In this issue of Blood, Horst and colleagues demonstrate that myeloid cells expressing the adhesion molecule CEACAM1 play a role in promoting angiogenesis and resolving inflammation.
In the adult, angiogenesis is essential to wound repair and inflammation and for highly specialized functions, such as the regeneration of the endometrium. Angiogenesis is also involved in tumor growth, chronic inflammatory diseases and atherosclerosis. Vast literature has emerged that investigates the cellular and molecular mechanisms underlying the formation of new vessels. One of the important findings over the past few years has been the identification of the role of different cell types in angiogenesis.
As with endothelial cells (ECs) and their bone marrow (BM) precursors, heterogeneous populations of BM-derived cells of the myeloid lineage contribute to the angiogenic process. Among the phenotypes described are Tie2-expressing immature monocytes, tumor-associated macrophages, CXCR4+ VEGFR1+ hemangiocytes, and Gr1+CD11b+ myeloid cells.1 These cell populations, originating from a common BM-derived precursor, circulate in peripheral blood until recruited to tissues by specific chemoattractants. Once there, their phenotype may change in response to local signals. The proangiogenic role of BM-derived myeloid cells has been demonstrated in several animal models and human clinical trials. However, the underlying mechanism remains controversial. Several distinct modes of action for the role of myeloid cells in promoting angiogenesis have been proposed,1 including the release of cytokines and growth factors with proangiogenic activity (see figure).
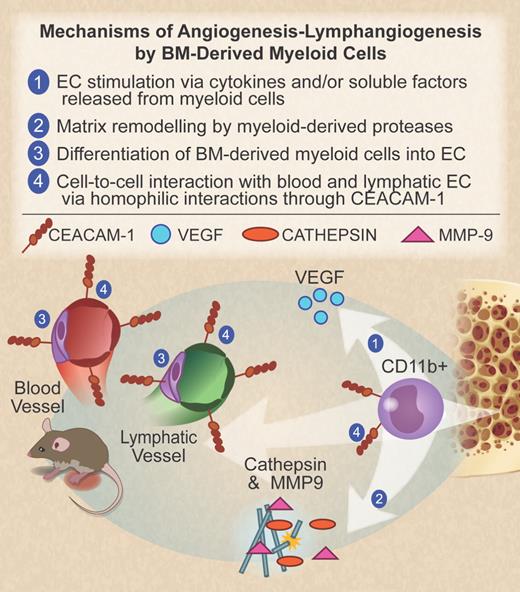
CD11b+ myeloid progenitor cells, originating from the bone marrow, migrate to the site of inflammation to promote angiogenesis and lymphangiogenesis. Several possible mechanisms are listed here. (1) Release of cytokines and growth factors with proangiogenic activity, including VEGF. (2) Production of proteases such as the cysteine protease cathepsin and matrix metalloprotease-9 (MMP-9), which mediate degradation of the extracellular matrix and release of VEGF from its membrane-bound form. (3) Myeloid cell differentiation into endothelial cells, a mechanism that is still debated. (4) Direct interaction between myeloid cells and blood or lymphatic endothelial cells through CEACAM1 in an homophilic manner, as described by Horst et al in the article that begins on page 6726. Professional illustration by Debra T. Dartez.
CD11b+ myeloid progenitor cells, originating from the bone marrow, migrate to the site of inflammation to promote angiogenesis and lymphangiogenesis. Several possible mechanisms are listed here. (1) Release of cytokines and growth factors with proangiogenic activity, including VEGF. (2) Production of proteases such as the cysteine protease cathepsin and matrix metalloprotease-9 (MMP-9), which mediate degradation of the extracellular matrix and release of VEGF from its membrane-bound form. (3) Myeloid cell differentiation into endothelial cells, a mechanism that is still debated. (4) Direct interaction between myeloid cells and blood or lymphatic endothelial cells through CEACAM1 in an homophilic manner, as described by Horst et al in the article that begins on page 6726. Professional illustration by Debra T. Dartez.
In this issue of Blood, Horst et al contribute to the debate around angiogenesis and lymphangiogenesis by describing a novel pathway.2 In this study, Horst et al identify a novel mechanism of adhesion-mediated stimulation of angiogenesis and lymphangiogenesis, which involves the adhesion molecule CEACAM1 (CEA-related cell-adhesion molecule). Using the CEACAM1-deficient mouse and a model of cutaneous inflammation, they find that BM-derived CD11b+ CEACAM1+ monocyte progenitor cells are involved in the resolution of inflammation by promoting the formation of blood and lymphatic vessels. The authors observe that in the CEACAM1−/− mice, the inflammatory response is more intense and prolonged. Both angiogenesis and lymphangiogenesis are reduced with consequent edema, probably due to impaired vascular drainage. They then proceed to demonstrate that this effect is due to the absence of CEACAM1+ BM-derived monocytes, since the phenotype can be corrected by BM transplants from B6 mice.
CEACAM1, a member of the immunoglobulin (Ig) superfamily of cell adhesion molecules, is expressed in microvessels of proliferating tissues, in tissues after wounding, and in solid human tumors.3 Endothelial CEACAM1 has been previously shown by this group and others to play a role in angiogenesis through various possible mechanisms, which include regulation of EC proliferation and migration via the integrin αvβ3 and angiogenic growth factors including VEGF.4 Other members of the Ig superfamily of endothelial adhesion molecules that support homophilic adhesion, such as CD31/PECAM and ICAM-2, have been shown to be required for effective angiogenesis in the adult while being dispensable for vasculogenesis and embryonic development.5,6 The novelty of the article by Horst et al is the description of a new mechanism that appears to rely on adhesion between CEACAM1+ myeloid progenitors and CEACAM1+ ECs. Thus, CEACAM1 might engage in a homophilic manner between leukocytes and the endothelium, mediating extravasation and/or signaling, or even incorporation into the vascular structure.
The same mechanism could be involved in the generation of lymphatic vessels: CEACAM1+ is also expressed in lymphatic neovessels, and Horst et al show that CEACAM1+ monocytes are required for lymphangiogenesis. The involvement of monocytes/macrophages and dendritic cells of monocytic lineage in the development of novel lymphatic vessels is known.7 The authors suggest that CEACAM1 may be a regulator of lymphatic-specific signaling pathways.
The reported expression of CEACAM1 by immature myeloid, lymphatic, and blood ECs might be a consequence of their common origin from the hemangioblast, as suggested for other molecules such as CD31/PECAM.8 However, another possibility is that ECs in the inflamed vessels originate by direct differentiation of myeloid progenitor cells. Myeloid cells have features that are similar to endothelial progenitor cells, they can acquire endothelial-specific markers, and their possible endothelial differentiation has been suggested.9 This intriguing mechanism deserves further study to define the precise relationship between myeloid and ECs. Another open question is the relative role of endothelial and myeloid CEACAM1 in regulating angiogenesis.
Finally, these results raise the possibility that other Ig superfamily members may be mediating angiogenesis through a similar mechanism, involving homophilic interaction between BM-derived myeloid cells and ECs.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal