Abstract
Reconstitution of the recipient lymphoid compartment following hematopoietic cell transplantation (HCT) is typically delayed. The present studies investigated the residual host CD4+CD25+Foxp3+ (Treg) compartment after several conditioning regimens, including T cell–depleted and T cell–replete HCT and observed (1) a small number of recipient Treg cells survived aggressive conditioning; (2) the surviving, that is, residual Tregs underwent marked expansion; and (3) recipient CD4+FoxP3+ cells composed the majority of the Treg compartment for several months post-syngeneic HCT. Notably, residual Tregs also dominated the compartment post-HCT with T cell–depleted (TCD) major histocompatibility complex–matched allogeneic bone marrow but not following T cell–replete transplantations. The residual Treg cell compartment was functionally competent as assessed by in vitro lymphoid suppression and in vivo autoimmune disease transfer assay. These observations support the notion that functional host Tregs initially occupy a niche in lymphopenic transplantation recipients, undergo significant expansion, and contribute to the compartment for an extended period before donor-derived CD4+FoxP3+ T cells eventually compose the majority of the compartment. In total, the findings suggest that the presence of host Tregs may be important to consider regarding elicitation of immune (eg, antitumor, vaccine) responses in recipients during the early post-transplant period involving autologous and certain allogeneic HCT regimens.
Introduction
Generation and function of the CD4+CD25+ T regulatory cell (Treg) compartment in normal mice are now understood to contribute an essential peripheral mechanism for the maintenance of self-tolerance and control of autoimmune disease.1-3 The capacity of these cells to regulate adaptive and innate immune responses has led to experimental studies investigating their potential use to regulate allogeneic transplantation responses.4-10 For example, experimental hematopoietic stem cell transplantations (HSCTs) have demonstrated that administration of donor Treg cells at the time of allogeneic HSCT can regulate graft-versus-host (GVH) and host-versus-graft (HVG) responses.5,7-9 With respect to HSCT, a clinically important issue concerns the reconstitution of the CD4 and CD8 lymphoid compartments after transplantation. Reconstitution of T cells is generally a slow process following allogeneic HSCT, typically complicated by immunosuppressive therapy and GVH disease.11-14 In addition, delay and abnormalities in T-cell numbers and function occur after autologous and syngeneic HSCT.15-17 Chemotherapy and radiation have been demonstrated to have a negative impact on thymic function (ie, as evidenced in the CD4+ T-cell compartment) dependent on age in nontransplanted patients.18 Thus, it has become increasingly appreciated that in certain patients including elderly persons who receive HSCT, in addition to thymic pathways of T-cell development, peripheral expansion of donor and surviving host populations are increasingly considered to contribute to the developing lymphoid pool.19
We have previously reported that host CD4+CD25+ T cells can suppress responses by host NK cells, which mediate resistance to engraftment by allogeneic bone marrow (BM) after a lethal (total body irradiation [TBI]) conditioning regimen and HSCT.20 Others have reported the ability of host Tregs to modulate T cell–dependent resistance.4 Several clinical reports have recently noted that antibody-mediated lymphoid depletion using Campath-1, anti-CD4, or RATG routinely results in residual phenotypic populations principally composed of memory cells including CD4+CD25+ T cells.21-24 Such studies demonstrate that host Treg cells survive, at least transiently, after such transplantations and have the capacity to mediate functional activity that regulates adaptive and innate immune responses. Based on these observations, we hypothesized that, dependent on the conditions of transplantation, at least some “residual” host Treg cells may persist after conditioning and BM transplantation. Furthermore, such surviving Treg cells could possess an initial advantage within this niche before development of de novo–derived Treg cells, resulting in the persistence and expansion of the former population.
In the present investigation, we found that host CD4+CD25+Foxp3+ T cells can be identified in recipients after syngeneic and allogeneic T cell–depleted (TCD) HCT after both ablative (> 9.0 TBI Gy) and nonablative conditioning. Although the transplantation of T cell–replete (TCR) syngeneic HCT yielded similar observations, the transplantation of allogeneic T cells resulted in the absence of these host Treg cells. Notably, for 6 to8 weeks after syngeneic or TCD allogeneic HCT, the Treg compartment was shown to be composed of a large component of host Treg cells derived from surviving cells, which underwent rapid expansion. Based on these findings, we speculate that surviving host Tregs initially occupy a niche in transplantation recipients, which permits their lymphopenic and/or antigen-driven expansion. Hence, these host Treg cells are present for a relatively extended period and contribute to the regulatory T-cell compartment at a significantly greater level than anticipated.
Methods
Animals
C57BL/6J (B6, H-2b), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1, H2b), B6.129S2-Cd4tm1Mak/J (B6-CD4−/−, H2b), B6.PL-Thy1a/CyJ (B6-Thy1.1, H2b), and C.B10-H2b/LiMcdj (BALB.B, H2b) mice were initially obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 × C57BL/10SgSnAi-[KO]γc-[KO]Rag2 (B6-Rag2−/−γc−/−, H2b) mice were purchased from Taconic Farms (Germantown, NY) and were 8 to 10 weeks of age at the time of injection. C57BL/6 IL-2Rβ−/− (B6-IL2Rβ−/−) mice were generated and maintained as previously described.25,26 Other than the B6-Rag−/−γc−/− recipients, all other mice were 8 to 16 weeks of age- and sex-matched for transplantations. All procedures involving animals were approved by the Animal Care and Use Committee at the University of Miami.
Antibodies
The following antibodies were used for flow cytometric analysis: anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD25 (PC61), anti-Thy1.1 (OX-7), anti-CD45.1 (A.20), anti-Thy1.2 (53-2.1), anti-BrdU monoclonal antibodies (mAbs) obtained from BD Biosciences (San Jose, CA) and anti-Foxp3 (FJK16s) mAb from eBioscience (San Diego, CA).
BM transplantation
Marrow from femurs and tibias were harvested from appropriate female donors. T cells were removed by incubation with anti-Thy1.2 mAb (HO13.4 ascites diluted 1:10) followed by 10% vol/vol Low-Tox M rabbit complement (Accurate Chemical & Scientific, Westbury, NY) at 37°C for 45 minutes. T cells present were reduced from approximately 2.0% to below the level of detection by fluorescence-activated cell sorter (FACS), ie, less than 0.2%. Recipient mice were administered various single or multiple split doses (3.0-14.0 Gy) of TBI from a Gammacell40 (37Cs source) at 35 to 40 cGy/min. Twenty-four hours after the last exposure, indicated doses of TCD-BM or TCD-BM supplemented with indicated numbers of lymph node cells or purified CD4+ and CD8+ peripheral T cells were infused intravenously and mice were maintained ad libitum on acidified water (pH < 2.2).
BrdU labeling/proliferation of Treg cells
For 5-color analysis, cells were incubated for 20 minutes at 4°C with Pacific Blue–CD4, phycoerythrin (PE)–Cy7-CD25, peridinin chlorophyll protein (PerCP)–Thy1.1 followed by 1 wash with Hank balanced salt solution (HBSS) containing 0.2% bovine serum albumin (BSA) and 150 mM NaN3. For analysis of BrdU incorporation, cells were incubated with BD Cytofix/Cytoperm buffer (BD Biosciences) for 20 minutes at 4°C and washed with Dulbecco phosphate-buffered saline (2.7 mM KCl, 0.370 mM KH2PO4, 1.4 M NaCl, 0.800 mM Na2HPO4) containing 3% fetal bovine serum (FBS), 0.1% saponin, and 0.09% NaN3 (wash buffer) followed by incubation for 10 minutes at 4°C with Cytoperm Plus Buffer (BD Biosciences) with 1 wash. Cells were incubated again with Cytofix/Cytoperm buffer for 5 minutes at 4°C, washed 1 time, and then incubated with fluorescein isothiocyanate (FITC)–conjugated isotype control or anti-BrdU for 20 minutes at room temperature followed by 1 wash. Cells were then incubated with rat IgG (0.01 mg/mL) for 15 minutes at 4°C followed by PE-Foxp3 for 30 minutes at 4°C and 1 wash with 1× permeabilization buffer (Foxp3 Kit; eBioscience). FACS analysis was performed as previously described27 using LSRII and Diva software (BD Biosciences).
Results
Host CD4+CD25+ Foxp3+ T-cell survival after high-intensity conditioning in experimental HCT recipients
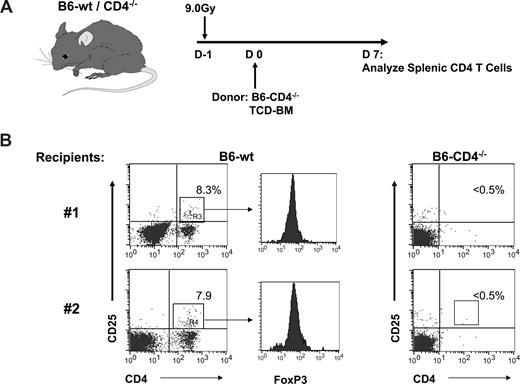
To initially address how ablative conditioning and HCT affect the host Treg compartment, a transplant was performed using CD4-deficient BM to prevent transfer of donor Tregs or their de novo generation from donor marrow progenitors in recipients post-HCT (Figure 1A). As anticipated, no CD4+CD25+ or CD4+Foxp3+ cells were observed in CD4−/− recipients 1 week posttransplant (Figure 1B right). In contrast, CD4+CD25+ Foxp3+ cells were identified in the spleen in B6–wild-type (wt) recipients after transplantation with this same CD4−/− marrow inoculum (Figure 1B left). To corroborate these results, B6-gfp marrow was transplanted into ablatively and reduced intensity conditioned syngeneic B6 recipients. Virtually no gfp+ (ie, donor) CD4+CD25+ Foxp3+ T cells could be detected 1 week after transplantation. In contrast, gfp− CD4+CD25+ Foxp3+ T cells were readily identified in the spleen of all recipients. Greater numbers of gfp− host cells were clearly present in recipients of 4.0 versus 9.0 Gy TBI (data not shown). These observations indicated the presence of host, that is, residual CD4+CD25+ Foxp3+ T cells, after 9.0 Gy TBI and BMT.
Recipient Treg cells are apparent in the spleens of lethally conditioned recipient mice 1 week post-transplant of Treg-deficient BM donors. (A) B6-wt or B6-CD4−/− mice were conditioned with 9.0 Gy TBI and 1 day later transplanted with TCD–bone marrow cells (BMC) from B6-CD4−/− donors. Lymph node cell CD4 T cells were assessed 1 week post-transplant. (B) Dot plots of spleen cells from 2 individual mice stained for CD4 and CD25. Numbers represent the percentage of CD4 T cells expressing CD25. Histograms represent FoxP3 staining of gated CD4+CD25+ T cells.
Recipient Treg cells are apparent in the spleens of lethally conditioned recipient mice 1 week post-transplant of Treg-deficient BM donors. (A) B6-wt or B6-CD4−/− mice were conditioned with 9.0 Gy TBI and 1 day later transplanted with TCD–bone marrow cells (BMC) from B6-CD4−/− donors. Lymph node cell CD4 T cells were assessed 1 week post-transplant. (B) Dot plots of spleen cells from 2 individual mice stained for CD4 and CD25. Numbers represent the percentage of CD4 T cells expressing CD25. Histograms represent FoxP3 staining of gated CD4+CD25+ T cells.
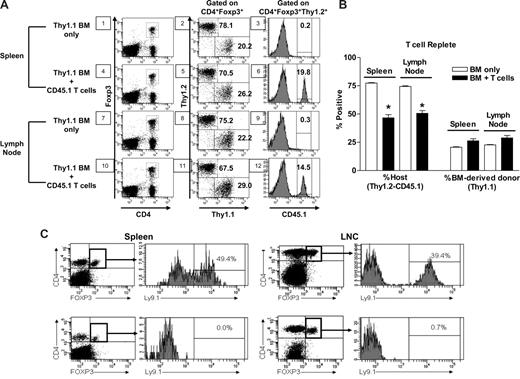
To begin to assess the length of time that host Treg cells could be identified after lethal conditioning, transplants were performed infusing donor CD45.2 TCD-BM into 9.5-TBI–conditioned CD45.1 recipients (Figure 2A). Lymph node cells were examined 5 weeks posttransplant when approximately 10% of the peripheral CD4+ pool was CD25+. Foxp3 analysis demonstrated that the majority of the CD4+CD25+ Treg cells present (∼80% of the Foxp3-expressing CD4+CD25+ T cells) were of host not donor (ie, CD45.2) origin. To assess the effect of transplanted T cells on the host Treg compartment, TCR marrow transplants were performed. Syngeneic congenic B6-Thy1.1 BM was depleted of T cells and 5 × 106 purified Thy1.2, CD45.1 (> 99%), CD4, and CD8 T cells were added and transplanted into 9.5 Gy TBI conditioned Thy1.2, CD45.2 recipients. One month post–TCR HCT, host Treg cells (Thy1.2+, CD45.2+) were readily detectable in these mice (Figure 3A) and composed almost 50% of the compartment (Figure 3B). The overall composition of this compartment versus HCT was altered, that is reduced approximately 25% (P < .001) as a result of a contribution by the mature (Thy1.2+, CD45.1+) Tregs contained in the graft, which contributed approximately 15% to 20% of the Treg compartment (Figure 3A). Recipients transplanted with TCR marrow thus contained Tregs from 3 distinct sources, ie, marrow → thymus-derived (Thy1.1+), mature donor cells in the graft (Thy1.2+, CD45.1+), and residual (Thy1.2+, CD45.2+) CD4+CD25+FoxP3+ cells.
Host Treg cells compose the predominant component of the CD4+FoxP3+ compartment in lymph nodes 5 weeks after autologous HCT. (A) B6-CD45.1 congenic mice were conditioned with 9.5 Gy TBI and 1 day later transplanted with TCD-BMC from B6-wt (CD45.2) donors. Lymph nodes were collected and CD4 T cells were assessed 5 weeks posttransplant. (Left panels) Dot plots from 2 individual mice stained for CD4 and CD25. Numbers outside of boxes represent the percentage of CD4+ T cells, which were CD25+. (Right panels) Dot plots of gated CD4+CD25+ cells analyzed for expression of donor CD45.2 and FoxP3. Numbers represent the percentage of positive staining cells in each quadrant. (B) BALB.B (H2b, Ly9.1+) mice were conditioned with 9.5 Gy TBI and 1 day later transplanted with TCD-BMC from major histocompatibility complex–identical B6 (H2b, Ly9.1−). The panels and numbers represent the same populations as those described in panel A with the exception that gated CD4+CD25+ cells were analyzed for expression of host Ly9.1 and FoxP3. The percentage of CD4 T cells expressing CD25 in the rectangles ranged between 12% and 18%.
Host Treg cells compose the predominant component of the CD4+FoxP3+ compartment in lymph nodes 5 weeks after autologous HCT. (A) B6-CD45.1 congenic mice were conditioned with 9.5 Gy TBI and 1 day later transplanted with TCD-BMC from B6-wt (CD45.2) donors. Lymph nodes were collected and CD4 T cells were assessed 5 weeks posttransplant. (Left panels) Dot plots from 2 individual mice stained for CD4 and CD25. Numbers outside of boxes represent the percentage of CD4+ T cells, which were CD25+. (Right panels) Dot plots of gated CD4+CD25+ cells analyzed for expression of donor CD45.2 and FoxP3. Numbers represent the percentage of positive staining cells in each quadrant. (B) BALB.B (H2b, Ly9.1+) mice were conditioned with 9.5 Gy TBI and 1 day later transplanted with TCD-BMC from major histocompatibility complex–identical B6 (H2b, Ly9.1−). The panels and numbers represent the same populations as those described in panel A with the exception that gated CD4+CD25+ cells were analyzed for expression of host Ly9.1 and FoxP3. The percentage of CD4 T cells expressing CD25 in the rectangles ranged between 12% and 18%.
Effect of TCR syngeneic and allogeneic HCT on the recipient's Treg compartment. B6 (CD45.2, Thy1.2) mice were conditioned with 9.5 Gy TBI and transplanted 1 day later with TCD-BM from B6-Thy1.1 congenic mice or TCR with 5 × 106 purified CD4+CD8− and CD4−CD8+ cells from CD45.1+Thy1.2+ B6 mice. Four weeks later, the CD4+FoxP3+ T-cell compartment was analyzed in the spleen and lymph nodes from 4 mice. (A) Data from a representative mouse illustrate (1) the residual host (CD4+FoxP3+Thy1.2+) Treg cells in recipients of TCD transplants (panels 2 and 8), as well as in recipients of TCR transplants (panels 5 and 11, top left quadrant by subtraction of percentage donor transplanted CD45.1+ Thy1.2+ (panels 6 and 12); (2) Treg cells transplanted in the donor inoculum (panels 6 and 12); and (3) donor BM (CD4+FoxP3+Thy1.1+) derived (panels 2, 5, 8, and 11, bottom right quadrants). (B) The summary of the average overall percentage contribution of residual Tregs, donor BM-derived Tregs, and transplanted donor Treg cells from both groups of transplant recipients (n = 4/group). *Significant difference between groups: P < .001, 1-way repeated measure ANOVA followed by Newman-Keuls multiple comparison test compared with BM only in the specified organ. (C) Lack of host CD4+FoxP3+ T cells in recipients of TCR allogeneic BM transplants. BALB.B (8.25 Gy TBI) recipients of 2 × 106 TCD BM alone (top panels), or together with 7 × 106 B6 lymph node T cells (bottom panels) were examined at 4 (spleen) and 5 (lymph nodes) weeks post-HCT for CD4+FoxP3+ Treg cells of host (Ly9.1+) or donor (Ly9.1−) origin. Data from an individual transplant recipient is presented as percentage CD4+FoxP3+ of host (vs donor) origin (histograms). Identical results were observed from 2 additional recipients at this time point.
Effect of TCR syngeneic and allogeneic HCT on the recipient's Treg compartment. B6 (CD45.2, Thy1.2) mice were conditioned with 9.5 Gy TBI and transplanted 1 day later with TCD-BM from B6-Thy1.1 congenic mice or TCR with 5 × 106 purified CD4+CD8− and CD4−CD8+ cells from CD45.1+Thy1.2+ B6 mice. Four weeks later, the CD4+FoxP3+ T-cell compartment was analyzed in the spleen and lymph nodes from 4 mice. (A) Data from a representative mouse illustrate (1) the residual host (CD4+FoxP3+Thy1.2+) Treg cells in recipients of TCD transplants (panels 2 and 8), as well as in recipients of TCR transplants (panels 5 and 11, top left quadrant by subtraction of percentage donor transplanted CD45.1+ Thy1.2+ (panels 6 and 12); (2) Treg cells transplanted in the donor inoculum (panels 6 and 12); and (3) donor BM (CD4+FoxP3+Thy1.1+) derived (panels 2, 5, 8, and 11, bottom right quadrants). (B) The summary of the average overall percentage contribution of residual Tregs, donor BM-derived Tregs, and transplanted donor Treg cells from both groups of transplant recipients (n = 4/group). *Significant difference between groups: P < .001, 1-way repeated measure ANOVA followed by Newman-Keuls multiple comparison test compared with BM only in the specified organ. (C) Lack of host CD4+FoxP3+ T cells in recipients of TCR allogeneic BM transplants. BALB.B (8.25 Gy TBI) recipients of 2 × 106 TCD BM alone (top panels), or together with 7 × 106 B6 lymph node T cells (bottom panels) were examined at 4 (spleen) and 5 (lymph nodes) weeks post-HCT for CD4+FoxP3+ Treg cells of host (Ly9.1+) or donor (Ly9.1−) origin. Data from an individual transplant recipient is presented as percentage CD4+FoxP3+ of host (vs donor) origin (histograms). Identical results were observed from 2 additional recipients at this time point.
To determine whether these results may have been strain dependent and/or limited to syngeneic HCT recipients, an allogeneic HCT was performed using TCD major histocompatibility complex–matched, minor histocompatibility antigen–mismatched B6 (H2b, Ly9.1−) marrow transplanted into 9.5 Gy conditioned BALB.B (H2b, Ly9.1+) recipients (Figure 2B). One month post- HCT, staining of CD4+CD25+ LN T cells for Foxp3 expression again revealed a significant preponderance of host (ie, Ly9.1+) CD4+CD25+ Foxp3+ cells T cells (Figure 2B). These results indicated that, for a significant time period, at least 4 to 5 weeks after ablative conditioning and TCD-allogeneic HCT, the regulatory compartment is predominantly composed of Treg cells of host origin. BM transplantations were then performed with the same strain combination using TCR or TCD B6 donor inoculum (Figure 3C). BALB.B recipients of allogeneic marrow alone (Figure 3C top panels) again contained significant numbers of host Treg cells (2.6 × 106 and 6.8 × 105 per spleen and lymph nodes, respectively). However, host Treg cells could barely be detected (<105 in spleen and lymph nodes) in recipients of B6 donor lymph node cells (Figure 3C bottom panels). Thus, host Treg cells did not persist in animals receiving allogeneic T cells, which mediate GVH responses posttransplant.
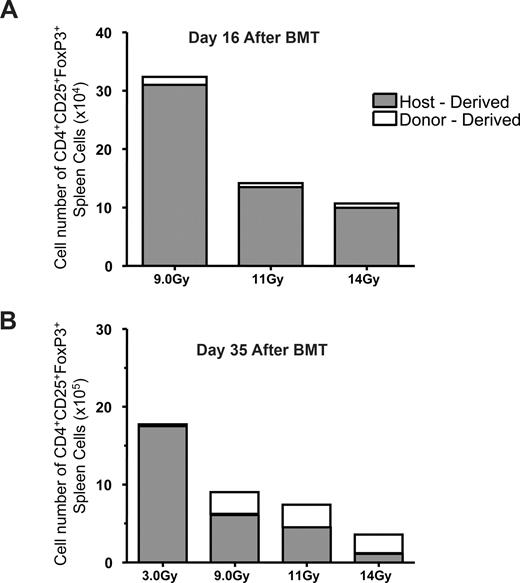
We next examined the post-HCT compartment in transplanted CD45 congenic mice after various levels of TBI. Not unexpectedly, when a reduced intensity conditioning level of 3.0 Gy was administered, the majority of Treg cells present 5 weeks post-HCT were indeed of host origin (Figure 4B). Split-dose administration (day −1, afternoon; day 0, morning) to reduce the toxicity to the gut and epithelial surfaces totaling 9.0, 11.0, and 14.0 Gy were then administered to expose recipients to supralethal levels of TBI. As observed, following 9.0 Gy, the post-HCT CD4+CD25+ Foxp3+ T-cell compartment was dominated by host cells 16 and 35 days after transplantation (Figure 4). As the level of irradiation was increased, lower overall numbers of Treg cells were observed in HCT recipients. After 11.0 Gy TBI, host Tregs continued to compose the majority of the CD4+CD25+ Foxp3+ T cells present during the first 5 weeks posttransplant. By 5 weeks post-HCT after 14 Gy TBI, some host Treg cells were still identified; however, donor Tregs now composed the majority of the CD4+CD25+Foxp3+ compartment.
Treg T cells in the spleens of mice after reduced-intensity and high- intensity TBI. B6-wt (CD45.2) recipient mice were conditioned with various doses of irradiation ranging from 3.0 to 14.0 Gy. With the exception of the 3.0 Gy (single dose), conditioning was delivered using 2 equivalent split doses beginning on day −1 with an overnight rest between doses. On day 0, conditioned mice were transplanted with 5 × 106 TCD-BM from B6-CD45.1 donors. Spleen cells from transplanted animals were analyzed 16 (A) and 35 (B) days post-HCT to determine the numbers of donor versus host CD4+CD25+Foxp3+ present. Results represent staining from pools of 4 mice per group.
Treg T cells in the spleens of mice after reduced-intensity and high- intensity TBI. B6-wt (CD45.2) recipient mice were conditioned with various doses of irradiation ranging from 3.0 to 14.0 Gy. With the exception of the 3.0 Gy (single dose), conditioning was delivered using 2 equivalent split doses beginning on day −1 with an overnight rest between doses. On day 0, conditioned mice were transplanted with 5 × 106 TCD-BM from B6-CD45.1 donors. Spleen cells from transplanted animals were analyzed 16 (A) and 35 (B) days post-HCT to determine the numbers of donor versus host CD4+CD25+Foxp3+ present. Results represent staining from pools of 4 mice per group.
Transplantations were performed using B6-CD45.1 BM administered to B6-CD45.2 recipients conditioned with 9.5 Gy TBI (Figure 5A). At day 20 post-HCT, a few donor-derived CD4+CD25+ FoxP3+ splenic T cells were detected but more than 90% of the total Treg cell numbers were of host origin (data not shown). The overall numbers of splenic host CD4+CD25+ FoxP3+ T cells increased by 5 weeks posttransplant, reaching approximately 106 (Figure 5A left panel). The same pattern of increased numbers of these host T cells was also observed in the lymph nodes (Figure 5A). Notably, the majority of the overall CD4 T-cell compartment was composed of donor cells by this time period and virtually all CD19+ B cells were also of donor origin (Figure 5A middle, right panels). In total, these results suggested that the Treg compartment was unique in that it appeared to be the only one examined containing a large fraction of cells derived from host origin after ablative conditioning and transplantation.
Host Treg cells compose the predominant component of the Treg compartment during the first month after lethal TBI and autologous donor TCD-BM transplant. (A) B6-wt (CD45.2) recipient mice were conditioned with 9.5 Gy TBI and were transplanted with 5 × 106 TCD-BM from B6-CD45.1 donors. Five weeks post-HCT (day 35), the spleen and lymph nodes were analyzed for the indicated populations (CD4+CD25+ FoxP3+ [left panel], CD4+ [middle panel], and CD19+ [right panel]) and the origin (donor, host) of each according to CD45.2 expression. The total cell numbers of each population were obtained using the percentage of positive staining from flow cytometric analyses and the total splenic mononuclear cell number. Results represent staining of indicated tissues from 2 individual B6-wt transplanted mice. (B) Kinetic analyses of host and donor Treg cells during the 2-month period after ablative conditioning and syngeneic TCD-BM transplant. B6-wt (Thy1.2) recipient mice were conditioned with 9.5 Gy TBI and transplanted with 5 × 106 TCD-BM from B6-Thy1.1 congenic donors. Spleens (B, left panel) and lymph nodes (axillary, cervical, brachial, and inguinal; B, right panel) were collected from 3 mice at each of the time points indicated, and the numbers of CD4+Foxp3+ T cells in each compartment of individual mice were determined by the percentage of positive staining and total numbers of mononuclear cells. The total cell numbers of each population were obtained using the percentage of positive staining for CD4, Foxp3, and Thy1.1 from flow cytometric analyses and the total splenic or lymph node cell mononuclear cell number. Results represent mean percentage positively stained cells plus or minus SEM (note, where error bars not apparent, SEM values did not extend outside of symbol) from groups of mice (n = 3/group) assessed from the indicated time points after HCT. Dotted lines represent the total numbers of donor plus host CD4+Foxp3+ T cells. There are typically 1 to 2 × 106 Treg cells in the splenic compartment of normal, adult B6 mice.
Host Treg cells compose the predominant component of the Treg compartment during the first month after lethal TBI and autologous donor TCD-BM transplant. (A) B6-wt (CD45.2) recipient mice were conditioned with 9.5 Gy TBI and were transplanted with 5 × 106 TCD-BM from B6-CD45.1 donors. Five weeks post-HCT (day 35), the spleen and lymph nodes were analyzed for the indicated populations (CD4+CD25+ FoxP3+ [left panel], CD4+ [middle panel], and CD19+ [right panel]) and the origin (donor, host) of each according to CD45.2 expression. The total cell numbers of each population were obtained using the percentage of positive staining from flow cytometric analyses and the total splenic mononuclear cell number. Results represent staining of indicated tissues from 2 individual B6-wt transplanted mice. (B) Kinetic analyses of host and donor Treg cells during the 2-month period after ablative conditioning and syngeneic TCD-BM transplant. B6-wt (Thy1.2) recipient mice were conditioned with 9.5 Gy TBI and transplanted with 5 × 106 TCD-BM from B6-Thy1.1 congenic donors. Spleens (B, left panel) and lymph nodes (axillary, cervical, brachial, and inguinal; B, right panel) were collected from 3 mice at each of the time points indicated, and the numbers of CD4+Foxp3+ T cells in each compartment of individual mice were determined by the percentage of positive staining and total numbers of mononuclear cells. The total cell numbers of each population were obtained using the percentage of positive staining for CD4, Foxp3, and Thy1.1 from flow cytometric analyses and the total splenic or lymph node cell mononuclear cell number. Results represent mean percentage positively stained cells plus or minus SEM (note, where error bars not apparent, SEM values did not extend outside of symbol) from groups of mice (n = 3/group) assessed from the indicated time points after HCT. Dotted lines represent the total numbers of donor plus host CD4+Foxp3+ T cells. There are typically 1 to 2 × 106 Treg cells in the splenic compartment of normal, adult B6 mice.
Groups of mice were then transplanted with donor TCD marrow from B6-Thy1.1 congenic mice was infused into 9.5 Gy TBI-conditioned B6 Thy1.2 recipients. At various time periods for 8 weeks posttransplant, splenic and lymph node CD4+CD25+FoxP3+ T cells were analyzed to determine the relative contributions of donor and host cells to this compartment. To assess the total CD4 regulatory compartment, we monitored the numbers of CD4+Foxp3+ T cells in these recipients (Figure 5B). Treg cells of host origin were found to be the predominant population 5 to 6 weeks posttransplant. Notably, de novo–derived donor Treg cells began to be detected between 2 and 3 weeks after transplantation and by 2 months post-HCT, composed the majority of the CD4+Foxp3+ T cells in the periphery (Figure 5B). By 1 month post-HCT, the overall level of CD4+Foxp3+ T cells was maintained at a relatively narrow range (see Figure 5, dotted lines).
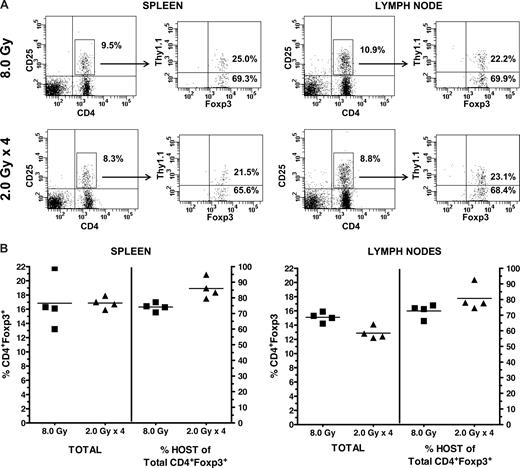
Syngeneic HCT experiments were then performed to examine how differing transplantation conditioning regimens impact residual host Treg cells. B6 mice were administered a single-dose 8.0 Gy TBI or 2.0 Gy TBI per day for 4 consecutive days before infusion of Thy1.1 TCD congenic marrow (Figure 6). Whereas some donor-derived Treg cells are apparent 1 month post-HCT, the overall Treg compartment (splenic, lymph node) of recipients (n = 4) of 8.0-Gy single-dose TBI contained primarily host CD4+FoxP3+ T cells (Figure 6). Analysis of the splenic and lymph node Treg compartments of individual animals (n = 4) receiving 4× split-dose TBI (Figure 6A) also demonstrated that, of the CD4+CD25+ T cells in the spleens and lymph nodes (ranging from 6.3% to 8.3% and 8.8% to 10.2%, respectively, of the CD4+ T cells), there was a clear predominance of host CD4+CD25+ FoxP3+ T cells (ranging from 65.6% to 82.4% and 68.4% to 85.5%, respectively). Analysis of individual recipients demonstrated the clear predominance of residual Tregs in multiple-day TBI-conditioned mice in both spleen and lymph nodes (Figure 6B). These levels were at least equivalent and tended to be slightly increased (significant in spleen but not LN) versus those in recipients receiving single, lethal dose exposure (Figure 6B). Syngeneic transplantations were also performed in which recipients were conditioned with busulfan (Bu) or cyclophosphamide (CyP; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We have found that 20 mg/kg body wt of Bu delivered on 4 consecutive days markedly diminishes the progenitor cell compartment while minimally diminishing Treg cells (D. Ross and R. B. L., personal communication, 2008). Results indicated that recipients of busulfan plus or minus CyP (200 mg/kg) contained a significant residual CD4+CD25+Foxp3+ component a month post-HCT with very few Treg cells derived from the BM. Interestingly, mice administered a regimen of both Bu and CyP also contained a significant population of host Treg cells. However, compared with recipients of either treatment alone, the Bu plus CyP mice had a far greater number of BM-derived Treg cells and hence more closely resembled the recipients of lethal TBI. Together, these results demonstrate that, after a variety of hematopoietic transplantation conditioning regimens, Treg cells of host origin are present in significant levels for several weeks post-HCT.
Recipient B6-wt (Thy1.2) mice were conditioned with either a single 8.0-Gy TBI dose (day −1) or 4 daily 2.0-Gy TBI doses (days −4, −3, −2, and −1). On day 0, all recipients received 5 × 106 B6-Thy1.1 TCD-BM. One month posttransplant, spleen and lymph nodes cells were harvested and analyzed for Foxp3+ cells of donor (Thy1.1+) and host (Thy1.1−) origin. (A) Representative Foxp3 phenotype of the CD4+CD25+ T cells and percentage of donor versus host cells in spleen (8.0 Gy: 25.0 vs 69.3; 2.0 Gy × 4: 21.5 vs 65.6) and lymph node (8.0 Gy: 22.2 vs 69.9; 2.0 Gy × 4: 23.1 vs 68.4). (B) Individual analysis of spleen and lymph nodes for the total percentage of the CD4+Foxp3+ T cells in 8.0 Gy vs 2.0 Gy × 4 recipients and the percentage of this population, which are of host origin; N = 4/group.
Recipient B6-wt (Thy1.2) mice were conditioned with either a single 8.0-Gy TBI dose (day −1) or 4 daily 2.0-Gy TBI doses (days −4, −3, −2, and −1). On day 0, all recipients received 5 × 106 B6-Thy1.1 TCD-BM. One month posttransplant, spleen and lymph nodes cells were harvested and analyzed for Foxp3+ cells of donor (Thy1.1+) and host (Thy1.1−) origin. (A) Representative Foxp3 phenotype of the CD4+CD25+ T cells and percentage of donor versus host cells in spleen (8.0 Gy: 25.0 vs 69.3; 2.0 Gy × 4: 21.5 vs 65.6) and lymph node (8.0 Gy: 22.2 vs 69.9; 2.0 Gy × 4: 23.1 vs 68.4). (B) Individual analysis of spleen and lymph nodes for the total percentage of the CD4+Foxp3+ T cells in 8.0 Gy vs 2.0 Gy × 4 recipients and the percentage of this population, which are of host origin; N = 4/group.
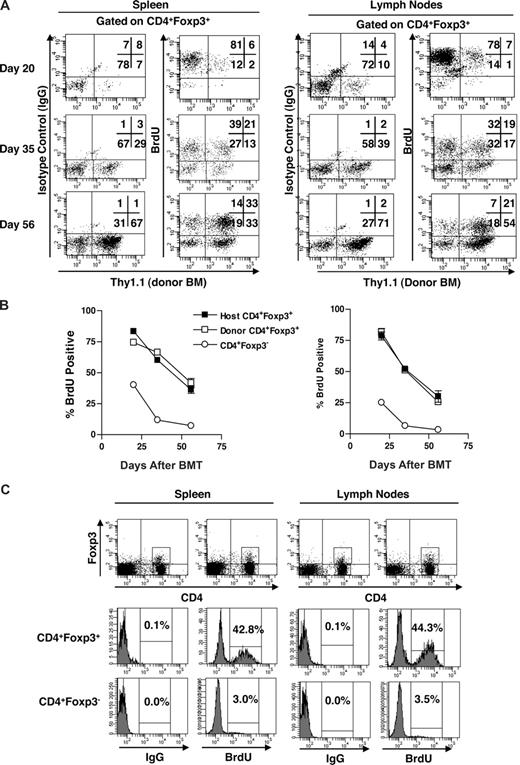
CD4+CD25+ FoxP3+ host and donor T cells incorporate BrdU in recipients following high-intensity conditioning and HCT
The findings indicating initial predominance of host Treg cells posttransplant may reflect their proliferation and expansion after ablative conditioning and HCT. To assess whether peripheral expansion of host Tregs was occurring post-HCT, transplanted B6-CD45.1 groups of recipients (n = 3/group) were placed on BrdU drinking water for 4 days before death at days 20, 35, and 56 post-HCT (Figure 7). CD4+Foxp3+ T cells were then assessed for BrdU uptake in both host and donor populations. Three weeks after HCT, > 80% (78 of 92) of the CD4+Foxp3+ host Treg cells in both the spleen and lymph node compartments incorporated BrdU (Figure 7A,B). By 5 weeks, 50% to 60% of host as well as donor Treg cells were dividing as assessed by BrdU incorporation. Finally, 2 months posttransplant, 30% to 40% of both CD4+Foxp3+ populations, now predominantly donor derived, were incorporating BrdU. Notably, the “final,” 2 months after HCT, BrdU levels were comparable with the uptake exhibited by peripheral CD4+FoxP3+ cells in normal, nontransplanted mice (Figure 7C). Interestingly, the CD4+Foxp3+ population in normal mice (n = 3/group) exhibits a significantly greater percentage of BrdU incorporation (ie, ∼1 log) compared with the CD4+Foxp3− population (Figure 7C). In addition, the level of BrdU incorporation by host Tregs at 3 weeks was extremely elevated, consistent with high proliferation and little cell death. In total, these proliferative results are consistent with the increased numbers of host Treg cells identified in recipients during the first 3 to 6 weeks posttransplant. Notably, even in the lymphopenic state, the Treg compartment exhibited a much higher proliferation than the CD4+FoxP3− population (Figure 7B).
Proliferation of CD4+FoxP3+ Treg population after ablative conditioning. B6 mice were subjected to 9.5 Gy TBI. On the following day, mice received 5 × 106 T-depleted BM cells isolated from Thy1.1 congenic B6 mice. Mice were killed 20, 35, and 56 days post-BMT and spleen and lymph nodes subjected to FAC analysis. Four days before death, mice were placed on BrdU (0.8 mg/mL) drinking water. (A) Representative dot plot showing the Thy1.1 (donor BM) staining and IgG of BrdU staining of the gated CD4+Foxp3+ cells in the spleen (left panels) and lymph nodes (right panels). (B) Percentage BrdU+ cells in the host (Thy1.1−) and donor (Thy1.1+) CD4+Foxp3+ and CD4+Foxp3− compartments in the spleen (left) and lymph nodes (right). (C) Wild-type B6 mice (n = 3) were placed on BrdU drinking water for 4 days, killed, and the spleen and lymph nodes subjected to FACS analysis. Representative dot plot showing CD4 and Foxp3 expression with corresponding histograms illustrating staining by isotype control (IgG) and anti-BrdU mAb of the gated CD4+FoxP3+ and CD4+Foxp3− populations in the spleen (left) and lymph nodes (right). Data in panel B are mean plus or minus SEM for 3 mice per group per time point.
Proliferation of CD4+FoxP3+ Treg population after ablative conditioning. B6 mice were subjected to 9.5 Gy TBI. On the following day, mice received 5 × 106 T-depleted BM cells isolated from Thy1.1 congenic B6 mice. Mice were killed 20, 35, and 56 days post-BMT and spleen and lymph nodes subjected to FAC analysis. Four days before death, mice were placed on BrdU (0.8 mg/mL) drinking water. (A) Representative dot plot showing the Thy1.1 (donor BM) staining and IgG of BrdU staining of the gated CD4+Foxp3+ cells in the spleen (left panels) and lymph nodes (right panels). (B) Percentage BrdU+ cells in the host (Thy1.1−) and donor (Thy1.1+) CD4+Foxp3+ and CD4+Foxp3− compartments in the spleen (left) and lymph nodes (right). (C) Wild-type B6 mice (n = 3) were placed on BrdU drinking water for 4 days, killed, and the spleen and lymph nodes subjected to FACS analysis. Representative dot plot showing CD4 and Foxp3 expression with corresponding histograms illustrating staining by isotype control (IgG) and anti-BrdU mAb of the gated CD4+FoxP3+ and CD4+Foxp3− populations in the spleen (left) and lymph nodes (right). Data in panel B are mean plus or minus SEM for 3 mice per group per time point.
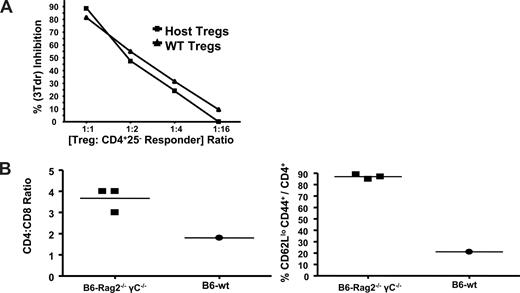
Residual Tregs are functional as assessed by in vitro suppressive activity and the failure to adoptively transfer autoimmune disease in recipients containing host CD4+CD25+ FoxP3+ T cells
To determine whether the host Tregs present in recipients post-HCT were functional suppressor cells, CD4+CD25+ T cells were obtained from B6 recipients who underwent lethal conditioning and a transplantation with TCD B6 B6-CD4−/− donor BM (Figure 8A). Purified host CD4+CD25+ T cells were assessed for their ability to inhibit proliferation by responding syngeneic CD4+CD25− B6 T cells, and their suppressive activity was virtually indistinguishable from that mediated by Treg cells obtained from normal B6 mice (Figure 8A). To assess host Treg cell function in vivo, BM from IL-2Rβ−/− (CD122−/−) donors was transplanted into 9.5 TBI- conditioned B6-wt or B6-Rag2−/−γc−/− recipients (Figure 8B). Autoimmune disease that develops in IL-2Rβ−/− mice is characterized by the clinical appearance of wasting and several lymphohematologic alterations, including CD4 T-cell expansion and an activated surface phenotype.25,28 B6-wt recipients of IL-2Rβ−/− marrow always appeared clinically healthy, whereas B6-Rag2−/−γc−/− recipients evidenced a haunched appearance, diarrhea, and weight loss (data not shown). In B6-Rag2−/−γc−/− recipients, CD4 T cells underwent significant expansion consistent with the enhanced CD4/CD8 ratio and marked increase in peripheral CD62Llo T cells. In contrast, these alterations were not detected in the B6-wt recipients. In total, these findings support the notion that host Treg cells present post-HCT are functionally capable of mediating immune regulation.
Residual Treg cells affect in vitro and in vivo regulatory function. (A) B6-wt mice were conditioned with 9.0 Gy TBI and 1 day later transplanted with TCD-BMC from B6-CD4−/− donors. Approximately 1 month (day 32) posttransplant, CD4+CD25+ T cells were purified from the spleens and lymph nodes of 2 recipients, pooled (0.5 × 106 Tregs/mouse), and assessed for regulatory activity. CD4+CD25+ T cells were also purified from normal, nontransplanted B6-wt mice for comparison. Various numbers of the CD4+CD25+ T-cell populations were cocultured in triplicate with syngeneic B6 CD4+CD25− responder T cells plus accessory cells together with anti-CD3 mAb. Cultures were pulsed with 3Tdr for the final 6 hours of incubation and harvested after 72 hours. Data are presented as the percentage inhibition based on the cpm of cultures with Treg cells versus cpm of cultures composed of responder cells, accessory cells, and anti-CD3 mAb without Treg cells. (B) B6-wt and B6-Rag2−/−γc−/− (H2b) recipient mice were conditioned with 9.0 Gy on day −1 and 1 day later (day 0) transplanted with 3 × 106 TCD-BM from B6 IL2Rβ−/− donors. Spleens were harvested from recipient animals 2 months post-BMT and analyzed for CD4 and CD8 T cells. In addition, CD4 T cells were analyzed for CD62L and CD44 expression. Elevated CD4/CD8 ratios and an activated CD4 T-cell phenotype are present in recipients expressing clinical signs of autoimmune disease. Data represent analysis of 3 individual B6-Rag2−/−γc−/− mice and a pool of 3 normal B6-wt recipients.
Residual Treg cells affect in vitro and in vivo regulatory function. (A) B6-wt mice were conditioned with 9.0 Gy TBI and 1 day later transplanted with TCD-BMC from B6-CD4−/− donors. Approximately 1 month (day 32) posttransplant, CD4+CD25+ T cells were purified from the spleens and lymph nodes of 2 recipients, pooled (0.5 × 106 Tregs/mouse), and assessed for regulatory activity. CD4+CD25+ T cells were also purified from normal, nontransplanted B6-wt mice for comparison. Various numbers of the CD4+CD25+ T-cell populations were cocultured in triplicate with syngeneic B6 CD4+CD25− responder T cells plus accessory cells together with anti-CD3 mAb. Cultures were pulsed with 3Tdr for the final 6 hours of incubation and harvested after 72 hours. Data are presented as the percentage inhibition based on the cpm of cultures with Treg cells versus cpm of cultures composed of responder cells, accessory cells, and anti-CD3 mAb without Treg cells. (B) B6-wt and B6-Rag2−/−γc−/− (H2b) recipient mice were conditioned with 9.0 Gy on day −1 and 1 day later (day 0) transplanted with 3 × 106 TCD-BM from B6 IL2Rβ−/− donors. Spleens were harvested from recipient animals 2 months post-BMT and analyzed for CD4 and CD8 T cells. In addition, CD4 T cells were analyzed for CD62L and CD44 expression. Elevated CD4/CD8 ratios and an activated CD4 T-cell phenotype are present in recipients expressing clinical signs of autoimmune disease. Data represent analysis of 3 individual B6-Rag2−/−γc−/− mice and a pool of 3 normal B6-wt recipients.
Discussion
In patients with limited thymic function as a consequence of disease or age, peripheral lymphocyte expansion can play a significant role in reconstitution of the T-cell compartment after HCT.19 Therefore, dependent on the transplantation parameters, including level of conditioning and TCD or TCR inoculum, donor as well as surviving host T-cell populations may contribute to the emergent lymphoid compartment.29,30 The CD4+CD25+ Treg compartment is now appreciated to contribute a crucial peripheral regulatory mechanism to maintain self-tolerance and hence its redevelopment after HCT is critical to appropriate immune reconstitution.1-3 T-cell reconstitution after conditioning and HCT, as a consequence of prolonged immune suppression, suboptimal thymic function, and other transplantation-related complications, is frequently delayed for months and sometimes years after clinical progenitor cell transplantation.31 Accordingly, the re-emergence of the de novo–derived thymic-dependent CD4+CD25+Foxp3+ T-cell compartment post-HCT might also be expected to reflect considerable delay. Using experimental HCT models, the present studies examined Treg cell presence in mice after various levels of conditioning and identified the presence of significant numbers of recipient Tregs even after ablative conditioning and HCT for several months posttransplant. In total, the findings here support the notion that at least some host Tregs survive various conditioning regimens and undergo rapid, early expansion before the emergence of significant numbers of thymic-derived de novo–generated donor Treg cells. Residual Treg cells were readily identified in syngeneic recipients of TCD or TCR grafts as well as in allogeneic recipients of TCD-BM. These host Treg cells that persist several months posttransplant are functional and provide regulatory activity, which can prevent the advent of autoimmune disease. The data therefore suggest that such host Tregs may be important to consider with regard to strategies designed early post-HCT to generate antitumor and antiviral immunity in patients who receive such types of HSCT.
Several studies have examined the survival of T cells after lymphocyte-depleting protocols, including antilymphocyte antibodies and TBI.32-36 Effector/memory cells (TM) have been found to exhibit greater survival compared with naive lymphocytes after irradiation-induced stress.34,35 Elevated levels of antiapoptotic molecules in TM cells versus naive T (TN) cells have been detected and proposed to contribute to such survival advantage.36 Recent studies have begun to examine the affects of lymphodepletion on the Treg compartment.21-24 Consistent with the aforementioned comments, those surviving cells with effector/memory characteristics appear to be the population capable of undergoing proliferation early after depletion with antilymphocyte antibody (CD52, RATG) regimens.21 Memory cells, including CD4+CD25+ T cells, were also found to dominate the T-cell pool in patients during the first several months in nontransplanted recipients, after depletion with anti-CD52.21 Notably, consistent with these clinical observations, strong depleting doses of anti-CD4 mAb in murine systems resulted in 6%, 28%, and 36% survival, respectively, of the naive, memory, and regulatory T-cell compartments.24 Analogous findings were reported after antimouse antilymphocyte antibody treatment in B6 strain recipients, which we have used in several of the studies reported here.22 In addition, as similarly observed for non-Treg effector/memory cells, this same study examined prosurvival Bcl-2 and Bcl-xL expression between CD4+CD25+ and CD4+CD25− T cells and reported significant up-regulation of Bcl-xL protein in the former population.22
Such observations regarding human and murine Tregs are noteworthy with respect to the present studies in which we observed that, after lymphodepletion via TBI, recipient Treg populations could be identified in significant percentage and numbers within the overall CD4+Foxp3+ compartment after HCT. Moreover, similar to the aforementioned observations, we found marked BrdU incorporation of these host Treg cells, which was consistent with an increasing number over the first 4 to 6 weeks post-transplant. Based on the early appearance of host Treg cells, within a week of lethal single- or split-dose TBI exposures and transplantation, it is apparent that some Treg cells do survive conditioning, present before any possible de novo production of thymic-derived host Tregs because of damage to the host progenitor cell and thymic compartments. Hence, we interpret the findings to indicate expansion of surviving host Tregs before the emergence of thymic-derived donor CD4+CD25+ T cells, which we begin to detect 2 to 3 weeks after transplantation. A similar high proliferation index was also observed in donor Treg cells present at this early time period and during the next 2 months post-transplant. These observations are consistent with the notion that, as host Treg proliferation diminished, together with the increasing emergence of newly derived donor Tregs and their own peripheral expansion, by 2 months post-transplant, donor Tregs ultimately composed the predominant component of the overall Treg compartment. Regarding expansion of surviving host and donor Tregs, the latter population is not limited to newly emergent, donor-derived thymic emigrants as donor Tregs present in TCR grafts also contributed to the compartment post-HCT (Figure 3A,B). Transplanted donor Treg cells probably immediately compete with residual Tregs for space within the niche and thus, when present, contribute to the development of donor-derived Treg predominance post-transplant.
Presently, it is not clear why host Tregs compared with conventional host T cells and B cells comprise a larger component of their respective overall compartments post-HCT. Even if a slightly greater percentage of host Tregs versus T conventional cells did survive conditioning, all lymphoid cells are exposed to a lymphopenic environment before the emergence of de novo–derived populations with which they would compete for survival and expansion. Currently, we do not know what drives residual Treg expansion after conditioning and HCT, but it is possible that the signals present post-transplant to drive lymphopenic T-cell expansion could be stronger for Treg cells versus naive T cells present. IL-2 appears to be important for peripheral maintenance of Tregs and in the lymphopenic conditions extant in newborn mice, anti-IL-2 treatment diminished peripheral Treg numbers.27,37-40 Other cytokines may be available in abundance (eg, IL-7, IL-15) because of lymphopenic conditions present post-transplant in our recipient mice, so it may be interesting to examine if such molecules contribute to host Treg levels. It is also intriguing to speculate that the overall damage after TBI may result in production of significant levels of self-antigens, perhaps derived from hematopoietic cell populations undergoing apoptosis.41,42 Cross-presentation of such antigens to surviving host Treg cells could conceivably result in a proliferative advantage for Tregs vs foreign antigen reactive conventional T cells. It may thus be interesting to investigate the TCR spectratype of these Tregs to assess for the presence of a skewed or restricted repertoire among the expanded Tregs present. Regardless of the nature of this population, it became apparent that, by one month posttransplant (Figure 5B), the total numbers of Tregs identified maintained a “fixed,” that is, relatively constant level in the compartments examined regardless of the relative contribution arising from host or donor origin. Such observations are consistent with the notion of a Treg cell “niche,” which supports a highly defined and tightly regulated overall level of this regulatory population.43
Results in the present study indicated that, in B6-wt mice where host Tregs are present posttransplant, autoimmune disease did not occur after infusion of IL-2Rβ–deficient BM. These findings are similar to a recent study, which reported that marrow from Foxp3 mutant scurfy mice failed to transfer disease after transplantation into lethally conditioned syngeneic wild-type mice, unless recipients were first depleted of host T cells.43 Delayed lymphocyte infusions exhibit decreased GVH-inducing capacity, and it has been proposed that this diminution may be attributable to diminished host inflammatory responses at later times after pretransplantation conditioning and perhaps diminished numbers of host antigen-presenting cells at the time of infusion.44,45 The present results suggest that in an environment in which there is a naturally high proliferative rate of Treg cells, such conditions may favor regulation of effector cell responses, thus contributing to the delayed lymphocyte infusions effect by helping to diminish the GVH disease potential of such inoculum.
Finally, the presence of significant levels of host Treg cells post- HCT may be worthwhile to consider with respect to differences in thymic function between mice and humans. Mice retain essentially normal thymic function well past sexual maturity, whereas humans are now appreciated to manifest significant age-related declines in thymic function beginning relatively early in life.30,46 Thus, dependent on the conditioning protocols for autologous HCT in older persons, limited thymic function may support the ability of endogenous Tregs to “compete” successfully for early niche space as there would probably be delayed development of new donor progenitor cell–derived Tregs.47,48 It may also be interesting to consider the consequence of autologous stem cell transplantations as rescue after intensive antitumor chemotherapy for older patients (eg, myeloma).49,50 Such infusions, typically of mobilized peripheral blood may result in transfer of some “host” Treg cells into an environment, which may also favor their occupation of the Treg niche for an extended time period posttransplant.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sylvester Cancer Center for their support of the Flow Cytometry Facility and Dr Mark Goodman (Department of Medicine, University of Miami, Miami, FL) for his help with obtaining busulfan for some studies.
This work was supported by National Institutes of Health (NIH) grants AI 46689 and CA 120776 (R.B.L.), and AI055815 (T.R.M.).
National Institutes of Health
Authorship
Contribution: A.L.B. and M.J. designed and performed experiments, collected and analyzed results, and revised the manuscript; J.C., L.d.A., and T.H.S. performed experiments and collected data; and T.R.M. and R.B.L. designed experiments, analyzed results, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert B. Levy, Department of Microbiology/Immunology and Medicine, Miller School of Medicine, University of Miami, PO Box 016960 (R138), Miami, FL 33101; e-mail: rlevy@med.miami.edu.


![Figure 5. Host Treg cells compose the predominant component of the Treg compartment during the first month after lethal TBI and autologous donor TCD-BM transplant. (A) B6-wt (CD45.2) recipient mice were conditioned with 9.5 Gy TBI and were transplanted with 5 × 106 TCD-BM from B6-CD45.1 donors. Five weeks post-HCT (day 35), the spleen and lymph nodes were analyzed for the indicated populations (CD4+CD25+ FoxP3+ [left panel], CD4+ [middle panel], and CD19+ [right panel]) and the origin (donor, host) of each according to CD45.2 expression. The total cell numbers of each population were obtained using the percentage of positive staining from flow cytometric analyses and the total splenic mononuclear cell number. Results represent staining of indicated tissues from 2 individual B6-wt transplanted mice. (B) Kinetic analyses of host and donor Treg cells during the 2-month period after ablative conditioning and syngeneic TCD-BM transplant. B6-wt (Thy1.2) recipient mice were conditioned with 9.5 Gy TBI and transplanted with 5 × 106 TCD-BM from B6-Thy1.1 congenic donors. Spleens (B, left panel) and lymph nodes (axillary, cervical, brachial, and inguinal; B, right panel) were collected from 3 mice at each of the time points indicated, and the numbers of CD4+Foxp3+ T cells in each compartment of individual mice were determined by the percentage of positive staining and total numbers of mononuclear cells. The total cell numbers of each population were obtained using the percentage of positive staining for CD4, Foxp3, and Thy1.1 from flow cytometric analyses and the total splenic or lymph node cell mononuclear cell number. Results represent mean percentage positively stained cells plus or minus SEM (note, where error bars not apparent, SEM values did not extend outside of symbol) from groups of mice (n = 3/group) assessed from the indicated time points after HCT. Dotted lines represent the total numbers of donor plus host CD4+Foxp3+ T cells. There are typically 1 to 2 × 106 Treg cells in the splenic compartment of normal, adult B6 mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/3/10.1182_blood-2008-08-173179/5/m_zh80240828390005.jpeg?Expires=1767708990&Signature=i-Ahstc7MUS8q8xP8keELv6P8w8l3TDby4OfIqB-H6~aLxjlQ7VR-x7AbjrpoBj6YJFtNsKL01ZHmSHt1Ogh7YnfXwjTNZT23NqeJCkBZvOj7PjWlJSBQXCi6YodXI8iqS4S9REof5xYmgKQtWuTLMxSOeRsNUzPr9Kxpt~xgi0r~q0u305wIPzaAbbo1qTyaE4B5~IhmwPkqyXc0-FpBZ7KaapdYPrilLhtfW08JFmW2iDnXzMf5-0X2QB-VhQgRypet8eShHarxGA6bsnL73pLzAH01OiBcOzEQxQtQsOCct~vh1gb9CBYPyxuEyT6PQ31y3tCeF0GoSdhMxh7OA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal