α-tocopherol (antioxidant) were partially effective (not shown). Thus, capillary morphogenesis of ECs is inhibited by oxygen-induced stress in the absence of CYP1B1, especially in the hyperoxic range (> 10%). Figure 5A demonstrates that TMS inhibition of CYP1B1+/+ ECs' capillary morphogenesis was overcome in the presence of NAC. The frequently used inhibitors of ROS production, particularly NADPH-oxidase (diphenyleneiodonium chloride and apocynin), xanthine oxidase (allopurinol), and 20-hydroxyeicosatetraenoic and epoxyeicosatrienoic acid (20-HETE and EET) synthases (17-octadecynoic acid),34 minimally affected the capillary morphogenesis of ECs in Matrigel regardless of CYP1B1 status. However, diphenyl-1-pyrenlphophine, an inhibitor of lipid peroxidation was partially effective in restoring capillary morphogenesis in null cells without affecting CYP1B1+/+ cells (not shown). In addition, retinol, all-trans retinoic acid, and AM580 (RXR agonist) failed to restore capillary morphogenesis of CYP1B1−/− EC (not shown).
Antioxidant (NAC) or low oxygen overcome CYP1B1 deficiency and restore capillary morphogenesis of CYP1B1−/− ECs. NAC can overcome TMS inhibition of CYP1B1+/+ EC capillary morphogenesis (A). CYP1B1+/+ ECs, control (A1), with 5 μM TMS (A2), with 1 mM NAC (A3), or with both TMS and NAC (A4) were plated in Matrigel as described in “Methods” (×40). Please note a significant increase in the mean number of branches in cells incubated with both NAC and TMS, compared with cells incubated with TMS alone. CYP1B1−/− ECs, with (B2) or without (B1) NAC were plated in Matrigel as described in “Methods.” Please note a significant increase in the mean number of branches in CYP1B1−/− ECs incubated with NAC. CYP1B1−/− ECs were plated in Matrigel and cultured under low oxygen (2%, C2), compared with room air (20% oxygen, C1). Please note CYP1B1−/− ECs undergo extensive capillary morphogenesis when cultured in 2% oxygen. All cultures were photographed after 17 hours in digital format. Quantitative assessment of the data is shown in panel D. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate the standard deviation; n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.
Antioxidant (NAC) or low oxygen overcome CYP1B1 deficiency and restore capillary morphogenesis of CYP1B1−/− ECs. NAC can overcome TMS inhibition of CYP1B1+/+ EC capillary morphogenesis (A). CYP1B1+/+ ECs, control (A1), with 5 μM TMS (A2), with 1 mM NAC (A3), or with both TMS and NAC (A4) were plated in Matrigel as described in “Methods” (×40). Please note a significant increase in the mean number of branches in cells incubated with both NAC and TMS, compared with cells incubated with TMS alone. CYP1B1−/− ECs, with (B2) or without (B1) NAC were plated in Matrigel as described in “Methods.” Please note a significant increase in the mean number of branches in CYP1B1−/− ECs incubated with NAC. CYP1B1−/− ECs were plated in Matrigel and cultured under low oxygen (2%, C2), compared with room air (20% oxygen, C1). Please note CYP1B1−/− ECs undergo extensive capillary morphogenesis when cultured in 2% oxygen. All cultures were photographed after 17 hours in digital format. Quantitative assessment of the data is shown in panel D. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate the standard deviation; n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.
We also evaluated the contribution of alterations in mitochondrial potential and/or electron transfer complexes in ROS generation and inhibition of capillary morphogenesis. The functionality of the mitochondria was determined by examining the expression of prohibitin-1. Prohibitin-1 is a highly conserved protein localized to the inner mitochondrial membrane and was recently implicated in modulation of the angiogenic capacity of EC.35 Decreased expression of prohibitin-1 resulted in increased mitochondrial production of ROS, through inhibition of complex I, and attenuation of EC migration and capillary morphogenesis in vitro and functional blood vessels in vivo. We observed similar levels of prohibitin-1 expression in CYP1B1+/+ and CYP1B1−/− retinal ECs, thus, suggesting a minimal role for mitochondria as a source of ROS in CYP1B1−/− ECs (not shown). We have also determined GSH levels in CYP1B1+/+ and CYP1B1−/− retinal ECs and retinal extracts using the GSH Bioluminescent Assay Kit (Promega). We observed no significant changes in GSH levels in the absence of CYP1B1, both in vivo and in cultured retinal EC. The levels of GSH in retinal EC were: CYP1B1+/+ = 36.94 ± 1.85 nM versus CYP1B1−/− = 36.53 ± 1.76 nM (P > .05; n = 3). The levels of GSH in retinas were: room air P21 CYP1B1+/+ = 78.96 ± 3.65 nM versus CYP1B1−/− = 73.5 ± 3.15 nM, and during OIR, P15 CYP1B1+/+ = 77.52 ± 3.75 nM versus CYP1B1−/− 78.93 ± 3.65 nM (P > .05; n = 3).
Increased HNE and TSP2 staining in retinas of CYP1B1−/− mice
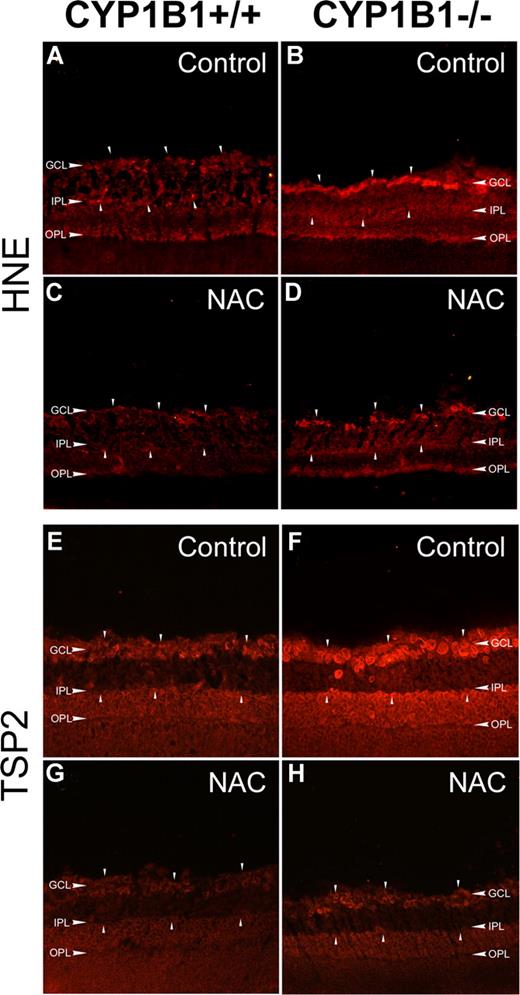
One of the most damaging effects of ROS production is initiation of lipid peroxidation, a radical chain reaction when ROS oxidize cellular membrane lipids. 4-Hydroxy-2-nonenal (HNE), one of the major aldehydic products of the peroxidation of membrane ω-6 PUFA, provides a measure of this process and can also contribute to oxidant stress-mediated cell injury.36 We next evaluated the presence of HNE in eye sections using immunofluorescence staining. Figure 6A and B show increased HNE staining in retinas prepared from P17 CYP1B1−/− mice compared with CYP1B1+/+ mice during OIR. The retina staining patterns observed here were consistent with those previously reported in eyes with increased oxidative stress,37 where strongest staining was observed in the inner retina, namely in the ganglion cell and inner plexiform layers.
Increased HNE and TSP2 staining in CYP1B1−/− mice retina. Frozen eye sections prepared from P17 CYP1B1+/+ (A,C,E,G) and CYP1B1−/− mice (B,D,F,H) exposed to OIR receiving solvent control (A,B,E,F) or NAC (C,D,G,H; 10 mg/kg in 0.1 mL IP) from P12 to P17, were stained with specific antibodies to HNE (A-D) and TSP2 (E-H) (×200). Please note the marked fluorescence staining for TSP2 and HNE (arrowheads) in CYP1B1−/− retina which are diminished upon administration of NAC compared with CYP1B1+/+ retinas. Sections were treated identically and images were obtained under identical conditions. These experiments were repeated twice with eyes from 4 different mice with similar results.
Increased HNE and TSP2 staining in CYP1B1−/− mice retina. Frozen eye sections prepared from P17 CYP1B1+/+ (A,C,E,G) and CYP1B1−/− mice (B,D,F,H) exposed to OIR receiving solvent control (A,B,E,F) or NAC (C,D,G,H; 10 mg/kg in 0.1 mL IP) from P12 to P17, were stained with specific antibodies to HNE (A-D) and TSP2 (E-H) (×200). Please note the marked fluorescence staining for TSP2 and HNE (arrowheads) in CYP1B1−/− retina which are diminished upon administration of NAC compared with CYP1B1+/+ retinas. Sections were treated identically and images were obtained under identical conditions. These experiments were repeated twice with eyes from 4 different mice with similar results.
To further confirm that these changes in retinas of CYP1B1−/− mice are due to increased oxidative stress, we evaluated the impact of NAC administration (10 mg/kg; intraperitoneally daily for 5 days from P12 to P17) on retinal HNE staining and neovascularization during OIR. Figure 6C and D show HNE staining of P17 retinal sections from CYP1B1+/+ and CYP1B1−/− mice treated with NAC. We observed a significant decrease in HNE staining in NAC-treated mice. We also examined TSP2 levels in retinas of control and NAC-treated P17 CYP1B1+/+ and CYP1B1−/− mice during OIR. Figure 6E and F shows increased TSP2 staining in retinas from CYP1B1−/− mice compared with CYP1B1+/+ mice. The strong TSP2 staining was also observed in the inner retina very similar to the HNE staining observed in retinas from CYP1B1−/− mice. However, TSP2 staining was significantly reduced in retinas of CYP1B1−/− mice incubated with NAC (Figure 6H).
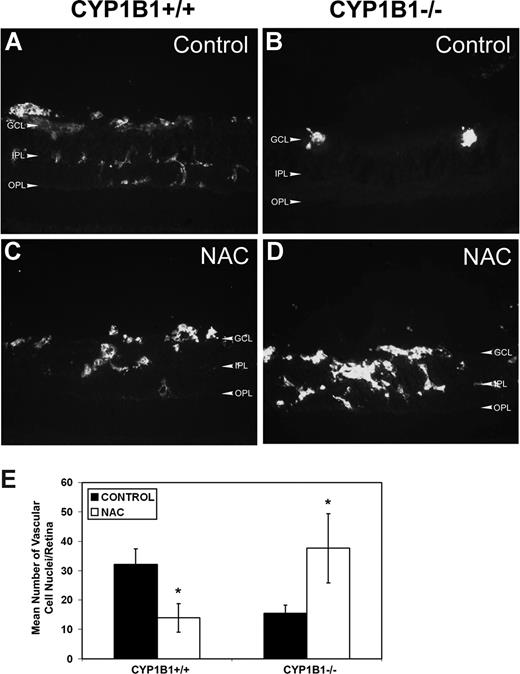
The significant decrease in HNE staining and TSP2 expression in retinas of CYP1B1−/− treated with NAC suggested that retinal neovascularization may now occur in these mice, as we observed in CYP1B1+/+ mice (Figure 1). We next assessed the degree of neovascularization in CYP1B1+/+ and CYP1B1−/− mice treated with NAC or solvent control by staining frozen retinal section with endoglin, a marker of ECs. We observed that the administration of NAC significantly reduced retinal neovascularization in CYP1B1+/+ mice (Figure 7A,C), as recently was demonstrated in wild-type mice.38 In contrast, administration of NAC restored significant retinal vascularization in CYP1B1−/− mice during OIR (Figure 7B,D). The quantitative assessment of the data are shown in Figure 7E (n = 15; *P < .05).
Restoration of retinal vascularization in CYP1B1−/− mice treated with NAC. Frozen eye sections from P17 CYP1B1+/+ (A,C) and CYP1B1−/− (B,D) mice exposed to OIR receiving solvent control (A,B) or NAC (10 mg/kg in 0.1 mL IP) from P12 to P17, were stained with anti-endoglin antibody (×400). Please note significant vascularization in CYP1B1−/− retina treated with NAC compared with solvent control. The degree of neovascularization was quantified as described in Figure 1 and shown in panel E (n = 15, *P < .05).
Restoration of retinal vascularization in CYP1B1−/− mice treated with NAC. Frozen eye sections from P17 CYP1B1+/+ (A,C) and CYP1B1−/− (B,D) mice exposed to OIR receiving solvent control (A,B) or NAC (10 mg/kg in 0.1 mL IP) from P12 to P17, were stained with anti-endoglin antibody (×400). Please note significant vascularization in CYP1B1−/− retina treated with NAC compared with solvent control. The degree of neovascularization was quantified as described in Figure 1 and shown in panel E (n = 15, *P < .05).
Discussion
This study describes a novel role for CYP1B1 as a modulator of EC oxidative state and regulator of angiogenesis. We show attenuation of pathologic angiogenesis in CYP1B1−/− mice. In addition, CYP1B1−/− ECs were less adherent and migratory, and failed to undergo capillary morphogenesis. We demonstrated that lack of CYP1B1 leads to increased intracellular oxidative stress in the endothelium and increased production of the angiogenesis inhibitor TSP2. Furthermore, we showed reexpression of CYP1B1, incubation with the antioxidant NAC, or culturing under low oxygen of CYP1B1−/− ECs restored their capillary morphogenesis and decreased TSP2 production. We also showed knockdown of TSP2 in CYP1B1−/− retinal ECs restored capillary morphogenesis. In vivo, administration of the antioxidant NAC was sufficient to reduce oxidative stress, decrease HNE staining and TSP2 expression, and restore retinal vascularization in CYP1B1−/− mice. Thus, our findings suggest that CYP1B1 metabolizes cell products that modulate intracellular oxidative stress, such that in its absence there is increased oxidative stress, enhanced production of TSP2, and inhibition of angiogenesis.
The proper regulation of EC adhesion and migration is essential during angiogenesis. CYP1B1 is constitutively expressed in both smooth muscle cells and ECs.22-24 However, the function of CYP1B1 in vascular cells requires investigation. To gain further insight into the role CYP1B1 plays in EC function, we successfully isolated retinal ECs from CYP1B1+/+ and CYP1B1−/− mice. These cells retained their EC markers in culture even after numerous passages. Most ECs rapidly organize and form capillary-like networks when plated in Matrigel, which recapitulates the later stages of angiogenesis with minimum cell proliferation. However, the CYP1B1−/− ECs failed to undergo capillary morphogenesis in Matrigel (Figure 2), and expressed increased levels of the antiangiogenic factor TSP2 (Figure 4E).39
TSP2 is a member of the TSP gene family of matricellular proteins and is closely related to TSP1. TSP2, like TSP1, is a potent endogenous inhibitor of angiogenesis.39,40 Mice deficient in TSP1 or TSP2 are viable but exhibit various vascular abnormalities including increased vascular density in many tissues and defects in wound healing.41-43 Purified TSP2 protein inhibits capillary EC migration in culture and basic fibroblast growth factor (bFGF)–induced corneal neovascularization in vivo.44 This is mediated, at least in part, through the ability of TSP2 to bind matrix metalloproteinase-2 (MMP2) promoting its internalization and reducing its activity.45-47 This is consistent with the attenuation of retinal pathologic angiogenesis, the adhesive and migratory defects observed in CYP1B1−/− ECs, and their inability to undergo capillary morphogenesis in Matrigel.
To demonstrate whether lack of CYP1B1 is sufficient to restore the proangiogenic defects observed in the null cell, we successfully reexpressed CYP1B1 in CYP1B1−/− ECs. The expression of CYP1B1 promoted capillary morphogenesis of CYP1B1−/− ECs (Figure 3C-E) and was concomitant with a significant decrease in TSP2 level (Figure 4F). In addition, the knockdown of CYP1B1 in wild-type cells resulted in the attenuation of capillary morphogenesis and increased TSP2 levels (Figures 3F,G,I and not shown). Thus, a reciprocal relationship may exist between CYP1B1 and TSP2 expression, such that in the absence of CYP1B1 up-regulation of TSP2 promotes an anitangiogenic state. In fact, we observed restoration of capillary morphogenesis in CYP1B1−/− retinal ECs infected with mouse specific TSP2-siRNAs (Figure 4I,J).
Rac1-induced NADPH oxidase–generated ROS stimulates TSP2 expression, but not TSP1, in human aortic ECs.32 Thus, TSP2 levels are sensitive to changes in the intracellular oxidative state. Given the nature of the CYP enzymes chemical reaction, we hypothesized that the lack of CYP1B1 results in increased levels of ROS and accumulation of oxygenated products. This set of deregulation enhances TSP2 release from ECs, thus promoting an antiangiogenic state. We showed that CYP1B1−/− ECs produce markedly more ROS than the CYP1B1+/+ cells (Figure 4). More interestingly, TMS-treated CYP1B1+/+ cells also exhibited higher levels of ROS in comparison to the untreated cells. Thus, lack of CYP1B1 expression and/or activity leads to accumulation of ROS and oxidative stress in ECs. Furthermore, the incubation of CYP1B1−/− ECs with the antioxidant NAC lowered TSP2 levels (Figure 4G); supporting the notion that higher TSP2 levels are a direct result of increased cellular oxidative stress. Although incubation of CYP1B1+/+ retinal ECs with TMS resulted in attenuation of capillary morphogenesis it did not result in significant expression of TSP2 (Figure 5A and not shown). This suggests that the effects of oxidative stress on TSP2 expression may require longer exposure to ROS, accumulation of oxygenated products, and/or diminished CYP1B1 protein expression/levels. These possibilities are a subject of future investigation.
ROS play an important role as intracellular mediators of vascular endothelial growth factor (VEGF) signaling in vitro and angiogenesis in vivo.48,49 However, when ROS are generated in high concentrations, they cause tissue injury and cell death, and are considered as risk factors for a variety of vascular diseases.50 The PUFAs are the major cellular target of ROS and readily provide an extractable proton to oxygen-free radicals. The resultant lipid radical is stabilized by the bis-allylic double bond system and rapidly accepts molecular oxygen to form peroxyl radicals. This initiates a series of complex, autocatalytic reactions that generate a variety of carbonyl compounds as their end products.51 Of these aldehydes, HNE is the most abundant and hence of greater biologic significance.36 The formation of HNE and related aldehydes is symptomatic of oxidative stress. We observed increased HNE as well as TSP2 staining in retinas of CYP1B1−/− mice compared with CYP1B1+/+ mice, especially during OIR (Figure 6). The TSP2 staining pattern correlated well with that of HNE staining, which concentrated in the inner retina (namely the ganglion and inner plexiform layers), as seen in AMD.37 The accumulation of HNE and its protein adducts in the retina indicates that lipid peroxidation products could contribute to the pathology and progression of AMD.52,53 Our studies indicate that HNE and its adducts may also impact appropriate vascularization of the retina, further exacerbating their pathologic impact. However, administration of the antioxidant NAC was sufficient to reduce HNE and TSP2 levels, resulting in restoration of retinal vascularization (Figures 6,7). These results suggest that the oxidative stress in response to lack of CYP1B1 is unique and perhaps different from simple exposure to high oxygen. Administration of NAC had a protective effect against retinal neovascularization during OIR in wild-type mice (Figure 7), as previously demonstrated.38
Other cytochrome P450s play major roles in vascular functions and angiogenesis.54 The most widely studied CYPs with vascular function belong to the CYP4A, 2B, 2C, and 2J families. Inhibitors of CYP4A suppress angiogenic responses, while CYP2C has important roles in hypoxia-induced EC function and angiogenesis.55-57 The majority of these CYP activities are mediated through metabolism of arachidonic acid and generation of eicosatetraenoic acids. Here, we show CYP1B1 also plays an important role during angiogenesis, and migration and capillary morphogenesis of retinal ECs. Our preliminary gene expression analysis of RNA prepared from eyes of CYP1B1+/+ and CYP1B1−/− mice by DNA microarray analysis indicated no alterations in the expression of other closely related CYP family members except CYP2A4, whose expression was up-regulated in null cells (our unpublished data, August 2006). However, the substrate specificity of murine CYP2A4 was recently shown to be very different from that of CYP1B1.58 There is not much known about the expression of CYP2A4 in the endothelium and its potential function in angiogenesis, especially in the absence of CYP1B1, and this requires future investigation. The lack of effects on capillary morphogenesis of retinal ECs in the presence of 17-ODYA suggest a minimal role for CYP4A and CYP2J, which catalyze the formation of 20-HETE and EET from arachidonic acid,34 in these processes.
Our hypothesis is that the accumulation of oxygenated products, mainly removed by CYP1B1, results in increased EC oxidative stress and TSP2 expression, which inhibits angiogenesis. Our data suggest that the contribution of NADPH-oxidase, xanthine-oxidase, mitochondria, or glutathione levels to ROS generation and oxidative stress in CYP1B1−/− ECs is minimal. We also observed increased expression of antioxidant enzymes such as superoxide dismutase and fatty aldheyde dehydrogenase, perhaps as a feedback mechanism to dampen the oxidative stress observed in null cells (our unpublished data). The incubation of CYP1B1−/− ECs under hypoxic conditions restored their ability to undergo capillary morphogenesis. Activity of CYP1B1 in these ECs substantially determines their sensitivity to oxygen. The parallel effects of NAC indicate that this sensor process is mediated by ROS and likely functions in proportion to oxygen concentration in the hyperoxia range (> 10%). This may also explain the lack of CYP1B1-deficiency effects on developmental vascularization and angiogenesis where oxygen concentration ranges from 2% to 9%.33 This contrasts with the HIF1α/proline hydroxylase sensor which begins to function only below 5% oxygen.59 The increased oxidative stress caused by the lack of CYP1B1 is responsible for the defects in pathologic angiogenesis in vivo and capillary morphogenesis of ECs in vitro. Therefore, understanding the mechanisms through which CYP1B1 modulates cellular oxidative state will provide important insight into regulation of angiogenesis and the basis for biorationale approaches to develop both inhibitors and enhancers of this process with potential future therapeutic applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants DK67120 (C.M.S.), ES09878 (C.B.M.), CA81493 (C.R.J.), EY16695 (N.S.), P30 CA014520 UW Paul P. Carbone Cancer Center Support Grant, P30 EY016665, and an unrestricted departmental award from Research to Prevent Blindness. N.S. is a recipient of research awards from American Diabetes Association (1-06-RA-123) and Retina Research Foundation. Y.T. was supported by American Heart Predoctoral Fellowship 0810200Z.
National Institutes of Health
Authorship
Contribution: Y.T. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the paper; E.A.S. performed research and collected and analyzed data; S.W. performed research and collected and analyzed data; C.M.S. performed research, collected and analyzed data, and critically revised the paper; C.B.M. analyzed data and critically revised and approved the paper; C.R.J. was involved in design of research and critically revised and approved the paper; and N.S. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nader Sheibani, University of Wisconsin School of Medicine and Public Health, Department of Ophthalmology and Visual Sciences, 600 Highland Avenue, K6/458 CSC, Madison, WI 53792-4673; email: nsheibanikar@wisc.edu.

![Figure 3. Expression of CYP1B1 restores the capillary morphogenesis defect observed in CYP1B1−/− EC. (A) Western blot analysis of whole cell lysates (20 μg) from CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1 at different virus input. β-catenin was used for loading control. (B) CYP1B1 activity assay of CYP1B1+/+ (incubated with or without TCDD) and CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1. Data in each bar are the mean relative luminescence (error bars indicate the standard deviation, n = 3, *P [CYP1B1+/+; control vs TCDD] and **P < .05 [CYP1B1−/−; vector vs CYP1B1]). The CYP1B1−/− ECs infected with adenovirus control (5 pfu/cell) (C), adenovirus expressing CYP1B1 (5 pfu/cell) (D), or CYP1B1+/+ ECs with vector control (5 pfu/cell) (E) were plated on Matrigel as described in “Methods” (×40). The capillary morphogenesis by CYP1B1+/+ cells infected with a retrovirus expressing control siRNA (F) or a mouse specific CYP1B1 siRNA 2015 (G) was similarly determined. The quantitative assessments of the data are shown in panels H and I. Data in each bar are the mean number of branches per 5 high-power fields (×100; error bars indicate SD). Note that the ability of CYP1B1−/− ECs to organize in Matrigel was significantly improved with reexpression of CYP1B1, while its siRNA knockdown resulted in attenuation of capillary morphogenesis in CYP1B1+/+ retinal ECs (n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/3/10.1182_blood-2008-03-145219/5/m_zh80050930310003.jpeg?Expires=1767740913&Signature=sjIjRj2lxOUtwSkFxCZj9Onmb~-Y7pFKP4lkZRu7-onc1tkX3u5QLCpXmk6tc01V9YTADXSnbo~2rvHbW1hiKaZWbrVbw3ER2t2Luk8FLEKvApLZxOcnZfVLyvep5lC0YnJuyDPjf0Uab4HQ8jsNCkgdprsCu8h82naskgLdZHMz2S82-gDJOBrl35T9z-n1D4nGcRTpHBAiyBP~5Sj~tafW9~EN~jjkiFpTP2pn989z7bwtmyGjRZ8~nchSh34AOZveokiK8L5A7C4nlC6FnYrFBD4w3lD~i~YAA2fcQ12NxKJ-YaySsRKUUkJ1JLvec02W4EakdUagrK0ANlOCFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. CYP1B1−/− ECs show higher oxidative stress with increased TSP2 expression. DHE staining of CYP1B1+/+ (A), CYP1B1−/− (B) ECs, and CYP1B1+/+ ECs incubated with TMS (C) (×400) are shown. The quantitative assessment of the is shown in panel D. The data in each bar are the mean fluorescence intensities determined as described in “Methods,” and error bars indicate standard deviation. A significant increased fluorescent intensity was observed in CYP1B1−/− cells (n = 20, *P < .05). CYP1B1+/+ cells incubated with TMS showed higher fluorescence compared with untreated CYP1B1+/+ cells, similar to CYP1B1−/− cells. The level of TSP2 was analyzed by Western blotting of serum-free conditioned medium prepared from CYP1B1+/+ and CYP1B1−/− ECs as described in “Methods” (E). A blot of cell lysates prepared from these cells was probed with β-catenin to control for loading. Western blot analysis of whole cell lysates of CYP1B1−/− ECs infected with the adenoviruses expressing empty vector or CYP1B1 is shown in F. Blots were probed with antibodies to TSP2, CYP1B1, and β-actin to control for loading. Please note expression of CYP1B1 in CYP1B1−/− ECs is inversely correlated with expression of TSP2. Western blot analysis of whole cell lysates from CYP1B1−/− ECs incubated with or without NAC for 2 days is shown in panel G. Blot was probed with TSP2, and β-actin was used as loading control. Western blot analysis of cell lysates prepared from CYP1B1−/− ECs expressing TSP2-specific siRNAs (2574 [I] or 3611 [J]) or control siRNA, probed with anti-TSP2 or β-actin (loading control) is shown in panel H. The capillary morphogenesis of these cells in Matrigel are shown in panels I-K. The quantitative assessment of the data are shown in panel L. Capillary morphogenesis was significantly restored in CYP1B1−/− cells expressing TSP2 siRNAs compared with control vector (n = 3, *P < .05). These experiments were repeated with 2 different preparations of ECs with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/3/10.1182_blood-2008-03-145219/5/m_zh80050930310004.jpeg?Expires=1767740913&Signature=0yTrpg~olwijXr6QpHpnZqQBCVYrq6km-7mIU1WytOnQuv5~Yp1IBd4mQn9aEKSoaUrcBJ7uIOXE~tF-t-6KPTOO~3we7MdP0-lCTcbXvxanmoEIRIjFa~CN~Ck9WqNzzQXOdx6C2gyhcaLWq~7yLFHWl7aQXJjoxuocwk7cNU2fhcxzb0bJJXAWgY8JcfbLcvvnRFSEPZmUN80NvfrDc1qlN7MBssPQBXMulu17gCD4qcRjPD7-lBFt9kI7XR21YVI2CBJmA~tf~c~h3BiI-S-Mdm300EViYlTWVs2GDpGP0vSs5YE-Zot9v7qP0Uc-xjMn5dKKBPKmdwIIJnLBGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal