Abstract
Myeloid cell leukemia-1 (Mcl-1) is an antiapoptotic member of the Bcl-2 protein family. Increased Mcl-1 expression is associated with failure to achieve remission after treatment with fludarabine and chlorambucil in patients with chronic lymphocytic leukemia (CLL). However, the influence of Mcl-1 expression has not been examined in CLL trials using chemoimmunotherapy. We investigated Mcl-1 protein expression prospectively as part of a phase 2 study evaluating the efficacy of pentostatin, cyclophosphamide, and rituximab in patients with untreated CLL. No significant difference by Mcl-1 expression was noted in pretreatment or response parameters. However, in patients with higher Mcl-1 expression, both minimal residual disease-negative status and progression-free survival was found to be significantly reduced (57% vs 19%, P = .01; 50.8 vs 18.7 months; P = .02; respectively). Mcl-1 expression may therefore be useful in predicting poor response to chemoimmunotherapy. These findings further support pursuing treatment strategies targeting this important antiapoptotic protein. (Because the trials described were conducted before the requirement to register them was implemented, they are not registered in a clinical trial database.)
Introduction
B-cell leukemia/lymphoma-2 (Bcl-2) family proteins are important regulators of apoptosis in cells of hematopoietic origin, including chronic lymphocytic leukemia (CLL) cells. The delicate balance between various family members, including Bcl-2, Noxa, Bim, and others, determines CLL cell fate.1-4 Myeloid cell leukemia-1 (Mcl-1) is a particularly intriguing member of this family that interacts with multiple other Bcl-2 family proteins and is dynamically regulated at both the mRNA and protein level. Mcl-1 modulation impacts response of CLL cells to various commonly used therapeutic agents, and loss of Mcl-1 is by itself sufficient to induce apoptosis in CLL cells.5-7 Recent reports have also revealed a correlation between lower Mcl-1 protein8 and mRNA levels9 with known biologic prognostic markers and improved outcomes in patients with CLL.
The addition of rituximab to CLL treatment regimens has substantially improved outcomes for a large subset of patients,10 and the use of rituximab or other therapeutic monoclonal antibodies will likely continue as a mainstay in the treatment of newly diagnosed CLL. We previously reported that combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab (PCR) has significant clinical activity with low accompanying toxicity in previously untreated CLL patients and is especially well tolerated in older patients in whom the use of fludarabine may be associated with prohibitive toxicities.11 As part of this study, we incorporated plans for prospective analysis of Mcl-1 protein to determine its prognostic impact in patients receiving PCR. Our results support the evaluation of Mcl-1 protein expression as a prognostic marker in larger studies using chemoimmunotherapy as well as the development of agents that target Mcl-1.
Methods
PCR clinical trial
Samples were obtained from a 2-center prospective phase 2 clinical trial conducted at Ohio State University (Columbus, OH) and Mayo Clinic (Rochester, MN).11 All patients had untreated, progressive CLL as defined by National Cancer Institute 1996 criteria.12 Patients provided written informed consent for correlative studies according to the Declaration of Helsinki on an Institutional Review Board–approved protocol for the collection and use of samples for research purposes from both participating institutions. Eligible patients received a regimen consisting of pentostatin (2 mg/m2), cyclophosphamide (600 mg/m2), and rituximab (375 mg/m2) given intravenously on day 1 of a 21-day cycle for a maximum of 6 cycles.11 Responses were assessed by National Cancer Institute 1996 criteria12 and included a bone marrow evaluation and 2-color flow cytometry 2 months after completion of therapy. Flow cytometry–negative status was defined as patients with less than or equal to 1% positive CD5+/CD19+ cells.
Mcl-1 expression analysis
Peripheral blood mononuclear cells were obtained from CLL patients immediately before treatment, and whole-cell extracts were immediately prepared and frozen for later analysis as published previously.13 Lysates were normalized for total protein content and analyzed by immunoblot with antibodies to Mcl-1 (sc-819; Santa Cruz Biotechnology, Santa Cruz CA) and GAPDH (MAB374; Millipore, Billerica, MA), followed by horseradish peroxidase–conjugated secondary antibodies (Bio-Rad, Hercules CA). Identical aliquots of lysate from the BJAB cell line were included on each immunoblot as a normalization control across assays. Detection was performed by chemiluminescence (Pierce Chemical, Rockford, IL), and band intensities were measured digitally using a ChemiDoc apparatus (Bio-Rad). All samples were run in duplicate, and Mcl-1/GAPDH ratios from each lane were averaged and calculated relative to the Mcl-1/GAPDH ratio in BJAB lysate.
Statistical analysis
This was a single-stage phase 2 trial examining efficacy of PCR therapy in previously untreated CLL. Mcl-1 expression was examined as a continuous variable to evaluate correlation with various outcome measures. Recursive partitioning analysis14 was used to establish an optimal cutoff point for comparative analyses. Progression-free (PFS) and overall survival were estimated using the Kaplan-Meier method.15 Differences were evaluated using the Fisher exact, Wilcoxon rank-sum, and the χ2 tests. A P value less than .05 was considered statistically significant.
Results and discussion
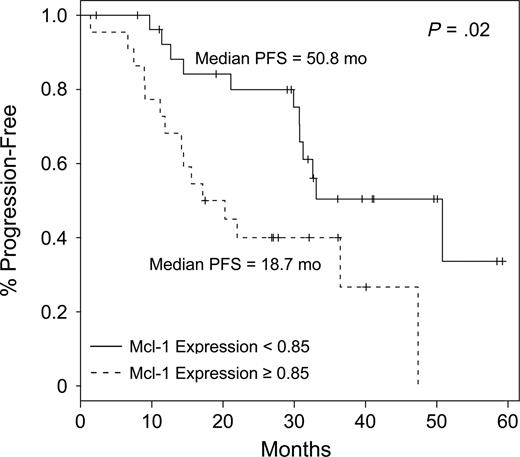
Of the 64 patients evaluated in this trial, clinical responses were seen in 58 (91%), with 26 (41%) complete responses (CRs), 14 (22%) nodular partial responses (nPRs), and 18 (28%) partial responses (PRs).11 Fifty of 64 patients (78%) had evaluable Mcl-1 data. Mcl-1 protein expression did not correlate with any pretreatment features or response to therapy. Similar results were also reported by Veronese et al, who did not find a correlation between Mcl-1 mRNA levels and known biologic prognostic markers of CLL.9 Recursive partitioning analysis14 was performed using Mcl-1 expression as a continuous variable, which revealed an optimal cutoff point of 0.85. At this cutoff, flow cytometry–negative status was significantly different between the 2 groups (P = .01; Table 1). As a continuous variable, Mcl-1 was not significantly associated with PFS (P = .22); but when using the 0.85 cutoff, median PFS was found to be significantly higher (P = .02) in patients with Mcl-1 levels of less than 0.85 (50.8 vs 18.7 months) Figure 1. This suggests that other biologic features in addition to Mcl-1 are contributing to treatment resistance and ultimately short response to chemoimmunotherapy with PCR. Our data represent what we believe to be the first prospective description of elevated Mcl-1 expression adversely influencing the likelihood of patients achieving cytometry-negative complete remissions and extended PFS. Mcl-1 expression was also independent of other known risk factors, such as CD38, ZAP70, and IgVH, indicating additional benefit for this measure in assessing patients. This may be the result of the relatively small number of patients included in our study, and further confirmatory analyses should be conducted in larger patient cohorts. Relevant to this, the patients treated on this trial were in the high-risk group as determined by IgVH gene mutation status and Rai stage.11
Mcl-1 levels and relationship to risk factors and outcome measures
| Variable . | Mcl-1 levels . | P . | |
|---|---|---|---|
| < 0.85, no. (%) . | ≥ 0.85, no. (%) . | ||
| Rai stage | .66 | ||
| Low | 1 (4) | 2 (9) | |
| Intermediate | 12 (43) | 10 (46) | |
| High | 15 (54) | 10 (46) | |
| Sex | .30 | ||
| Male | 24 (86) | 16 (73) | |
| Female | 4 (14) | 6 (27) | |
| Age, y | .22 | ||
| < 70 | 18 (64) | 18 (82) | |
| ≥ 70 | 10 (36) | 4 (18) | |
| ECOG PS | .42 | ||
| 0 | 18 (64) | 10 (46) | |
| 1 | 9 (32) | 9 (41) | |
| 2 | 1 (4) | 2 (9) | |
| 3 | 0 (0) | 1 (5) | |
| CD38 (> 30) | 10 (36) | 6 (27) | .56 |
| ZAP-70+ cells (> 20) | 7 (32) | 7 (33) | .92 |
| IgVH status unmutated (> 98) | 19 (68) | 16 (76) | .75 |
| FISH | .82* | ||
| Normal | 2 (7) | 2 (9) | |
| +12 | 8 (29) | 3 (14) | |
| 11q− | 6 (21) | 6 (27) | |
| 17p− | 1 (4) | 1 (5) | |
| 6q− | 0 (0) | 1 (5) | |
| 13q− | 10 (36) | 9 (41) | |
| Other | 1 (4) | 0 (0) | |
| No. of genetic defects | .37* | ||
| 0 | 2 (7) | 2 (9) | |
| 1 | 13 (46) | 15 (68) | |
| 2+ | 13 (46) | 5 (23) | |
| Bone marrow pattern | .37 | ||
| Diffuse | 9 (39) | 11 (55) | |
| Not diffuse | 14 (61) | 9 (45) | |
| Outcome measures | |||
| MRD negative | 13 (57) | 4 (19) | .01* |
| Responders | 23 | 19 | .16 |
| CR | 15 (65) | 7 (37) | |
| nPR | 5 (22) | 6 (32) | |
| PR | 3 (13) | 6 (32) | |
| Median PFS | 50.8 mo | 18.7 mo | .02 |
| Variable . | Mcl-1 levels . | P . | |
|---|---|---|---|
| < 0.85, no. (%) . | ≥ 0.85, no. (%) . | ||
| Rai stage | .66 | ||
| Low | 1 (4) | 2 (9) | |
| Intermediate | 12 (43) | 10 (46) | |
| High | 15 (54) | 10 (46) | |
| Sex | .30 | ||
| Male | 24 (86) | 16 (73) | |
| Female | 4 (14) | 6 (27) | |
| Age, y | .22 | ||
| < 70 | 18 (64) | 18 (82) | |
| ≥ 70 | 10 (36) | 4 (18) | |
| ECOG PS | .42 | ||
| 0 | 18 (64) | 10 (46) | |
| 1 | 9 (32) | 9 (41) | |
| 2 | 1 (4) | 2 (9) | |
| 3 | 0 (0) | 1 (5) | |
| CD38 (> 30) | 10 (36) | 6 (27) | .56 |
| ZAP-70+ cells (> 20) | 7 (32) | 7 (33) | .92 |
| IgVH status unmutated (> 98) | 19 (68) | 16 (76) | .75 |
| FISH | .82* | ||
| Normal | 2 (7) | 2 (9) | |
| +12 | 8 (29) | 3 (14) | |
| 11q− | 6 (21) | 6 (27) | |
| 17p− | 1 (4) | 1 (5) | |
| 6q− | 0 (0) | 1 (5) | |
| 13q− | 10 (36) | 9 (41) | |
| Other | 1 (4) | 0 (0) | |
| No. of genetic defects | .37* | ||
| 0 | 2 (7) | 2 (9) | |
| 1 | 13 (46) | 15 (68) | |
| 2+ | 13 (46) | 5 (23) | |
| Bone marrow pattern | .37 | ||
| Diffuse | 9 (39) | 11 (55) | |
| Not diffuse | 14 (61) | 9 (45) | |
| Outcome measures | |||
| MRD negative | 13 (57) | 4 (19) | .01* |
| Responders | 23 | 19 | .16 |
| CR | 15 (65) | 7 (37) | |
| nPR | 5 (22) | 6 (32) | |
| PR | 3 (13) | 6 (32) | |
| Median PFS | 50.8 mo | 18.7 mo | .02 |
Exact P value.
Previous studies of chemotherapy have shown varied results when examining Mcl-1 as a biomarker.8,9 For example, higher levels of Mcl-1 have been associated with failure to attain CR after chemotherapy with fludarabine and chlorambucil,1,16 whereas a higher Mcl-1/Bax ratio (but not Mcl-1 level alone) was associated with poor response to rituximab in a small number of variably treated patients.17 The US Intergroup Phase III Trial (E2997) revealed no statistically significant association of Mcl-1 level or Mcl-1/Bax ratio with either CR or PFS, although trends toward this were observed and lower Mcl-1/Bax ratios were associated with overall response.18 This discordance may be the result of logistic issues that prevented immediate preparation of whole-cell extracts in this large multi-institutional trial. Mcl-1 is well recognized to have a short half-life both at the mRNA and protein level, and immediate processing is recommended to reduce variability in Mcl-1 assessments. All samples for this study were freshly isolated and processed immediately on-site.
Mcl-1 represents not only a biomarker, but also a survival protein that can be targeted with therapeutic agents. We recently showed, using Mcl-1–specific small interfering RNA, that Mcl-1 down-regulation alone is sufficient to promote mitochondrial membrane depolarization and apoptosis in CLL cells.5 This Mcl-1 reduction also enhances CLL cell sensitivity to rituximab-mediated direct and complement-dependent cytotoxicity, but not antibody-dependent cellular cytotoxicity.5 Agents that target Mcl-1 protein expression in CLL cells, such as flavopiridol19,20 and the novel agent silvestrol,21 may therefore be of significant benefit in combination chemoimmunotherapy.
Our data further highlight the value of assessing Mcl-1 expression before therapy in prospective studies to establish its definitive role as a predictor of response to chemoimmunotherapy. If these results are confirmed, combination of chemoimmunotherapy with targeted therapy toward Mcl-1 holds tremendous promise for the management of patients with CLL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (RO1 CA 95241), the American Society of Clinical Oncology, the Leukemia & Lymphoma Society, and the D. Warren Brown Foundation.
National Institutes of Health
Authorship
Contribution: F.T.A. analyzed the data and wrote the manuscript; N.E.K. is the principal investigator of the clinical trial from which samples were derived, and analyzed data and edited the manuscript; M.E.D. performed the immunoblot analysis; W.W. and S.M.G. performed the statistical analysis; N.L., T.S.L., T.D.S., C.S.Z., and T.G.C. are coinvestigators on the clinical study; D.F.J. oversaw the IgVH characterization of patient samples; R.C.T. performed IgVH mutational analysis; C.R.S. received, processed, and analyzed patient samples; M.R.G. is a coinvestigator on the clinical study and provided input on trial design and correlative studies; N.A.H. oversaw the FISH characterization of samples; G.L. conducted the flow cytometric analysis of patient samples; J.C.B. is the coprincipal investigator of the clinical trial, was responsible for research concept, analyzed data, and edited the manuscript; and D.M.L. oversaw protein analysis of patient samples and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Lucas, 410 West 12th Avenue, Room 455, The Ohio State University Comprehensive Cancer Center Building, Columbus OH 43210; e-mail: david.lucas@osumc.edu.
References
Author notes
*J.C.B. and D.M.L. are senior authors who contributed equally to the design and implementation of this research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal