Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a potent adjuvant in cancer vaccination; however, the specific role of endogenous GM-CSF remains unknown. We performed cell-based vaccination in 2 tumor models. First, we vaccinated C57BL/6 mice lacking either GM-CSF, IL-5, or beta-common chain (βc), a receptor subunit essential for GM-CSF and IL-5 signaling, with melanoma cells engineered to produce GM-CSF. Tumor vaccination was effective in both GM-CSF−/− and IL-5−/− mice, showing that protective immunization is independent of both endogenous cytokines. However, all βc−/− animals developed tumor. Loss of tumor immunity in βc−/− mice does not reflect global impairment in cell-mediated immunity, as contact hypersensitivity reaction to haptens is unaltered. The importance of tumor cell–derived GM-CSF was highlighted by recruitment of dendritic cells at the vaccination site in wild-type, GM-CSF−/−, and IL-5−/− but not in βc−/− mice. In the second model, vaccination with unmodified RENCA cells showed similar results with efficient immunization in BALB/c wild-type and GM-CSF−/−, whereas all βc−/− animals died. Altogether, our results strongly suggest that although endogenous GM-CSF and IL-5 are not required to induce tumor immunity, signaling through βc receptor is critically needed for efficient cancer vaccination in both genetically modified GM-CSF–secreting tumor cells and a spontaneously immunogenic models.
Introduction
Recent insights into the cellular and molecular mechanisms underlying the host antitumor response have led to the development of several strategies for enhancing antitumor immunity.1-3 Regardless of the antigenic sources (naked DNA, peptide, protein, antigen-loaded dendritic cells, whole cells), granulocyte-macrophage–colony stimulating factor (GM-CSF) has been shown to increase the immune response both in animal models and clinical trials.4-6 It is now widely used as an adjuvant in immunotherapy protocols. We and others have shown that vaccination with irradiated tumor cells engineered to secrete GM-CSF stimulates the generation of potent, specific, and long-lasting antitumor immunity in multiple murine tumor models.7-10 Moreover, this vaccination scheme consistently induces dense CD4+ and CD8+ T-lymphocyte and plasma cell infiltrates, in metastatic lesions of patients with advanced melanoma. These inflammatory reactions result in extensive tumor necrosis, fibrosis, and edema.11 In addition to melanoma, clinical trials using GM-CSF–secreting tumors cells have been reported in patients with several tumor types including non–small cell lung carcinoma,12,13 pancreatic,14 prostate15 and renal cell carcinoma.16 Despite the data from animal models and phase 1 clinical trials, the critical role of GM-CSF is not well characterized and several reports have raised concern about potential detrimental effects of this cytokine.17 Indeed high doses of GM-CSF may prevent optimal immunization due to the expansion of myeloid-derived suppressor cells.18 This has been further supported by the findings of Filipazzi et al, who have identified the presence of myeloid suppressor cells in melanoma patients treated with subcutaneous administration of recombinant GM-CSF.19 Moreover, GM-CSF induces the expression of milk fat globule EGF-8 in antigen-presenting cells, which plays a critical role in the maintenance of FoxP3+ regulatory T cells (Tregs).20 A deeper understanding of the functions of GM-CSF should help guide the use of this cytokine in immunotherapy.
The increased immunogenicity of GM-CSF–secreting tumor cells may be related to the ability to recruit and mature dendritic cells (DCs).21 Although the critical role of DCs in priming antigen-specific responses is well established,22 several studies have identified specific DC characteristics that are critical in the induction of a potent antitumor vaccination activity.23 For example, although both GM-CSF and Flt3-ligand induce the marked expansion of DCs,24,25 we have shown that GM-CSF–secreting tumor cells promoted higher levels of protective immunity than vaccination with FLT3-L–secreting tumor cells.10 The superior efficacy of GM-CSF–secreting vaccines is in part associated with the higher expression of B7-1 (indicative of a better maturation) and CD1d (which evokes the involvement of natural killer T [NKT] cells) on DCs.10 We have also shown that tumor protection induced by GM-CSF–secreting tumor cell vaccine was abrogated in CD1d-deficient mice, whereas vaccinated wild-type (WT) mice mount protective tumor immunity.26 The abrogation of tumor protection in CD1d-deficient mice is associated with impaired T-cell cytokine response to tumor cells including GM-CSF, IL-5, IL-10, and IL-13, whereas T-cell IFN-γ secretion and tumor-specific cytotoxicity remained unchanged.26
Previous mouse studies exploiting gene-targeting techniques or neutralizing antibodies have established that both CD4+ and CD8+ T cells are required for efficient vaccination.7,9 Other investigations have revealed a central role for CD4+ T cells in the production of IFN-γ and IL-4 and the activation of eosinophils and macrophages to produce nitric oxide, and reactive oxygen species in GM-CSF–secreting tumor vaccination.9 Indeed, multiple effector mechanisms, including tumor-induced cytotoxicity, Th1 and Th2 cytokine production, high titer IgG antibodies to surface and intracellular tumor determinants, and the selective destruction of the tumor vasculature have been attributed to the GM-CSF–secreting tumor cell vaccines.27
Studies of adoptive T-cell therapy provide an alternative approach to identify specific effector functions associated with tumor protection. Although both Th1 and Th2 cells can mediate tumor destruction, in many model systems tumor-induced T-cell production of GM-CSF and IFN-γ are tightly correlated with antitumor efficacy.2,28 In fact, GM-CSF secretion and cytotoxic activity of tumor-activated T cells are closely linked with the ability of ex-vivo expanded tumor-infiltrating lymphocytes to mediate clinical responses in patients with metastatic melanoma.29 Together, these studies reveal important roles for GM-CSF in both the priming and effector phases of antitumor responses.
Surprisingly, the role of endogenous GM-CSF in tumor immunity has not been addressed. GM-CSF is known as a potent hematopoietic growth factor for granulocyte and macrophage expansion and it also induces dendritic cell recruitment and maturation. Most of GM-CSF's activities are redundant with other hematopoietic growth factors such as IL-3, M-CSF, G-CSF, and FLT3-L; furthermore, analysis of mice lacking GM-CSF did not reveal major hematopoietic defect.30 GM-CSF–deficient mice showed modestly reduced numbers of DC populations in hematopoietic organs and tissue.31 Study of GM-CSF–deficient mice revealed abnormal alveolar macrophage function with decreased surfactant clearance, leading to alveolar proteinosis.30 Spontaneous tumors were not described but increase in mortality to several pathogens, mainly encapsulated organisms, has been reported.32,33
In an effort to further clarify the requirement for endogenous GM-CSF in antitumor immunity, we used 2 distinct antitumor vaccination models in 2 different mouse strains. In the first model, we compared the immunization induced by subcutaneous injection of irradiated B16 melanoma tumor cells genetically engineered to secrete GM-CSF in C57BL/6 mice deficient in GM-CSF (GM-CSF−/−), IL-5 (IL-5−/−), or βc (βc−/−) and WT littermates. The βc is a receptor subunit common for GM-CSF, IL-5, and IL-3. Both GM-CSF and IL-5 signaling are abolished in βc-deficient mice, whereas IL-3 activity is maintained because of an additional β subunit, specific for IL-3 (β-IL-3).34 To further strengthen the importance of βc signaling in tumor immunization, we have also tested vaccination in the spontaneously immunogenic renal adenocarcinoma RENCA model in BALB/c strain. Vaccination with irradiated, unmodified RENCA tumor cells was performed in GM-CSF−/−, IL-3−/−, GM-CSF + IL-3−/−, and βc−/− in BALB/c background as well as WT littermates.
Methods
Animals
Mice deficient in GM-CSF,30 βc,35 IL-3,36 and both IL-3 and GM-CSF37 were backcrossed at least 9 generations onto the C57BL/6 and BALB/c strains. IL-5–deficient mice38 were generated in a pure C57BL/6 background. Animals studied were at least 10 weeks of age and all experiments were performed in accordance with local animal care regulations. The experimental protocols were accepted and approved by the Office Vétérinaire Cantonal, the regulatory body for animal experimentation at the Geneva University Hospital.
Antibodies
All monoclonal antibodies (mAbs) and isotype controls were purchased from BD Pharmingen (San Diego, CA) unless stated: phycoerythrin (PE)–conjugated anti-CD11c (hamster IgG, clone HL3) and isotype control (hamster IgG, clone A19-3); FITC-labeled mAbs including anti–Gr-1 (rat IgG2b, clone RB6-8C5) and anti-CD11b (rat IgG2b, clone M1/70); allophycocyanin (APC)–labeled anti-CD8 (rat IgG2a, clone 53-6-7); Alexa-Fluor488–conjugated anti-F4/80 (rat IgG2a, clone BM8; Caltag Laboratories, Burlingame, CA), and isotype controls (rat IgG2a, clone R35-95 and rat IgG2b, clone A95-1).
Cell lines
B16-F10 melanoma cells (syngeneic to C57BL/6 mice) and RENCA cells (syngeneic to BALB/c) were cultured in DMEM with 10% FCS and penicillin/streptomycin (complete medium). B16-F10 cells secreting GM-CSF (B16-GM), FLT3-L (B16-FL), or IL-3 (B16-IL-3) cells were generated by retroviral-mediated gene transfer, as previously described.10 GM-CSF secretion was approximately 150 ng/106 cells per 24 hours, as determined by enzyme-linked immunosorbent assay (ELISA; BD Pharmingen, as indicated by the manufacturer). All the cell lines were confirmed to be mycoplasma free (Mycoplasma Detection Kit Enzyme immunoassay; Roche Laboratories, Mannheim, Germany).
Tumor models
For tumorogenicity experiments, C57BL/6 and BALB/c mice were injected subcutaneously in the interscapular region with 1 × 105 live B16 and 2 × 106 live RENCA cells, respectively. Animals were killed when tumors reached 10 mm in diameter or became ulcerated. For vaccination experiments, C57BL/6 mice were injected subcutaneously in the abdomen with 1 × 106 irradiated (35 gray) B16-GM cells and challenged 7 days later with 5 × 105 live B16 cells injected subcutaneously in the upper back; BALB/c mice were injected subcutaneously in the abdomen with 1 × 106 irradiated (35 gray) RENCA cells and challenged 7 days later with 5 × 106 live RENCA cells injected subcutaneously in the upper back. Mice were followed for 3 months after tumor challenge. In all experiments, confirmation of genotype was performed by polymerase chain reaction (PCR) analysis using somatic DNA (data not shown).
Cytokine assays
Tumor-induced cytokine production was measured as previously described.10 Briefly, splenocytes (106 cells) were harvested 7 days after vaccination with irradiated B16-GM cells, depleted of erythrocytes, and cultured with irradiated (100 gray) B16 cells (2 × 104) in 2 mL complete medium supplemented with 10 units/mL IL-2. Supernatants were harvested after 5 days and assayed for GM-CSF, IL-5, IL-10, IL-13, and IFN-γ by ELISA using the appropriate monoclonal antibodies (Endogen, Woburn, MA and BD Biosciences, Erembodegem, Belgium).
GM-CSF ELISA
The GM-CSF secretion from RENCA cells was determined by ELISA Kit (OptEIA from BD Biosciences, Erembodegem, Belgium), as indicated by the manufacturer. Briefly, the RENCA cells were irradiated at 35 gray and seeded at a density of 106 cells per well. The GM-CSF release in the supernatant was measured after 24 hours.
Cytokine measurement using CBA
Irradiated B16-F10, B16-GM, B16-FL, and B16-IL-3 cells were seeded in triplicates at a density of 106 cells per well during 24 hours. Evaluation of cytokine secretion was done using Th1/Th2 and inflammatory cytometric bead array kits (BD PharMingen) by flow cytometry according to the manufacturer's instructions and analyzed by BD cytometric bead assay (CBA) system (BD PharMingen). Standard curves were determined for each cytokine from a range of 20 to 5000 pg/mL. The following cytokines were measured: IL-2, IL-4, IL-5, IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1), IFN-γ, tumor necrosis factor-α (TNF-α), and IL-12p70.
Contact hypersensitivity
WT and βc−/− mice from both C57BL/6 and BALB/c strains were sensitized epicutaneously on day 0 with 70 μL 4% 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone; Sigma-Aldrich, St Louis, MO) in acetone–olive oil (4:1). Mice were challenged 5 days later on the right ear with 20 μL 0.5% oxazolone in acetone–olive oil and with carrier acetone–olive oil alone on the left ear. The ear thickness was measured on both ears with a micrometer at 24 hours after challenge. Results are presented as the increased thickness of the hapten-treated ear minus the nonspecific swelling (carrier-treated ear). Data represent mean values of 5 mice per group. Similar results were obtained in 3 independent experiments.
Histopathology
Vaccination sites were removed at day 5 and processed for pathologic examination. Tissues were fixed in 10% neutral buffered formalin, routinely processed and embedded in paraffin, cut to 5-micrometer thickness, and stained with hematoxylin and eosin (H&E). Images were viewed with an Axioskop 2 plus microscope (Göttingen, Germany) and taken with an AxioCam HRc serie1.6 camera (Carl Zeiss, Jena, Germany). Software used was AxioVision 3.1 (Carl Zeiss).
Flow cytometric analysis of cells at the vaccination site
Mice were immunized subcutaneously with 106 irradiated tumor cells (B16 or B16-GM). Four days later, mice were killed, the site of vaccination was dissected, and cells were extracted as described previously.39 Briefly, tissue was minced with scalpel and enzymatically digested with PBS containing 2 mg/mL collagenase I (Sigma-Aldrich) and 2 mg/mL hyaluronidase I (Sigma-Aldrich) at 37°C during 30 minutes. After mechanical dissociation using a syringe piston, the cells were filtered through a cell strainer (Falcon). The filtered suspension was centrifuged and cells were incubated with the indicated antibodies and analyzed on a FACSCalibur cytometer (Becton Dickinson, Mountain View, CA). Living cells were identified by the nonpermeant DNA dye 7-amino-actinomycin D (Sigma-Aldrich). Data were analyzed with WINMDI software written by J. Trotter (Scripps, La Jolla, CA) and Cell-Quest software (Becton Dickinson). Fluorescence-activated cell sorting (FACS) analysis of the vaccination site was performed in at least 6 mice in each background.
Statistics
Tumor vaccination and contact hypersensitivity experiments were repeated at least 3 times with a minimum of 4 mice per groups and gave similar results. The 2-tailed Student t test was used to evaluate P values between experimental groups. A P value less than .05 was considered statistically significant.
Results
Comparison of tumor immunity among WT, GM-CSF−/−, IL-5−/−, and βc−/− mice
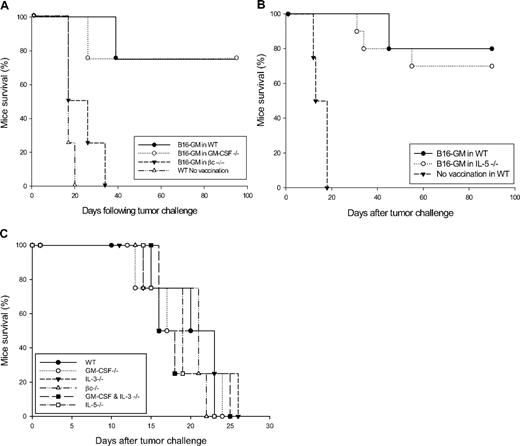
Because GM-CSF production by T cells involved in vaccination and adoptive therapy is closely associated with tumor protection, we first evaluated the ability of GM-CSF–deficient mice to generate antitumor immunity. In these experiments, adult female C57BL/6 GM-CSF−/− animals and littermate controls were vaccinated with 106 irradiated B16-GM and challenged 1 week later with 5 × 105 live B16 cells. Consistent with previous reports, vaccinated WT mice efficiently rejected tumor challenge (long-term protection of 75% of animals), whereas nonimmunized WT animals uniformly developed progressive tumors (Figure 1A). Interestingly, vaccinated GM-CSF−/− mice showed tumor protection that was equivalent to immunized WT controls, with 75% of animals rejecting tumor challenge (Figure 1A).
GM-CSF–secreting tumor cell vaccination in WT, GM-CSF, IL-5, or βc knockout C57BL/6 mice. (A) GM-CSF−/−, βc−/−, WT, or (B) IL-5−/− and WT C57BL/6 mice were immunized subcutaneously on the abdomen with 106 irradiated, B16-GM cells (5 per group). One week later, the vaccinated mice as well as the unvaccinated WT controls, were challenged subcutaneously on the back with 5 × 105 live B16 cells. The difference observed in survival time between B16-GM–vaccinated βc−/− mice and unvaccinated WT controls is not statistically significant. This experiment is representative of 3 independent experiments. (C) Tumorogenicity of B16-F10 cells in different knockout mice. Survival of WT and indicated knockout C57BL/6 mice inoculated with 5 × 105 B16-F10 cells. This experiment is representative of 3 independent experiments.
GM-CSF–secreting tumor cell vaccination in WT, GM-CSF, IL-5, or βc knockout C57BL/6 mice. (A) GM-CSF−/−, βc−/−, WT, or (B) IL-5−/− and WT C57BL/6 mice were immunized subcutaneously on the abdomen with 106 irradiated, B16-GM cells (5 per group). One week later, the vaccinated mice as well as the unvaccinated WT controls, were challenged subcutaneously on the back with 5 × 105 live B16 cells. The difference observed in survival time between B16-GM–vaccinated βc−/− mice and unvaccinated WT controls is not statistically significant. This experiment is representative of 3 independent experiments. (C) Tumorogenicity of B16-F10 cells in different knockout mice. Survival of WT and indicated knockout C57BL/6 mice inoculated with 5 × 105 B16-F10 cells. This experiment is representative of 3 independent experiments.
Studies have shown that although βc mediates most GM-CSF signaling, some evidence suggests that the α chain alone may transduce signals.40 As melanoma cells express the α chain in the absence of βc, part of the vaccination activity of GM-CSF–secreting melanoma cells may involve autocrine effects.41 To learn more about the pathways stimulated by GM-CSF–secreting tumor cells, we have evaluated the ability of βc−/− mice to generate tumor immunity. Although the tumorigenicity of B16 cells in WT, GM-CSF−/−, βc−/−, and GM-CSF + IL-3 double knockout mice appeared the same (Figure 1C), vaccination with irradiated B16-GM cells failed to induce any tumor protection in βc−/− mice (Figure 1A). All mutant animals rapidly developed growing tumors at the challenge site, with kinetics comparable with unvaccinated WT animals (Figure 1A). Because βc is also implicated in IL-5 signaling,35,42 we therefore evaluated the role of endogenous IL-5 to generate tumor immunity. IL-5−/− mice vaccinated with irradiated B16-GM cells showed a 75% survival rate, in comparison with an 80% survival rate in WT mice (Figure 1B). Tumor immunity obtained after efficient vaccination is sustained in WT, GM-CSF−/−, and IL-5−/− mice. All the protected animals rejected secondary tumor challenge performed on day 60 (data not shown).
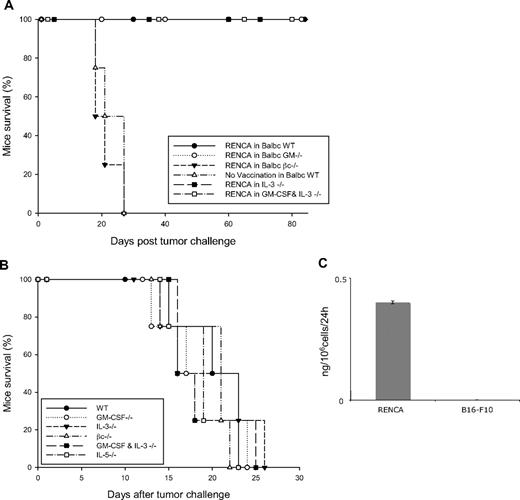
Similar experiments were performed in the BALB/c background, using the RENCA tumor cells. BALB/c WT and GM-CSF−/− mice, immunized with 106 irradiated RENCA cells, were protected in a comparable manner from subsequent 5 × 106 live RENCA cell challenge (Figure 2A). Similarly to the result obtained in the C57BL/6 strain, the loss of protective immunity is also observed in BALB/c βc−/− mice that were vaccinated with irradiated RENCA cells. Whereas all vaccinated βc−/− animals succumb to tumor challenge, all vaccinated WT and GM-CSF−/− mice showed protective antitumor response (Figure 2A). We then evaluated the tumorogenicity of RENCA cells in different knockout mice and found out that this remains identical among WT, GM-CSF−/−, βc−/−, and GM-CSF + IL-3−/− double knockout mice (Figure 2B).
Tumor protection of RENCA cells in WT and different knockout BALB/c mice. (A) WT and indicated knockout BABL/c mice were vaccinated with 106 irradiated RENCA cells and challenged 1 week later with 5 × 106 RENCA tumor cells. The survival curve indicates percentage of mice that survived the challenge. The graphic represents data of 4 independent experiments (n = 4 mice/group). (B) Tumorogenicity of RENCA cells in different knockout mice. WT and indicated knockout BALB/c mice were inoculated on the back with 2 × 106 RENCA tumor cells. Mice were killed when tumor size reached 15 mm in diameter or was ulcerated. This experiment is representative of 3 independent experiments. (C) Spontaneous production of GM-CSF by RENCA cells. Irradiated RENCA or B16-F10 cells (106) were seeded in a 10-mm plate and GM-CSF release was detected by enzyme-linked immunosorbent assay (ELISA) from their supernatant after 24 hours. The graph is representative of 3 independent experiments.
Tumor protection of RENCA cells in WT and different knockout BALB/c mice. (A) WT and indicated knockout BABL/c mice were vaccinated with 106 irradiated RENCA cells and challenged 1 week later with 5 × 106 RENCA tumor cells. The survival curve indicates percentage of mice that survived the challenge. The graphic represents data of 4 independent experiments (n = 4 mice/group). (B) Tumorogenicity of RENCA cells in different knockout mice. WT and indicated knockout BALB/c mice were inoculated on the back with 2 × 106 RENCA tumor cells. Mice were killed when tumor size reached 15 mm in diameter or was ulcerated. This experiment is representative of 3 independent experiments. (C) Spontaneous production of GM-CSF by RENCA cells. Irradiated RENCA or B16-F10 cells (106) were seeded in a 10-mm plate and GM-CSF release was detected by enzyme-linked immunosorbent assay (ELISA) from their supernatant after 24 hours. The graph is representative of 3 independent experiments.
As vaccination with RENCA cells induces tumor immunity in WT and GM-CSF−/− but failed in βc−/− mice, we hypothesized that GM-CSF protein should be present during the priming phase of vaccination. We, therefore, assessed the possibility of GM-CSF secretion, necessary for the induction of antitumor response, from RENCA tumor cells. Supernatant of irradiated RENCA cells was analyzed by ELISA and murine GM-CSF secretion was confirmed at a low but detectable level (Figure 2C). In contrast, unmodified B16-F10 melanoma cells do not release any GM-CSF (Figure 2C). We can, therefore, postulate that the murine GM-CSF present in the supernatant of RENCA cells might be responsible for the induction of protective tumor immunization.
Tumor immunity in IL-3−/− and GM-CSF + IL-3−/− mice
We have previously described that BALB/c IL-3–deficient mice showed no impairment in tumor vaccination using the RENCA model.36 Here we present additional data showing similar results in mice lacking both GM-CSF and IL-3. Similarly to WT littermates, GM-CSF + IL-3−/− mice have no defect in tumor immunization and are protected from subsequent tumor challenge (Figure 2A).
βc−/− mice immunization with different cytokine-secreting tumor cells
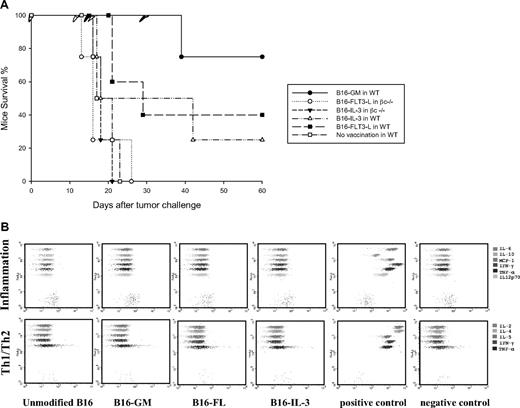
In the next set of experiments, we aimed to study the ability of other cytokines to immunize βc−/− mice. We, therefore, immunized WT and βc−/− mice either with irradiated B16-IL-3, B16-FL, or B16-GM. In WT animals, immunization with B16-IL-3 and B16-FL induces 40% and 25% (respectively) of survival upon tumor challenge compared with 75% tumor protection after B16-GM vaccination (Figure 3A). This is consistent with the previously published data where GM-CSF remains the most potent cytokine in the induction of tumor protection.7 On the contrary, none of these cytokine-producing cells was able to protect βc−/− mice, with all mice showing progressive tumor growth upon challenge (Figure 3A).
Tumor protection in WT and βc knockout mice vaccinated with different cytokine-producing tumor cells. (A) Survival of C57BL/6 mice vaccinated subcutaneously with irradiated cytokine-producing B16 cells. Mice were vaccinated with 106 B16 cells secreting GM-CSF, FLT3-L, or IL-3 and challenged 1 week later with 5 × 105 viable unmodified B16 cells. The graph is representative of 3 independent experiments. (B) Cytokine production by the modified B16 tumor cell lines used in the experiments. Cytokine-producing B16 cells were irradiated and seeded in triplicate at a density of 106 cells per well. Supernatant of cells was collected after 24 hours and IL-2, IL-4, IL-5, IL-6, IL-10, IL12p70, MCP-1, IFN-γ, and TNF-α were measured using inflammation and Th1/Th2 CBA kits. The positive and negative controls represent the DMEM complete medium and the 1250 pg/mL CBA standard curve, respectively. Each plot is representative of 1 single cell supernatant and illustrates 1 of the 2 independent experiments.
Tumor protection in WT and βc knockout mice vaccinated with different cytokine-producing tumor cells. (A) Survival of C57BL/6 mice vaccinated subcutaneously with irradiated cytokine-producing B16 cells. Mice were vaccinated with 106 B16 cells secreting GM-CSF, FLT3-L, or IL-3 and challenged 1 week later with 5 × 105 viable unmodified B16 cells. The graph is representative of 3 independent experiments. (B) Cytokine production by the modified B16 tumor cell lines used in the experiments. Cytokine-producing B16 cells were irradiated and seeded in triplicate at a density of 106 cells per well. Supernatant of cells was collected after 24 hours and IL-2, IL-4, IL-5, IL-6, IL-10, IL12p70, MCP-1, IFN-γ, and TNF-α were measured using inflammation and Th1/Th2 CBA kits. The positive and negative controls represent the DMEM complete medium and the 1250 pg/mL CBA standard curve, respectively. Each plot is representative of 1 single cell supernatant and illustrates 1 of the 2 independent experiments.
To determine whether the introduction of the different transgenes into B16-F10 could induce the secretion of other types of cytokine that might interfere with their respective properties, we analyzed the supernatants of cells for the presence of IL-2, IL-4, IL-5, IL-6, IL-10, MCP-1, IFN-γ, TNF-α, and IL12p70 using CBA system. Irradiated B16 WT, B16-FL, B16-IL3, and B16-GM cells (106) were cultured during 24 hours; their supernatant was collected and analyzed with the inflammation and Th1/Th2 kit of CBA system for the presence of the indicated cytokines (Figure 3B). We have not detected any of the above cytokines in the supernatant of different B16-F10 transformed cells; all the tested values were under the detection limit.
Characterization of effector function after GM-CSF–based tumor vaccination in different knockout mice
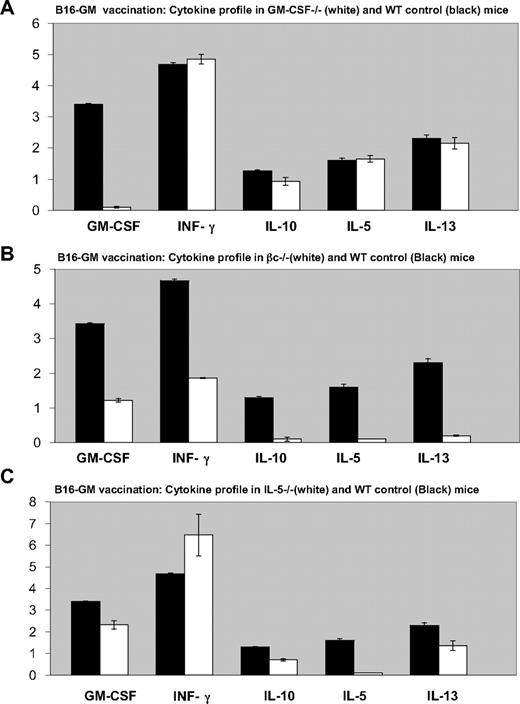
Vaccination with irradiated B16-GM cells stimulated comparable antitumor effector mechanisms in GM-CSF−/− mice as in WT controls. The tumor-induced production of IFN-γ, IL-5, IL-10, and IL-13 from splenocytes in GM-CSF−/− mice was similar to control animals, although the mutant animals were unable to secrete GM-CSF (Figure 4A). To delineate the basis for the loss of antitumor immunity in βc−/− mice, we characterized the generation of antitumor effector mechanisms. In contrast to WT or GM-CSF−/− animals, βc−/− mice showed reduced production of IFN-γ, GM-CSF, IL-5, IL-10, and IL-13 (Figure 4B). Furthermore, consistent with the results of tumor immunity (Figure 1B), the development of antitumor effector response in immunized IL-5−/− mice was comparable with WT animals (Figure 4C). In particular, the production of IFN-γ, GM-CSF, IL-10, and IL-13 was unimpaired, although the mutant animals were unable to secrete IL-5 (Figure 4C).
Comparison of tumor-induced cytokine profile in B16-GM vaccinated mice. Quantification of cytokines release by ELISA in cell suspension supernatants from spleen of B16-GM immunized C57BL/6 mice (■) or knockouts (A) GM-CSF−/− (B) βc−/− and (C) IL-5−/− mice (□), cocultured with irradiated B16 cells during 5 days, in the presence of IL-2. Values are in nanograms per milliliter. Error bars represent the standard deviation from triplicate samples of 1 single experiment. Similar results were obtained on 3 independent experiments.
Comparison of tumor-induced cytokine profile in B16-GM vaccinated mice. Quantification of cytokines release by ELISA in cell suspension supernatants from spleen of B16-GM immunized C57BL/6 mice (■) or knockouts (A) GM-CSF−/− (B) βc−/− and (C) IL-5−/− mice (□), cocultured with irradiated B16 cells during 5 days, in the presence of IL-2. Values are in nanograms per milliliter. Error bars represent the standard deviation from triplicate samples of 1 single experiment. Similar results were obtained on 3 independent experiments.
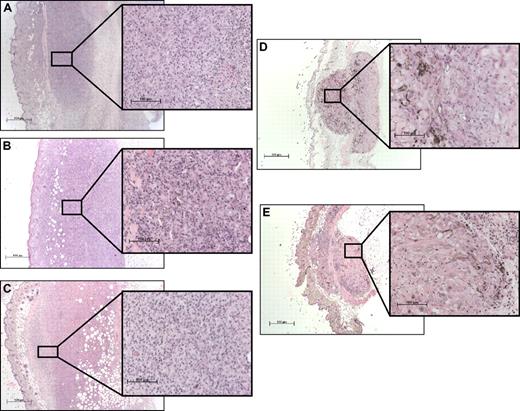
Histopathologic analysis of the immunization site in different knockout mice vaccinated with B16-GM
To understand the lack of vaccination efficacy in βc−/− mice, we performed histopathologic analyses of the site of tumor antigen capture 5 days after B16-GM immunization. This enabled us to investigate potential defects in the early phase of the response. In WT (Figure 5A) and GM-CSF−/− (Figure 5B) animals, B16-GM cells elicited a robust cellular infiltrate and inflammation at the site of vaccination. Central necrosis of the vaccination site is also observed. Histopathologic analysis of the vaccination sites in IL-5−/− animals was similar to WT mice (Figure 5C) except for reduced eosinophils. In contrast, the vaccination site in βc−/− mice showed minimal infiltrates and no inflammation (Figure 5D). Interestingly, this reaction was comparable with the response evoked in WT mice by vaccination with irradiated unmodified B16 cells (Figure 5E).
Histopathologic analysis of the vaccination site. (A) WT, (B) GM-CSF−/−, (C) IL-5−/−, and (D) βc−/− mice were immunized subcutaneously on the abdomen with 106 irradiated B16-GM cells. As control, WT mice were immunized with 106 irradiated B16 cells (E). Five days later, tissue samples were collected and fixed in formalin before paraffin embedding. Samples were then stained with hematoxylin and eosin. Magnification: 10 × 2.5 and 10 × 20.
Histopathologic analysis of the vaccination site. (A) WT, (B) GM-CSF−/−, (C) IL-5−/−, and (D) βc−/− mice were immunized subcutaneously on the abdomen with 106 irradiated B16-GM cells. As control, WT mice were immunized with 106 irradiated B16 cells (E). Five days later, tissue samples were collected and fixed in formalin before paraffin embedding. Samples were then stained with hematoxylin and eosin. Magnification: 10 × 2.5 and 10 × 20.
βc−/− mice immunized with GM-CSF–secreting tumor cells failed to recruit myeloid DCs at the site of vaccination
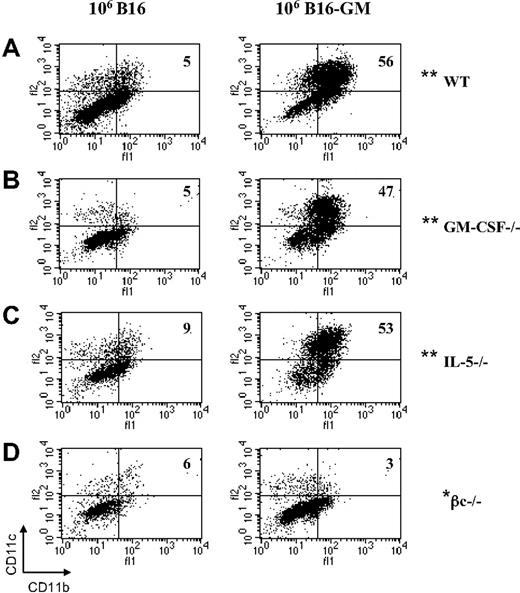
It has been shown that GM-CSF–secreting tumor cells induce the generation of potent antitumor immunity by increasing the local recruitment and maturation of myeloid-derived DCs.10 We therefore evaluated the capacity of immunized GM-CSF−/−, IL-5−/−, and βc−/− mice to recruit DCs at the vaccination site.
WT mice vaccinated with irradiated B16-GM tumor cells have significantly increased numbers of CD11c+CD11b+ DCs (36% ± 3.4%; SEM of 6 independent experiments) compared with WT animals vaccinated with irradiated unmodified B16 tumor cells (3.4% ± 1.9%; SEM of 5 independent experiments; Figure 6A). Furthermore, all recruited CD11b+CD11c+ DCs were CD8α− (data not shown).
Flow cytometric analysis of DC recruitment at the site of vaccination after B16 or B16-GM immunization. C57BL/6 WT (A), GM-CSF−/− (B), IL-5−/− (C), and βc−/− (D) mice were immunized with irradiated B16 or B16-GM (106 cells). Four days later cells at the vaccination sites were isolated and subsequently analyzed by flow cytometry for the presence of CD11c+, CD11b+, and CD8α+ DCs. Results are percentages and are representative of 1 of at least 3 animals per group. The difference observed between B16 and B16-GM in each group was highly significant (**P ≤ .005) except for the βc−/− group (*P = .45).
Flow cytometric analysis of DC recruitment at the site of vaccination after B16 or B16-GM immunization. C57BL/6 WT (A), GM-CSF−/− (B), IL-5−/− (C), and βc−/− (D) mice were immunized with irradiated B16 or B16-GM (106 cells). Four days later cells at the vaccination sites were isolated and subsequently analyzed by flow cytometry for the presence of CD11c+, CD11b+, and CD8α+ DCs. Results are percentages and are representative of 1 of at least 3 animals per group. The difference observed between B16 and B16-GM in each group was highly significant (**P ≤ .005) except for the βc−/− group (*P = .45).
Similarly to WT animals, DC recruitment was increased after B16-GM immunization in GM-CSF−/− (43% ± 3.3%; SEM of 6 independent experiments; Figure 6B) and IL-5−/− (39% ± 3.9%; SEM of 6 independent experiments) mice (Figure 6C). GM-CSF−/− (5% ± 0.5%; SEM of 6 independent experiments; Figure 6B) and IL-5−/− mice (3% ± 0.6%; SEM of 5 independent experiments; Figure 6C) vaccinated with unmodified B16 tumor cells showed a low percentage of DC recruitment that was similar to WT mice. In contrast, βc−/− mice fail to recruit DCs after B16-GM vaccination (4% ± 0.9%; SEM of 3 independent experiments; Figure 6D). This recruitment was similar to the vaccination of βc−/− mice with B16 WT tumor cells (4.25% ± 0.6%; SEM of 3 independent experiments; Figure 6D). Together, these observations suggest that the loss of antitumor immunity in βc−/− mice is not due to the lack of IL-5 signaling and establish a requirement for βc signaling in the early phase of GM-CSF–based vaccines.
Contact hypersensitivity reaction in βc−/− mice
To address whether βc−/− mice demonstrate a general impairment in cell-mediated immunity, we assessed the ability of these mice to generate contact hypersensitivity (CHS) in both mouse strains. This reaction is a form of delayed-type hypersensitivity in which hapten-protein conjugates are presented by cutaneous DCs, after their migration to regional lymph nodes, to hapten-specific CD4+ and CD8+ T lymphocytes.43 Upon secondary hapten challenge, sensitized T cells initiate a local inflammatory response. As shown in Figure 7, βc−/− mice mounted contact hypersensitivity reactions that were equivalent to WT controls in both C57BL/6 and BALB/c background.
Contact hypersensitivity reactions in βc−/− and WT mice. C57BL/6 and BALB/c mice were sensitized with oxazolone on the abdomen and foot pads on day zero. Five days later, mice were challenged on the right ear with 0.5% oxazolone in acetone–olive oil. The left ear was treated with the carrier (acetone–olive oil) alone. The ear thickness was measured with a micrometer at 24 hours after challenge. Results are presented as the increased thickness of the hapten-treated ear minus the nonspecific swelling (carrier-treated ear). Data represent mean values of 5 mice per group. Similar results were obtained in 3 independent experiments.
Contact hypersensitivity reactions in βc−/− and WT mice. C57BL/6 and BALB/c mice were sensitized with oxazolone on the abdomen and foot pads on day zero. Five days later, mice were challenged on the right ear with 0.5% oxazolone in acetone–olive oil. The left ear was treated with the carrier (acetone–olive oil) alone. The ear thickness was measured with a micrometer at 24 hours after challenge. Results are presented as the increased thickness of the hapten-treated ear minus the nonspecific swelling (carrier-treated ear). Data represent mean values of 5 mice per group. Similar results were obtained in 3 independent experiments.
Discussion
Many studies of tumor vaccination and adoptive T-cell therapy indicate important roles of GM-CSF in both the priming and the effector phases of antitumor responses. GM-CSF is thought to be one of the most potent adjuvants and is used in many tumor immunization schemes including DNA-, peptide-, tumor cell–, or dendritic cell–based vaccination. Despite numerous demonstrations of strong immunostimulatory effects in animal models and clinical trials, some reports have raised concerns regarding the detrimental effect of this cytokine when used at high concentration levels.17 In animal studies, GM-CSF has proven active when delivered or produced at the vaccination site. The adjuvant or antitumor immunization effect is thought to be maximal when prolonged and sustained release can be achieved at the inoculation site. Indeed, sustained local release of GM-CSF at the vaccination site by GM-CSF–secreting cells proved to be efficient in the induction of tumor immunity in many animal models when using cells engineered to release 90 to 300 ng/106 cells per 24 hours.7,44 In contrast, local release of high-dose GM-CSF at the vaccination site or injected intraperitoneally has been shown to block the immune response leading to the down-regulation of immune defense in antitumor reactions.18 In addition, high doses of GM-CSF injected subcutaneously have shown to be detrimental, resulting in the expansion of myeloid suppressor cells.19
The role of endogenous GM-CSF for generating and maintaining crucial hematopoietic cell types involved in immune responses has not been addressed fully. The design of this study aims at a better understanding of the selective role of endogenous GM-CSF and GM-CSF produced locally at the vaccination site by the implanted tumor cells and does address the role of other cytokines and chemokines.
Our experiments were undertaken in an effort to learn more about the requirements for GM-CSF signaling in antitumor immunity. The results showed that host-derived GM-CSF is dispensable for both the priming and the effector phases of GM-CSF–based tumor cell vaccines in 2 different antitumor immunization models using either genetically modified GM-CSF–secreting B16 tumor cell line in C57BL/6 or the spontaneously immunogenic RENCA tumor cell line in BALB/c. In the B16-GM model, the local release of exogenous GM-CSF by irradiated genetically modified tumor cells at the vaccination site is sufficient to trigger an efficient immune response in WT and GM-CSF−/− mice. These findings further imply that endogenous GM-CSF is not required for the development and survival of the various cell populations involved in GM-CSF–based antitumor immunization in at least 2 distinct tumor models.
In contrast, our experiments in βc-deficient mice demonstrated that GM-CSF signaling is crucial during the priming phase of vaccination. The tumor protection elicited by both GM-CSF–secreting B16 cells and the unmodified RENCA cells was completely abrogated in βc−/− mice. This was associated with a failure to develop granulocyte, macrophage, DC, and lymphocyte infiltrates as a consequence of vaccination. The β-subunit of the GM-CSF receptor is identical for IL-3 and IL-5 receptors. The α subunits of GM-CSF, IL-3, and IL-5 receptors are distinct and the α/β heterodimer forms a high-affinity receptor for the respective cytokines. In mice, a second βc for IL-3 has been identified (β-IL-3), which binds to the IL-3α subunit with low affinity, and forms a high-affinity receptor to transmit the proliferation signal.34 Mice lacking the βc subunit are therefore lacking both GM-CSF and IL-5 signaling but have adequate IL-3 signaling. In addition, we have previously shown that IL-3–deficient mice do not have any defect in tumor vaccination in the RENCA tumor model.36
Our results formally established that α chain signaling is not sufficient for the generation of antitumor immunity in these models and that specific functions mediated through the βc subunit receptor are required. Previous studies have demonstrated that the GM-CSF α chain receptor alone is insufficient to mediate in vitro survival of hematopoietic cells.45 Future experiments involving the adoptive transfer of defined cell populations from WT animals should help further elucidate the cellular requirements for effective priming.
Because βc is involved in both IL-5 and GM-CSF signaling, one explanation for the loss of tumor immunity was impaired IL-5 function. However, our results argue strongly against this possibility, by showing that the vaccine responses were not diminished in IL-5−/− mice. Furthermore, our results fail to confirm a previous report suggesting a significant role for IL-5 in this system.9 Although the basis for the discrepancy between the 2 studies is currently unclear, age-related B-cell defects in IL-5−/− mice or the amount of GM-CSF produced at the vaccine site may contribute to differences in immunization.38,46
The compromised tumor protection in βc−/− mice did not reflect a requirement for host-derived GM-CSF, as the efficiency of tumor vaccination in GM-CSF−/− mice was indistinguishable from WT controls. The generation of antitumor effectors in immunized GM-CSF−/− animals was also similar to WT littermates. Although these findings show that host-derived GM-CSF is dispensable for GM-CSF–based tumor vaccination, they do not preclude the possibility that the coordinated activities of GM-CSF and other factors are required for optimal tumor protection. Indeed, our previous studies of mice deficient in both GM-CSF and IL-3 established overlapping roles for these cytokines in hematopoiesis and immunity.37 Furthermore the close interrelation between inflammation and cancer is well illustrated by the study of mice lacking both GM-CSF and IFN-γ. The inability to uptake apoptotic cells by GM-CSF−/− antigen-presenting cells (APCs) led to autoimmunity via decreased numbers of regulatory T cells (Tregs), whereas double knockout mice lacking both GM-CSF and IFN-γ showed a marked increase in the incidence of both solid and hematologic tumors.20,47
Finally, tumor vaccination in βc−/− mice was completely inefficient and this was associated with a marked decrease in IL-5, IL-10, and IL-13 production and lack of inflammatory cells influx at the vaccination site (Figures 4B, 5D). This is probably due to the lack of DC recruitment at the site of vaccination in βc−/− mice (Figure 6D) that is mandatory in the initiation of effective antitumor responses. The critical role of βc signaling is further illustrated by the loss of protective immunity in the RENCA model. As production of GM-CSF by cancer cell lines has been reported for solid tumors including renal cell carcinoma,48 our hypothesis was that spontaneous release of GM-CSF by RENCA cells may trigger the priming phase. Indeed, analysis of supernatant from irradiated RENCA cells revealed a spontaneous production of GM-CSF. These results point to a unexpected role of GM-CSF signaling in immunogenicity. This is also illustrated by the inability of βc−/− mice to develop protective immunity when using B16 cells secreting other cytokines such as FLT3-L or IL-3. Our results parallel the recently published data revealing the critical role of βc signaling in lung inflammation and Th2 responses.49 Nevertheless βc−/− mice do not have generalized severe impairment in cell-mediated immunity as the contact hypersensitivity reaction is similar to WT control. The CHS data showed that at least some antigen-specific T-cell responses do not rely upon βc function. These observations point to a specific defect crucial for cell-based antitumor immunization. Working hypotheses include the lack of recruitment and/or differentiation of a subclass of DCs critically needed for the coordination of an efficient cell-based tumor immunization. In addition, a recent paper demonstrated a novel role for GM-CSF as being a potent driver of Th17 cells.50 It is therefore interesting to determine whether GM-CSF–based tumor rejection is mediated via Th17 effector responses and whether this response is abolished in βc−/− mice. Experiments analyzing the protective effect of other known potent adjuvants (cytokines and chemokines) in mice lacking GM-CSF signaling will be of great interest. Additional studies will help to better characterize the molecular defect responsible for the loss of antitumor immunity observed in βc−/− mice and may bring further understanding to improve cell-based antitumor immunization schemes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Glenn Begley for providing the βc−/− mice and for his comments and helpful discussions and Paul Walker for carefully reading the paper.
This work was supported by the Swiss National Science Foundation (PNR 37) (Berne, Switzerland), La Ligue genevoise contre le cancer (Geneva, Switzerland), la Fondation pour la lutte contre le cancer (Zurich, Switzerland; N.M.), the Cancer Research Institute/Partridge Foundation (New York, NY), and CA 74886 (G.D). G.D. is a Clinical Scholar of the Leukemia & Lymphoma Society (White Plains, NY).
Authorship
Contribution: S.Z. performed research and wrote the paper; F.S. and P.M. analyzed data; P.L. performed research; M.A.-L. provided new reagents and analyzed data; M.K. provided critical reagents; G.D. designed research and analyzed data; and N.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolas Mach, Oncology Division, Department of Internal Medicine, Geneva University Hospital, 24 Rue Micheli-du-Crest, 1205 Geneva, Switzerland; e-mail: nicolas.mach@medecine.unige.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal