Abstract
Detailed genomic studies have shown that cytogenetic abnormalities contribute to multiple myeloma (MM) pathogenesis and disease progression. Nevertheless, little is known about the characteristics of MM at the epigenetic level and specifically how microRNAs regulate MM progression in the context of the bone marrow milieu. Therefore, we performed microRNA expression profiling of bone marrow derived CD138+ MM cells versus their normal cellular counterparts and validated data by qRT-PCR. We identified a MM-specific microRNA signature characterized by down-expression of microRNA-15a/-16 and overexpression of microRNA-222/-221/-382/-181a/-181b (P < .01). We investigated the functional role of microRNA-15a and -16 and showed that they regulate proliferation and growth of MM cells in vitro and in vivo by inhibiting AKT serine/threonine-protein-kinase (AKT3), ribosomal-protein-S6, MAP-kinases, and NF-κB-activator MAP3KIP3. Moreover, miRNA-15a and -16 exerted their anti-MM activity even in the context of the bone marrow milieu in vitro and in vivo. These data indicate that microRNAs play a pivotal role in the biology of MM and represent important targets for novel therapies in MM.
Introduction
Multiple myeloma (MM) is the leading cause of death in hematologic malignancies.1,2 Despite advances in understanding the molecular pathogenesis of MM and promising new therapies, only 25% to 35% of patients respond to therapies in the relapsed and refractory setting.3 In addition, although detailed genomic studies have shown that MM is characterized by cytogenetic abnormalities,4,5 little characterization of this disease at the epigenetic level has been performed to date, and the role of microRNAs (miRNAs) in MM has not been fully described. Therefore, multilevel genetic characterization of MM is required to improve our understanding of the underlying molecular changes that lead to the initiation and progression of this disease; as well as to develop novel therapeutic agents that specifically target epigenetic abnormalities of clonal plasma cells.
miRNAs represent a class of small, noncoding RNAs of 18 to 24 nucleotides in length6 that act as negative regulators of gene expression by binding to the 3′ untranslated region of the target mRNAs with partial sequence complementarity, thereby leading to translational repression.7 To date, around 700 miRNAs have been discovered in humans.8 Mature miRNAs play a pivotal role in regulating physiologic processes such as development, cell differentiation, apoptosis, and cell proliferation.9 Moreover, they have been described to play crucial roles in pathogenesis of both solid tumors and hematologic malignancies.10-12 It has recently been reported that miRNAs regulate MM pathogenesis, as shown by overexpression, among others, of miRNA-17∼92 cluster (located on chromosome 13) in MM patients.13 Deletion of chromosome 13 [(del(13)] predicts a significantly reduced survival in patients with MM14 ; however, MM miRNA signature in patients with relapsed/refractory disease, with del(13), and the role of miRNAs in targeting MM cells in the context of the bone marrow (BM) microenvironment, has not been described to date.
In the present study, we evaluated miRNA expression patterns in MM using liquid phase Luminex microbead miRNA profiling (Austin, TX). We showed that selected miRNAs were specifically altered in MM patients compared with healthy controls and identified a specific miRNA signature that characterizes this disease. We then focused on the functional role of miRNA-15a and -16, 2 of the decreased or totally absent miRNAs in MM which are located in chromosome 13. We found that miRNA-15a and -16 regulate proliferation and growth of MM cells in vitro and in vivo by inhibiting AKT serine/threonine protein kinase (AKT3), ribosomal-protein-S6, MAP-kinases, and NF-kB activator MAP3KIP3. Moreover, miRNA-15a and -16 exerted anti-MM activity even in the context of the BM microenvironment. In addition, to define the prognostic role of miRNA in MM, we showed that 3 of the deregulated miRNAs significantly correlated with prognostic factors as predicted by the International Staging System for MM.15 These data enhance our understanding of the biologic role of miRNAs in the pathogenesis of MM and provide the basis for the development of new miRNA-targeted therapeutic strategies in this disease.
Methods
Cells
Primary MM cells were obtained from BM samples from patients with relapsed/refractory MM (n = 15) enrolled in a phase II clinical trial of single agent mTOR inhibitor RAD001 (Everolimus; Novartis, Basel, Switzerland) at Mayo Clinic College of Medicine, Rochester, MN (clinical trial NCT00618345). All samples were obtained pretherapy. Plasma cells were obtained using CD138+ microbead selection (Miltenyi Biotec, Auburn, CA) as previously described.16 FISH analysis as been performed as described.14 Similarly, CD138+ cells were isolated from the BM of 4 healthy donors and used as controls. Primary MM endothelial cells (MMECs) were obtained from BM of patients with MM, as described.17 Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients and healthy volunteers in accordance with the Declaration of Helsinki protocol. MM cell lines (MM.1S, RPMI8226) and human umbilical vein endothelial cells (HUVEC; Lonza, Walkersville, MD) were used in this study. All human MM cell lines, primary patient MM cells, HUVECs, and MMECs were cultured as in previous studies.16,17 GFP+/Luc+ MM.1S cells were generated using lentivirus infection, as previously described.18
DNA synthesis and cytotoxicity assay
Proliferation rate and cytotoxicity on MM.1S cells (pre-miR-15a, -16-1 precursors–, and control probe–transfected) and nontransfected MM.1S cells were measured by DNA synthesis using [3H]-thymidine uptake (Perkin Elmer, Boston, MA) and by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) dye absorbance, respectively, as previously described.19
Cell cycle analysis
Pre-miR-15a, -16-1 precursors–, and control probe–transfected and nontransfected MM.1S cells were stained with propidium iodine (PI; Sigma-Aldrich, St Louis, MO), and cell cycle was determined using an Epics (Coulter Immunology, Hialeah, FL) flow cytometry, as previously described.19
Adhesion assay
We performed an in vitro adhesion assay coated with primary MM BM stromal cells, and MM cells (pre-miR-15a, -16-1 precursors–, and control probe–transfected and nontransfected cells) were tested. Noncoated wells served as a negative control. Calcein AM was used to measure adherent cells, and the degree of fluorescence was measured using a spectrophotometer (485-520 nm).19
Transwell migration assay
Migration was determined using the transwell migration assay (Costar, Corning, NY), as previously described. MM.1S cells (pre-miR-15a, -16-1 precursors–, and control probe–transfected and nontransfected cells) were placed in the migration chambers in the presence of SDF-1 (R&D Systems, Minneapolis, MN). After 2 hours of incubation, cells that migrated to the lower chambers were counted as described.19
Effect of miRNA-15a and -16 on paracrine MM cell growth in the BM
To evaluate the effect of miRNA-15a and -16 on growth of MM cells adherent to BM stromal cells (BMSCs), BMSCs were cultured in the presence or absence of either pre-miR-15a, -16-1 precursors–, and control probe–transfected or nontransfected MM.1S cells. DNA synthesis was measured using [3H]-thymidine uptake assay, as described.19
Immunoblotting
Whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyldene fluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA). The antibodies used for immunoblotting included anti–phospho(p)-Akt (Ser473), -Akt1, -Akt3, -p-ERK1/2 (Thr202/Tyr204), -ERK1/2, –p-S6 ribosomal, p-IkB, p-TAK1, p-Rb, -cyclinD1, -cyclinD2, -cdc25a (Cell Signaling Technology, Danvers, MA), -TAB3, and -β actin (Santa Cruz Biotechnology, Santa Cruz, CA). Nuclear extracts of the cells were prepared using the Nuclear Extraction Kit (Panomics, Redwood City, CA) and subjected to immunoblotting with anti–p-p65, -p50/p105, -p52/p100, -RelB, (Cell Signaling Technology), and antinucleolin (Santa Cruz Biotechnology) antibodies.
NF-κB activity
NF-κB activity was investigated using the Active Motif TransAM NFκB Family Kit, a DNA-binding enzyme-linked immunosorbent assay (ELISA)–based assay (Active Motif North America, Carlsbad, CA). Briefly, MM.1S cells (miR-15a-, miR-16-1-precursors probe–, and control probe–transfected and nontransfected cells) were cultured in presence or absence of TNF-α (10 ng/mL) for 20 minutes. NFκB-p65, -p50, -p52, and -RelB transcription factors binding to the related consensus sequence on the plate-bound oligonucleotide were studied from nuclear extracts, following the manufacturer's procedure.
Immunofluorescence
The effect of miRNA-15a and -16 on TNF-α–induced nuclear translocation of p-p65 was examined by immunocytochemical method. Briefly, MM cells (miR-15a–, miR-16-1-precursors probe–, and control probe–transfected and nontransfected MM.1S cells) were stimulated with TNF-α (10 ng/mL) for 20 minutes. Immunocytochemical analysis was performed using an epifluorescence microscope (Nikon Eclipse E800; Nikon, Avon, MA) and a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX), as previously described.20
microRNA expression profiling
RNA was isolated using an RNeasy kit (QIAGEN, Valencia, CA), as reported in previous miRNA studies.21-23 Quality control of RNA was done using RNA 6000 Nano assay on the Agilent 2100 Bioanalyzer (Santa Clara, CA). All RNA samples showed high quality, without RNA degradation or DNA contamination. The expression of 318 miRNAs was investigated using liquid phase Luminex microbead miRNA profiling, as described.24 Briefly, 500 ng of total RNA was labeled with biotin using the FlexmiR MicroRNA Labeling Kit (Luminex, Austin, TX), which labeled all RNA molecules, including small RNA, by first using calf intestinal phosphatase for removal of 5′-phosphatases from the terminal end of the miRNAs. In the second step, a biotin label was then attached enzymatically to the 3′-end of the miRNAs in the total RNA sample. After an enzyme inactivation step, the sample was hybridized with beads containing one of 100 different fluorophores and coated with oligonucleotides complementary to each known miRNA. Addition of streptavidin-phycoerythnn (Invitrogen, Carlsbad, CA) then yielded fluorescence with wavelengths and amplitudes characteristic of the identity and quantity of miRNAs, respectively. Normalization of arrays and calculation of median fluorescence intensity was performed according to the manufacturer's instructions.
Quantitative reverse transcription-PCR
Stem-loop quantitative reverse transcription-PCR (qRT-PCR) for mature miRNAs (TaqMan microRNA Assays, Applied Biosystems, Foster City, CA) was performed as described25 on an Applied Biosystems AB7500 Real Time PCR System. All PCR reactions were run in triplicate, and miRNA expression, relative to RNU6B, was calculated using the 2−ΔΔCt method.26
miRNA transfection
MM.1S and RPMI8226 cell lines were transfected with either precursor (pre)–miR-15a, -16-1, or control probe (Ambion, Austin, TX) at final concentration of 40 nM, using Lipofectamine 2000 following manufacturer's instructions (Invitrogen).27,28 Culture medium was changed 8 hours after transfection and replaced with RPMI 10% FBS. Cells were then used for functional assays at different time points (24, 48, and 72 hours). Both nontransfected or control probe–transfected MM.1S and RPM I8226 cell lines were used as controls. Efficiency of transfection was validated by qRT-PCR and microRNA assay.
In vitro and in vivo angiogenesis assays
In vivo imaging
In vivo video rate confocal microscopy and 2-photon microscopy of MM cell (MM.1S/GFP+) homing to BM vasculature of the skull was analyzed using fluorescence confocal microscopy, as previously described.30 A skin flap was made in the scalp of the mice to expose the underlying dorsal skull surface. Images of the tumors were captured in approximately 1-hour-long sessions. High-resolution images with cellular detail were obtained through the intact mouse skull at depths of up to 250 μm from the surface of the skull using a 30×/0.9 NA water-immersion objective lens (Lomo, St Petersburg, Russia). Eight images of the BM niches (2 images/each niche) were captured 25 minutes after injection, and a map of the BM niches was consolidated. High-resolution images with cellular detail were obtained through the intact mouse skull using a 30×/0.9 NA water-immersion objective lens. For imaging of GFP+ tumor cells, GFP was excited with a 491-nm solid-state laser (Dual Calypso; Cobolt AB, Stockholm, Sweden) and detected with a photomultiplier tube through a 528/19-nm bandpass filter (Semrock, Rochester, NY). Blood vessels were imaged using Evan Blue (Sigma-Aldrich) excited with a 635-nm laser and captured through a 695/27.5-nm bandpass filter (Omega Optical, Brattleboro, VT). The frame rate of the confocal microscope was 30 frames per second. Images were captured after averaging 30 frames using a Macintosh computer equipped with an Active Silicon snapper card (Active Silicon, Chelmsford, MA). Approval of these studies was obtained by the Dana Farber Cancer Institute and Massachusetts General Hospital Institutional Animal Care and Use committees.
Detection of tumor progression by bioluminescence imaging
Luc+/GFP+ MM.1S cells (4 × 106/mouse) were injected to the tail vein of SCID mice. Mice were injected with 75 mg/kg of Luciferin (Xenogen, Hopkington, MA), and tumor growth was detected by bioluminescence 10 minutes after the injection. The home-built bioluminescence system used an electron multiplying CCD (Andor Technology Limited, Belfast, United Kingdom) with an exposure time of 30 seconds, and an electron multiplication gain of 500 voltage gain × 200, 5-by-5 binning, and with background subtraction. Images were analyzed using Image-J software (National Institutes of Health, Bethesda, MD).
Statistical analysis
miRNA expression data were analyzed according to manufacturer's instructions (Luminex). The expression patterns of unfiltered data were performed using unsupervised hierarchical clustering of samples, based on centroid linkage and 1-correlation distance metric, using dChip (www.dchip.org). To further define those miRNAs differentially expressed between groups (patients vs normal), the data were filtered on significance of differences using ANOVA test (P < .01). To identify specific predicted miRNAs-targeted mRNAs, TargetScan, PicTar, and miRanda algorithms were used.31,32 To reduce the numbers of false positives, only putative target genes predicted by the 3 algorithms were considered.
To describe the distribution of miRNA levels in MM patients compared with controls, mean values were compared using Mann-Whitney U rank sum test. The expression of deregulated miRNAs among all MM patients relative to the normal counterparts has been considered. MM patients have been categorized according to either International Staging System for MM15 (I vs II vs III). The mean level of deregulated miRNAs has been compared using the Mann-Whitney U rank sum test. All statistical tests are 2-sided, and all analyses were performed with SPSS software v12.0 (SPSS Inc, Chicago, IL).
Results
Specific miRNA expression signature distinguishes MM cells from their normal cellular counterparts
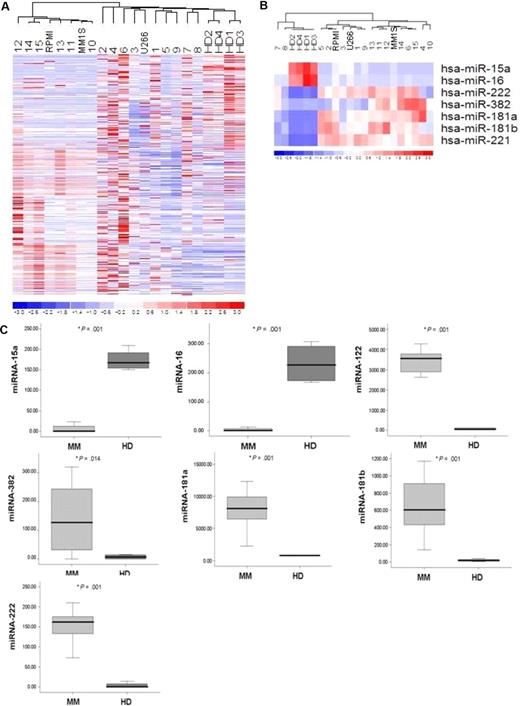
The expression of 318 miRNAs was profiled in BM CD138+ selected MM cells from 15 patients with relapsed/refractory MM in comparison with CD138+ cells isolated from BM of healthy donors and in MM cell lines (MM.1S, RPMI8226, U266). The clinical characteristics of the evaluated patients' samples are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Unsupervised hierarchical clustering of samples was performed on all unfiltered data. The generated heat map for the entire population of miRNAs showed differences between miRNA expression profiles of MM samples compared with normal counterparts (Figure 1A).
microRNA expression pattern in MM and normal donors. (A) miRNA analysis was performed on total RNA isolated from bone marrow (BM) CD138+ MM cells, normal BM (HD)–derived CD138+ counterparts, and MM cell lines (MM.1S, RPMI8226, U266). The heat map was generated after unsupervised hierarchical cluster analysis of all unfiltered data. Differential expression of miRNA is shown by the intensity of red (up-regulation) versus blue (down-regulation). (B) Supervised hierarchical clustering analysis was performed using the ANOVA test. Differential expression of miRNA patterns is shown by the intensity of red (up-regulation) versus blue (down-regulation). (C) Differential distribution of each indicated miRNA in all MM patients (MM) compared with healthy donors (HD): mean values were compared using Mann-Whitney U rank sum test; bars indicate standard errors; P value (*) for each miRNA is indicated.
microRNA expression pattern in MM and normal donors. (A) miRNA analysis was performed on total RNA isolated from bone marrow (BM) CD138+ MM cells, normal BM (HD)–derived CD138+ counterparts, and MM cell lines (MM.1S, RPMI8226, U266). The heat map was generated after unsupervised hierarchical cluster analysis of all unfiltered data. Differential expression of miRNA is shown by the intensity of red (up-regulation) versus blue (down-regulation). (B) Supervised hierarchical clustering analysis was performed using the ANOVA test. Differential expression of miRNA patterns is shown by the intensity of red (up-regulation) versus blue (down-regulation). (C) Differential distribution of each indicated miRNA in all MM patients (MM) compared with healthy donors (HD): mean values were compared using Mann-Whitney U rank sum test; bars indicate standard errors; P value (*) for each miRNA is indicated.
To identify statistically significant differences in miRNA expression profiles between patient and normal donor samples, supervised clustering analysis was performed. We identified 7 miRNAs with specific differential expression signatures between relapsed/refractory MM patients and healthy subjects. Specifically, miRNA analysis showed increased miRNA-222/-221/-382/-181a/-181b expression (P < .01) and decreased miRNA-15a and -16 expression in MM patients (P < .01; Figure 1B). miRNAs-222, -221, -382, -181a, -181b were significantly increased in the entire cohort of MM patients compared with control samples, whereas miRNA-15a and -16 were significantly decreased in all patient samples compared with control samples (P ≤ .01; Figure 1C).
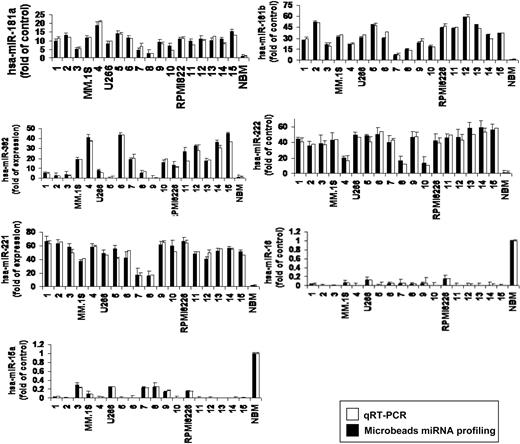
To further validate the results from miRNA expression profiling, qRT-PCR was performed on matched samples. We showed expression patterns similar to those observed in miRNA analysis (Figure 2). We next analyzed the predicted gene targets for each up- or down-regulated miRNA, using algorithms (miRanda, TargetScan, and PicTar) commonly used to predict human miRNA gene targets31,32 and specifically identified genes predicted by all 3 methods. Predicted targets of the increased miRNAs in MM patients included tumor suppressors, cytokine signaling suppressors, proapoptotic factors, NF-κB suppressors, and tyrosine phosphatases. Conversely, predicted target genes for the decreased miRNAs in MM included angiogenic cytokines, AKTserine/threonine protein kinase-3 (AKT3), ribosomal-protein-S6, MAP-kinases, and NF-κB activator MAP3KIP3 (Table S2).
Validation of miRNA expression levels by qRT-PCR. The amount of miRNA-181a, -181b, -382, -222, -221, -15a, and -16 in MM samples compared with normal controls, evaluated by qRT-PCR (□) and microbead miRNA profiling (■). Results are expressed as fold change of the miRNA expression in bone marrow (BM) MM CD138+ cells with respect to average miRNA expression from 4 normal donor BM CD138+ cells. qRT-PCR data were obtained using the ΔΔCt method, with normalization to the reference RNU6B microRNA. Bars represent SDs.
Validation of miRNA expression levels by qRT-PCR. The amount of miRNA-181a, -181b, -382, -222, -221, -15a, and -16 in MM samples compared with normal controls, evaluated by qRT-PCR (□) and microbead miRNA profiling (■). Results are expressed as fold change of the miRNA expression in bone marrow (BM) MM CD138+ cells with respect to average miRNA expression from 4 normal donor BM CD138+ cells. qRT-PCR data were obtained using the ΔΔCt method, with normalization to the reference RNU6B microRNA. Bars represent SDs.
miRNA15a and 16-1 modulate proliferation and cell cycle progression of MM cells
miRNA-15a and -16-1 are located on chromosome 13q14, an area commonly deleted in MM. Indeed, we found a total absence of miRNA-15a and -16-1 in those patients with chromosome 13 deletion [del(13)] with a significantly decreased expression in the remaining patients without del(13). Therefore, we studied the functional role of miRNA-15a and -16 in MM cells by testing precursors (pre)–miRNA15a- and pre-miRNA16-1–transfected MM.1S and RPMI8226 cells compared with controls (control probe–transfected or nontransfected MM.1S cells). Efficiency of transfection was evaluated by qRT-PCR at 24, 48, 72 hours after transfection (Figure S1A).
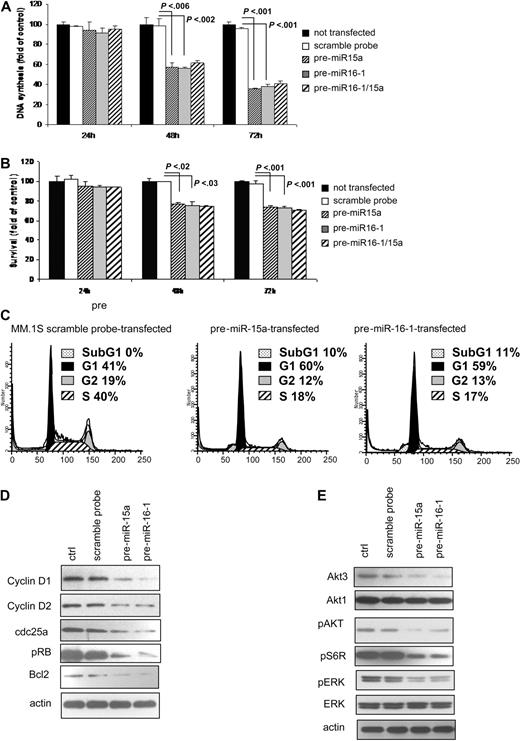
We showed that DNA synthesis was significantly reduced in pre-miRNA15a, and -16-1–transfected cells compared with control at both 48 and 72 hours (P < .006; Figure 3A). At 72 hours, pre-miRNA15a and -16-1 induced 62% and 60% reduction, respectively, in MM cell proliferation (P < .001; Figure 3A). No additive or synergistic effects were observed when cells were transfected with both pre-miRNA-15a and -16-1 (Figure 3A). The effect of miRNA-15a and -16 on survival of MM cells was evaluated. There was a slight induction of cytotoxicity (24%) in both pre-miRNA-15a– and -16-1–transfected cells (P < .03; Figure 3B), without differences at 48 and 72 hours. No additive or synergistic effects were observed in MM cells transfected with both pre-miRNA-15a and -16-1 (Figure 3B). Similar data were observed in pre-miRNA-15a– and -16-1–transfected RPMI8226 cell lines (Figure S2A).
miRNA-15a and -16 modulates proliferation and cell cycle of MM cells. (A,B) MM cells (pre-miRNA-15a–, -16-1 probe–, and control probe–transfected and nontransfected MM.1S) were harvested at 24, 48, and 72 hours after transfection; DNA synthesis and cytotoxicity were assessed by thymidine uptake and MTT assays, respectively. Nontransfected MM.1S cells were used as controls. P values are indicated. (C) Cells were first arrested and synchronized in G2/M phase by growth in 80-nM nocodazole for 16 hours. Cells were then washed and regrown using fresh media. After 6 hours, cell cycle analysis was performed by propidium iodide staining. (D) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected) were harvested at 24 hours after transfection. Whole cell lysates were subjected to Western blotting using anti–cyclin D1, –cyclin D3, cdk6, –p-Rb, -Bcl2, and -actin antibodies; nontransfected MM.1S cells were used as controls (ctrl). (E) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected) were harvested at 24 hours after transfection. Whole cell lysates were subjected to Western blotting using anti–phospho(p)-AKT, -AKT1, -AKT3, –p-ERK, -ERK, p-S6R, and -actin antibodies; nontransfected MM.1S cells were used as controls (ctrl).
miRNA-15a and -16 modulates proliferation and cell cycle of MM cells. (A,B) MM cells (pre-miRNA-15a–, -16-1 probe–, and control probe–transfected and nontransfected MM.1S) were harvested at 24, 48, and 72 hours after transfection; DNA synthesis and cytotoxicity were assessed by thymidine uptake and MTT assays, respectively. Nontransfected MM.1S cells were used as controls. P values are indicated. (C) Cells were first arrested and synchronized in G2/M phase by growth in 80-nM nocodazole for 16 hours. Cells were then washed and regrown using fresh media. After 6 hours, cell cycle analysis was performed by propidium iodide staining. (D) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected) were harvested at 24 hours after transfection. Whole cell lysates were subjected to Western blotting using anti–cyclin D1, –cyclin D3, cdk6, –p-Rb, -Bcl2, and -actin antibodies; nontransfected MM.1S cells were used as controls (ctrl). (E) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected) were harvested at 24 hours after transfection. Whole cell lysates were subjected to Western blotting using anti–phospho(p)-AKT, -AKT1, -AKT3, –p-ERK, -ERK, p-S6R, and -actin antibodies; nontransfected MM.1S cells were used as controls (ctrl).
Predicted targets of miRNA-15a and -16-targeted mRNAs, using miRanda, TargetScan, and PicTar algorithms, included cyclinD1, cyclinD2 and CDC25A. We, therefore, examined cell cycle profiling using PI staining in control probe– and pre-miRNA-15a and -16–transfected cells. Cells were first arrested and synchronized in G2/M phase by growth in 80 nM nocodazole for 16 hours33 ; absence of nocodazole-induced cytotoxicity was observed on treated cells, as assessed by MTT (Figure S1B). Pre-miRNA-15a and -16-1–transfected cells showed increased numbers of cells in G1 phase (60% and 59%, respectively, vs 41% in control), decreased numbers of cells in S phase (18% and 17%, respectively vs 40% in control), and a modest increase in subG1 phase (10% and 11%, respectively; Figure 3C). Similar data were observed in pre-miRNA-15a– and -16-1–transfected RPMI8226 cell line (Figure S2B). We further confirmed the functional role of miRNA-15a and -16 in regulating cell cycle at the protein level and found that they inhibited cyclinD1, cyclinD2, and CDC25A expression, as well as the level of phosphorylated-Rb (Figure 3D). Taken together, these data demonstrate changes in cell cycle regulatory proteins responsible for G1 arrest. Moreover, since miRNA-15a and -16 target BCL2,34 we confirmed that miRNA-15 and miRNA-16-1 reduced BCL2 expression, which may be responsible for miRNA-15a- and 16-induced subG1 phase in transfected MM cells (Figure 3D). Other predicted miRNA-15a- and 16-targeted mRNAs include members of the AKT serine/threonine protein kinase family (AKT3), as well as ribosomal protein S6 and MAP kinases. Therefore, we examined the impact of miRNA-15a and -16 on signaling cascades regulating proliferation of MM cells. We found that pre-miRNA-15a– and -16-1–transfected cells showed inhibition of AKT3, pAKT, pS6R, and pERK (Figure 3E). The observed changes in pAKT protein level may be a consequence of down-regulated total AKT3 protein level.35,36
miRNA-15a and -16-1 inhibit NF-κB pathway
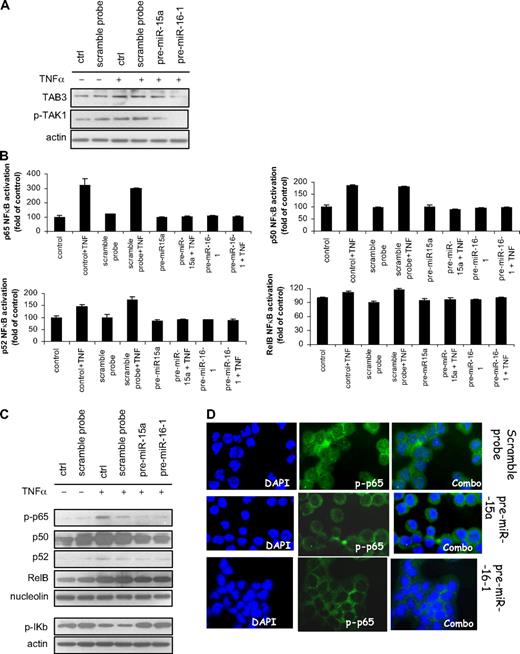
It has previously been shown that NF-κB activation plays a pivotal role in promoting growth and survival of MM cells,37-39 as well as in other plasma cell dyscrasias.40 Predicted miRNA-15a- and -16-targeted mRNAs include MAP3KIP3, also known as TAB3, which acts as activator of NF-κB by interacting with TAK1.41-43 We, therefore, sought to examine the functional relevance of miRNA-15a and miRNA-16 on NF-κB activity in MM cells. We first demonstrated that miRNA-15a and -16 target TAB3, as shown by inhibition of TAB3 at protein level. Despite a stronger inhibition of TAB3 upon pre-miRNA-16-1 transfection, compared with pre-miRNA-15a, TNF-α–induced TAB3 phosphorylation was inhibited by transfecting MM cells with either pre-miRNA-15a or -16-1, compared with control (Figure 4A).
miRNA-15a and -16 target NF-κB in MM cells. (A) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 24 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes. Whole cell lysates were subjected to Western blotting using anti-TAB3, phospho(p)-TAK1, and -actin antibodies; nontransfected MM.1S cells were used as control (ctrl). (B) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 24 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes; nontransfected MM.1S cells were used as control (ctrl). NF-κB-p65, p50, -p52, -RelB transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was measured in nuclear extracts. All results represent means (± SD) of triplicate experiments. (C) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 12 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes. Nuclear and cytoplasmic protein extracts were subjected to Western blotting using anti–phospho(p)-65, -p50, -p52, -RelB, -nucleolin, –p-IkB, and -actin antibodies; nontransfected MM.1S cells were used as control (ctrl). (D) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 24 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes; nontransfected MM.1S cells were used as control (ctrl). Immunocytochemical analysis was assessed using anti–phospho-NF-κB-p65 antibody, with DAPI used to stain nuclei.
miRNA-15a and -16 target NF-κB in MM cells. (A) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 24 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes. Whole cell lysates were subjected to Western blotting using anti-TAB3, phospho(p)-TAK1, and -actin antibodies; nontransfected MM.1S cells were used as control (ctrl). (B) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 24 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes; nontransfected MM.1S cells were used as control (ctrl). NF-κB-p65, p50, -p52, -RelB transcription factor-binding to its consensus sequence on the plate-bound oligonucleotide was measured in nuclear extracts. All results represent means (± SD) of triplicate experiments. (C) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 12 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes. Nuclear and cytoplasmic protein extracts were subjected to Western blotting using anti–phospho(p)-65, -p50, -p52, -RelB, -nucleolin, –p-IkB, and -actin antibodies; nontransfected MM.1S cells were used as control (ctrl). (D) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S) were harvested at 24 hours after transfection and treated with and without TNF-α (10 ng/mL) for 20 minutes; nontransfected MM.1S cells were used as control (ctrl). Immunocytochemical analysis was assessed using anti–phospho-NF-κB-p65 antibody, with DAPI used to stain nuclei.
We next investigated the NF-κB-p65, -p50, -p52, and -RelB DNA-binding activity in MM.1S cells, studying nuclear extracts from pre-miRNA-15a– and -16-1–transfected compared with control nontransfected and control probe–transfected MM cells using Active Motif assay. We found that TNF-α treatment induced NF-κB-p65, -p50, and -p52 recruitment to the nucleus in MM control cells, which was inhibited in pre-miRNA-15a– and -16-1–transfected cells (Figure 4B). Similar data were observed in pre-miRNA-15a– and -16-1–transfected RPMI8226 cell line (Figure S2C). In addition, immunoblotting from nuclear extracts of pre-miRNA-15a– and -16-1–transfected cells demonstrated that TNF-α–induced NF-κB-p-p65, -p50, and -p52 activation was inhibited compared with scramble probe– or nontransfected cells. Moreover, we found increased phosphorylation of the inhibitory protein IkB in the cytoplasm of pre-miRNA-15a and -16-1–transfected cells. We further confirmed that phospho-p65 translocation from the cytoplasmic compartment to the nucleus was inhibited in pre-miRNA-15a– and -16-1–transfected cells, resulting in a significant increase in p-p65 expression in the cytoplasmic compartment as shown by immunofluorescence (Figure 4C). These data suggest a possible role of miRNA-15a and -16 in regulating the canonical and noncanonical NF-κB pathways in MM cells.
miRNA-15a and -16-1 exert antiangiogenic activity in vitro and in vivo
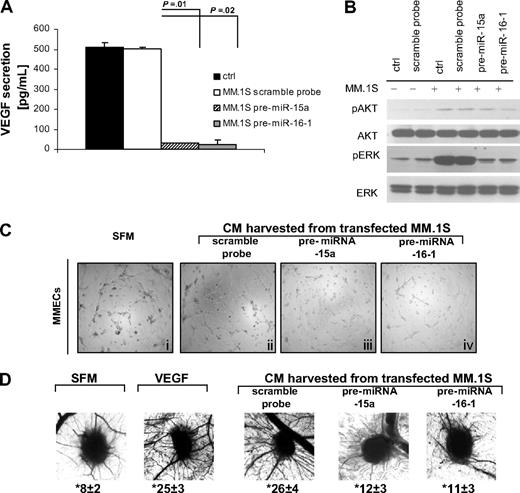
It has been demonstrated that BM neo-angiogenesis plays a crucial role in the pathogenesis and progression of MM. Vascular endothelial growth factor (VEGF) constitutes one of the major proteins with proangiogenic activity; and it has been reported that MM cells support endothelial cell proliferation by releasing VEGF in a paracrine manner.44,45 Moreover, predicted miRNA-15a– and -16–targeted genes are proangiogenic genes and include VEGF. We, therefore, evaluated the functional role of miRNA-15a and -16 on the modulation of angiogenic phenotype. We first showed that VEGF secretion was significantly decreased in the conditioned medium (CM) of pre-miRNA-15a– and -16-1–transfected MM.1S cells, compared with scramble probe- or nontransfected cells used as control (P ≤ .02; Figure 5A). Similar data were observed in pre-miRNA-15a– and -16-1–transfected RPMI8226 cell line (Figure S2D). In addition, either scramble probe– and nontransfected MM.1S triggered activation of ERK and AKT pathways in endothelial cells (ECs), which was inhibited when ECs were cultured with pre-miRNA-15a– and -16-1–transfected MM.1S cells (Figure 5B). We next observed that the spontaneous ability of primary MMECs seeded on a ECM matrix (Figure 5Ci) in absence of serum to induce the formation of capillaries in vitro was enhanced in the presence of CM obtained from scramble probe–transfected MM.1S (Figure 5Cii) versus reduced if seeded in presence of CM obtained from pre-miRNA-15a– and 16-1–transfected MM.1S cells (Figure 5Ciii,iv). Taken together, these data indicate that miRNA-15a and -16 inhibit MM cell-triggered endothelial cell growth and capillary formation in vitro.
miRNA-15a and -16 target endothelial cells in vitro and in vivo. (A) VEGF concentrations were measured in triplicate, by ELISA, in conditioned media obtained from MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S). (B) HUVECs were cultured in presence or absence of MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S). Nontransfected MM.1S cells were used as control (ctrl). Whole cell lysates were subjected to Western blotting using anti–phospho(p)-AKT, -AKT, p-ERK, -ERK, and -actin antibodies. (C) MMECs were seeded on top of ECM-matrix, in presence of CM obtained from either control probe–, pre-miRNA-15a, or -16-1–transfected MM.1S cells. Serum-free medium (SFM) was used as control. Tube formation was assessed using an inverted light microscope. Photographs are representative of 3 independent experiments. (D) Angiogenic responses induced by gelatin sponges loaded with CM obtained from either control probe–, pre-miRNA-15a, or -16-1–transfected MM.1S cells, in the in vivo CAM model. SFM and VEGF (10 ng/mL) were used as negative and positive control, respectively. Original magnification: ×50. Vessel counts at the sponge-CAM boundary are indicated below each image.
miRNA-15a and -16 target endothelial cells in vitro and in vivo. (A) VEGF concentrations were measured in triplicate, by ELISA, in conditioned media obtained from MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S). (B) HUVECs were cultured in presence or absence of MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S). Nontransfected MM.1S cells were used as control (ctrl). Whole cell lysates were subjected to Western blotting using anti–phospho(p)-AKT, -AKT, p-ERK, -ERK, and -actin antibodies. (C) MMECs were seeded on top of ECM-matrix, in presence of CM obtained from either control probe–, pre-miRNA-15a, or -16-1–transfected MM.1S cells. Serum-free medium (SFM) was used as control. Tube formation was assessed using an inverted light microscope. Photographs are representative of 3 independent experiments. (D) Angiogenic responses induced by gelatin sponges loaded with CM obtained from either control probe–, pre-miRNA-15a, or -16-1–transfected MM.1S cells, in the in vivo CAM model. SFM and VEGF (10 ng/mL) were used as negative and positive control, respectively. Original magnification: ×50. Vessel counts at the sponge-CAM boundary are indicated below each image.
To further validate the antiangiogenic impact of miRNA-15a and -16, the antiangiogenic activity of both miRNAs was also evaluated in vivo using the chicken embryo chorioallantoic membrane assay. Sponges loaded with either VEGF or CM from scramble probe–transfected MM cells triggered the formation of neo-vessels and vessel count, which was significantly reduced when CM from miRNA-15a– and -16-1–transfected MM cells were used (Figure 5D).
miRNA-15a and -16-1 exert anti-MM activity even in the context of BM milieu
Because the BM microenvironment confers growth and induces drug resistance in malignant cells,46 we next investigated whether miRNA-15a and -16 could affect MM cell growth in the context of the BM milieu. Previous studies have shown that the PI3K/Akt pathway regulates adhesion and migration in B cells47 ; we have found that miRNA-15a– and -16–targeted mRNAs include members of the AKT serine/threonine protein kinase family (AKT3) as well ribosomal protein S6, a downstream target of AKT. We next confirmed this at protein level (Figure 3E). We, therefore, examined the functional role of miRNA-15a and -16 on migration and adhesion of MM cells in vitro. Specifically, pre-miRNA-15a– and -16-1–transfected MM.1S cells as well as scramble probe– or nontransfected MM.1S cells were incubated for 2 hours in the presence of SDF-1. Pre-miRNA-15a– and 16-1–transfected cells showed inhibition in migration in response to SDF-1 (P ≤ .04; Figure 6A). Similarly, significant inhibition of adhesion of MM to primary BM stromal cells (BMSCs) was observed (P ≤ .006; Figure 6B). Subsequently, we examined the effect of miRNA-15a and -16 on growth of MM in the presence or absence of primary BMSCs. Transfected (either with control probe– or pre-miRNA-15a and -16-1) and nontransfected MM.1S cells were cultured with in the presence or absence of primary BMSCs for 48 hours. We confirmed that primary BMSCs triggered MM cell growth: adherence of both nontransfected or control probe–transfected MM.1S cells to BMSCs triggered 35% and 42% increases in proliferation, respectively, using [3H]-thymidine uptake. Importantly, we did not observe any significant increase in the proliferation rate of pre-miRNA-15a– and -16-1–transfected cells, indicating that they overcome the growth advantage from BMSCs (Figure 5D). To further corroborate the role of miRNA-15a and -16-1 in targeting MM cells in the context of BM microenvironment, we next investigated the effect of these miRNAs on migration in vivo, using a previously described in vivo homing model.30 Four weeks after injection of pre-miRNA-15a–, pre-miRNA-16-1–, and scramble probe–transfected GFP+/Luc+MM.1S cells in vivo, the number of pre-miRNA-15a and -16-1–transfected MM cells that homed and proliferated in vivo in BM was markedly lower than the scramble probe–transfected MM cells (Figure 6D). We confirmed that MM cells were efficiently transfected in vivo, as evaluated in cells isolated 4 weeks postinjection from BM of mice injected with either pre-miRNA-15a or -16-1–transfected MM.1S, using stem-loop qRT-PCR for human miRNA-15a and -16 (Figure S1C). We next confirmed that disruption of adhesion of MM cells to BM niches reduces tumor progression in mice, shown using bioluminescence imaging of the mice. Specifically, mice injected with scramble probe–transfected MM.1S showed significant tumor growth, whereas tumor burden was significantly reduced in mice injected with either pre-miR15a– or pre-miR-16-1–transfected MM.1S cells (Figure 6E; P < .001).
miRNA-15a and -16 target MM cells in the context of bone marrow (BM) milieu in vitro and in vivo. MM cells (pre-miRNA-15a-, -16-1 precursors probe–, control probe–transfected MM.1S cells) were harvested at 24 hours after transfection. Nontransfected MM.1S cells were used as control (ctrl). (A) Transwell migration assay. SDF-1 (30 nM) was placed in the lower chambers and migration was determined after 2 hours. Control probe–transfected and not-transfected cells showed significant migration with SDF-1 30 nM, whereas the pre-miRNA-15a and -16-1 probe–transfected cells showed minimal migration in response to SDF-1. (B) Adhesion assay to primary BMSCs. Control probe–transfected and nontransfected cells showed significant increase in adhesion to BMSCs, compared with control (BMSCs-non coated wells). Pre-miR-15a and -16-1 probe–transfected cells showed lower adhesion to BMSCs. (C) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S cells) cells were cultured for 48 hours in the presence or absence of BMSCs, and cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (SD) of triplicate experiments. (D) In vivo confocal imaging. GFP+ MM cells were transfected using either control-probe or pre-miRNA-15a, -16-1 probes, and then injected in mice 24 hours after transfection. GFP+ MM cells (green color) were excited with a 491-nm solid-state laser. Blood vessels (red color) were imaged using Evans blue excited with a 635-nm laser. Images of parasagittal vasculature and GFP+ tumor cells in the mouse skull BM. Numbers 1 through 8 indicate the 8 areas of the BM niches that were imaged. Control probe–transfected cells homed and adhered more than the miRNA-15a, -16-1–transfected counterparts. (E) Bioluminescence imaging (BLI) demonstrates tumor progression in the control mice with minimal to no BLI signal in the mice injected with miRNA-15a, -16-1–transfected MM cells. (a: dorsal image; b: ventral image). BLI signal has been quantitated using ImageJ software (P values are shown; *, **P < .001).
miRNA-15a and -16 target MM cells in the context of bone marrow (BM) milieu in vitro and in vivo. MM cells (pre-miRNA-15a-, -16-1 precursors probe–, control probe–transfected MM.1S cells) were harvested at 24 hours after transfection. Nontransfected MM.1S cells were used as control (ctrl). (A) Transwell migration assay. SDF-1 (30 nM) was placed in the lower chambers and migration was determined after 2 hours. Control probe–transfected and not-transfected cells showed significant migration with SDF-1 30 nM, whereas the pre-miRNA-15a and -16-1 probe–transfected cells showed minimal migration in response to SDF-1. (B) Adhesion assay to primary BMSCs. Control probe–transfected and nontransfected cells showed significant increase in adhesion to BMSCs, compared with control (BMSCs-non coated wells). Pre-miR-15a and -16-1 probe–transfected cells showed lower adhesion to BMSCs. (C) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S cells) cells were cultured for 48 hours in the presence or absence of BMSCs, and cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (SD) of triplicate experiments. (D) In vivo confocal imaging. GFP+ MM cells were transfected using either control-probe or pre-miRNA-15a, -16-1 probes, and then injected in mice 24 hours after transfection. GFP+ MM cells (green color) were excited with a 491-nm solid-state laser. Blood vessels (red color) were imaged using Evans blue excited with a 635-nm laser. Images of parasagittal vasculature and GFP+ tumor cells in the mouse skull BM. Numbers 1 through 8 indicate the 8 areas of the BM niches that were imaged. Control probe–transfected cells homed and adhered more than the miRNA-15a, -16-1–transfected counterparts. (E) Bioluminescence imaging (BLI) demonstrates tumor progression in the control mice with minimal to no BLI signal in the mice injected with miRNA-15a, -16-1–transfected MM cells. (a: dorsal image; b: ventral image). BLI signal has been quantitated using ImageJ software (P values are shown; *, **P < .001).
Association between miRNA profiling and prognostic factors
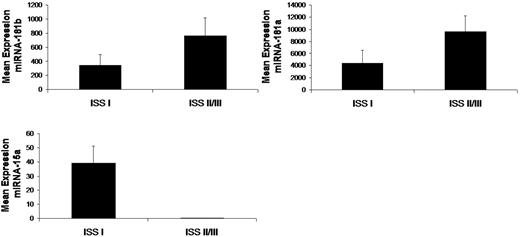
To further investigate the clinical relevance of miRNAs in MM in terms of prognosis, we correlated miRNA expression levels with prognostic factors according to ISS.15 We compared the mean level of each miRNA within the MM patients ISS I with ISS II and III groups. There was no statistical difference between ISS II and III groups; therefore, these groups were pooled. Specifically, absence of miRNA15a expression and overexpression of miRNA-181a and -181b correlated with worse prognosis as predicted by ISS II/III (Figure 7).
Differentially expressed miRNAs are associated with prognosis in MM patients. Significant differential distribution of each indicated miRNA among subgroups of MM patients with differential clinical-prognostic features according to ISS (I vs II/III; In = 4; IIn = 8; IIIn = 3). Mean values were compared using Mann-Whitney U rank sum test; bars indicate SEs. P value for each miRNA is indicated.
Differentially expressed miRNAs are associated with prognosis in MM patients. Significant differential distribution of each indicated miRNA among subgroups of MM patients with differential clinical-prognostic features according to ISS (I vs II/III; In = 4; IIn = 8; IIIn = 3). Mean values were compared using Mann-Whitney U rank sum test; bars indicate SEs. P value for each miRNA is indicated.
In addition, all the patients analyzed in this study were uniformly treated on a clinical trial of the mTOR inhibitor RAD001 in a phase II trial in multiple myeloma. Given that there were only 2 partial responses on this trial, correlation with clinical response was not performed.
Discussion
Several studies suggest that miRNAs are critical in delineating biologic alterations as well prognostic factors in solid and hematologic tumors.10-12 It has recently been reported that miRNAs regulate MM pathogenesis, providing important functional insights regarding the role of miRNAs with distinctive signature in MM and MGUS patients, characterized by increased expression of miRNAs with oncogenic properties.13 Authors showed overexpression, among others, of miRNA-17∼92 cluster (located on chromosome 13). Deletion of chromosome 13 [(del(13)] predicts a significantly reduced survival in patients with MM14 ; however, MM miRNA signature in patients in the relapsed/refractory setting, carrying del(13), and the role of miRNAs in targeting MM cells in the context of the BM microenvironment, has not been described to date. In the present study, we have characterized a specific miRNA signature that differentiates relapsed/refractory MM patients from healthy subjects. The altered MM-related miRNA signature in this subset of patients is characterized by increased expression of miRNA-181a, -181b, -222, -221, -382, and decreased expression miRNA-15a and -16.
Targets of overexpressed miRNAs include tumor suppressor genes as well as genes encoding proteins acting as negative regulators of cell proliferation and survival. Indeed, several genes with oncosuppressive activities were identified including PPP3CC, BCL11B, DEAD box polypeptide 3, and Pdcd4. In addition, we also found genes encoding proteins that inhibit cell proliferation, such as mortality factor 4, cyclin-dependent kinase inhibitor 1C, and thyrosine phosphatases. Other predicted targets included suppressors of cytokine signaling (SOCS), such as SOCS1 and SOCS6, whose inhibition has been implicated in the pathogenesis of several tumors, as SOCS proteins act as negative regulators of STAT-induced activities including cell proliferation, survival, and angiogenesis.48
For miRNAs with decreased expression, expected targets include genes encoding for proteins with oncogenic activities or targets that act as positive regulators of cell proliferation and survival. Indeed, several mRNAs with oncogenic functions and cell cycle regulators were found, such as RAS oncogene family members, RET-proto-oncogens, cyclinD1, cyclinD2, cyclinE, cdc25a, and E2F transcription factor 7. In addition, proangiogenic genes were also included within the down-expressed miRNAs, such as VEGF and bFGF. Most importantly, signaling cascade-related genes known to be constitutively activated in MM as well as in other B-cell malignancies were targeted, including AKT serine/threonine protein kinase family (AKT3), ribosomal protein S6 and MAP kinases, and NF-κB activator MAP3KIP3.
The 2 miRNAs with most significantly decreased expression included miRNA-15a and -16, which are described as a cluster located at chromosome 13q14, which is commonly deleted in solid tumors, chronic lymphocytic leukemia, lymphomas, and over 50% of patients with MM.14,49,50 Previous reports showed decreased expression of miRNA-15a and -16 in solid tumors as well as in hematologic malignancies.51-53 We found that miRNA-15a and -16 expression was lower in MM clonal plasma cells compared with control and totally absent in those patients carrying del(13). Therefore, we sought to investigate the role of miRNA-15a and -16 as tumor suppressors in this disease. Indeed, we demonstrated that they both act as crucial regulators of MM cell proliferation in vitro and in vivo. Importantly, we confirmed the efficacy of miRNA-15a and -16 targeting MM cells in the context of BM microenvironment in vitro. Moreover, in vivo studies confirmed significant inhibition of proliferation of pre-miRNA-15a– and -16-1–transfected MM cells.
Endothelial cells and angiogenesis regulate tumor proliferation in MM in the context of the BM milieu. Several cytokines are secreted by MM cells and BM endothelial cells, resulting in autocrine and paracrine growth loops between MM cells and BM milieu.44,45 As a direct consequence, MM cell-supported endothelial cell growth is characterized by increased BM angiogenesis, which further promotes MM progression and acquired MM cell resistance to conventional and novel therapies.42,43 Algorithms commonly used to predict human miRNA gene targets,31,32 specifically identified bFGF and VEGF as miRNA-15a and -16–targeted proangiogenic genes. It has been demonstrated that VEGF represents one of the major proangiogenic cytokines responsible for the induction of neo-angiogenesis in MM patients,44,45 whereas MM cells are not a good source of bFGF.54 Therefore, we sought to investigate the functional relevance of miRNA-15a and -16 as antiangiogenic miRNAs. We demonstrated that they reduced VEGF secretion from MM cells at the protein level, thereby reducing MM cell–induced proangiogenic activity on endothelial cells. These studies were further confirmed in vivo. Therefore, the antiangiogenic role of miRNA-15a and -16 may contribute, at least in part, to their anti-MM activity.
Although this study was performed using samples obtained from patients enrolled on a clinical trial of the mTOR inhibitor RAD001, we did not perform studies to correlate miRNA expression with response as only 2 patients demonstrated partial response on this trial. All of the samples analyzed were from BM of patients before therapy with RAD001. Given that RAD001 exerts antiangiogenic activity, future studies to determine the role of this agent on miRNA expression in MM tumor samples and endothelial cells from the BM of these patients posttherapy should be performed.
Together, these studies confirm that miRNA-15a and -16 are critical regulators of MM pathogenesis both directly by targeting clonal plasma cells, and indirectly by reducing BM neo-angiogenesis and the interaction between tumor cells and BM milieu. These studies provide the basis for the development of new miRNA-targeted therapies for MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Rafael Fonseca (Mayo Clinic, Scottsdale, AZ) for helpful discussions.
This work was supported by in part by RO1 CA125690-01, Claudia Adams Barr Foundation (Boston, MA); Italian Association for Cancer Research (Milan, Italy); and The Doctors Cancer Foundation (New Rochelle, NY).
National Institutes of Health
Authorship
Contribution: A.M.R., X.L., C.P.L., B.J.R., T.E.W., K.C.A., and I.M.G. designed and performed research, analyzed data, and wrote the paper; A.S., B.T., J.R., X.J., H.T.N., M.R.M., and D.R. performed research; and A.K.A. and F.A. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, MD, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.

![Figure 6. miRNA-15a and -16 target MM cells in the context of bone marrow (BM) milieu in vitro and in vivo. MM cells (pre-miRNA-15a-, -16-1 precursors probe–, control probe–transfected MM.1S cells) were harvested at 24 hours after transfection. Nontransfected MM.1S cells were used as control (ctrl). (A) Transwell migration assay. SDF-1 (30 nM) was placed in the lower chambers and migration was determined after 2 hours. Control probe–transfected and not-transfected cells showed significant migration with SDF-1 30 nM, whereas the pre-miRNA-15a and -16-1 probe–transfected cells showed minimal migration in response to SDF-1. (B) Adhesion assay to primary BMSCs. Control probe–transfected and nontransfected cells showed significant increase in adhesion to BMSCs, compared with control (BMSCs-non coated wells). Pre-miR-15a and -16-1 probe–transfected cells showed lower adhesion to BMSCs. (C) MM cells (pre-miRNA-15a–, -16-1 probe–, control probe–transfected MM.1S cells) cells were cultured for 48 hours in the presence or absence of BMSCs, and cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (SD) of triplicate experiments. (D) In vivo confocal imaging. GFP+ MM cells were transfected using either control-probe or pre-miRNA-15a, -16-1 probes, and then injected in mice 24 hours after transfection. GFP+ MM cells (green color) were excited with a 491-nm solid-state laser. Blood vessels (red color) were imaged using Evans blue excited with a 635-nm laser. Images of parasagittal vasculature and GFP+ tumor cells in the mouse skull BM. Numbers 1 through 8 indicate the 8 areas of the BM niches that were imaged. Control probe–transfected cells homed and adhered more than the miRNA-15a, -16-1–transfected counterparts. (E) Bioluminescence imaging (BLI) demonstrates tumor progression in the control mice with minimal to no BLI signal in the mice injected with miRNA-15a, -16-1–transfected MM cells. (a: dorsal image; b: ventral image). BLI signal has been quantitated using ImageJ software (P values are shown; *, **P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/26/10.1182_blood-2009-01-198408/4/m_zh89990938240006.jpeg?Expires=1767783957&Signature=4S5Nn~r5yfwLhpqMh4f5~bbmI~s06TyFUeI1tCys5QCvCIMo6fcpVu9QsyELc7NE6I4HGbJeJzkzt25ytvMFDuXmDIKJrIxUZj3ybW~tAyAG9Hf-TVESJvmW1x7P2SfDODhG8CfaYSzg944kmdDfqGVsOt2Zr~Wz0Gxu-2ZMeiAd7FaKHSZXYFH~6uRQ7f3vMceExxiWp7OCZcVljWFa5WsgcTl0CHxGyoHYfvV8voGLELy5Rm11Yl9rt2ua0LpQ-NPZGwr1maPIQ4dj5-EfKzRw3E5GPFQIRtSbkONMG3Tfko~HegieHMxzZhg9Ur3N2g8D6YJYbxqs0L7YsnvL6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal